

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071777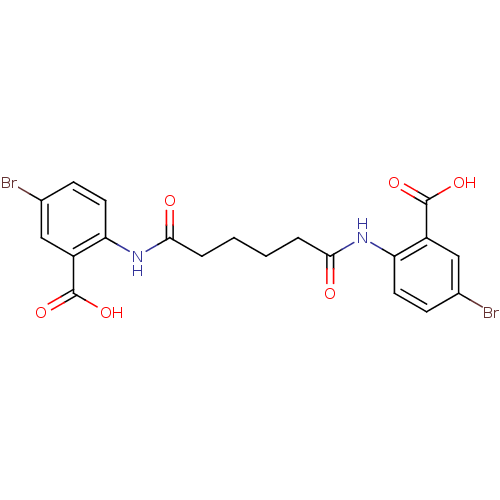 (5-bromo-2-({6-[(4-bromo-2-carboxyphenyl)amino]-6-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071776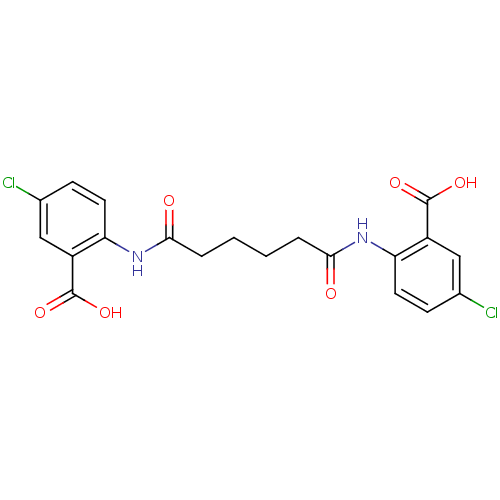 (2-({6-[(2-carboxy-4-chlorophenyl)amino]-6-oxohexan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071777 (5-bromo-2-({6-[(4-bromo-2-carboxyphenyl)amino]-6-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071766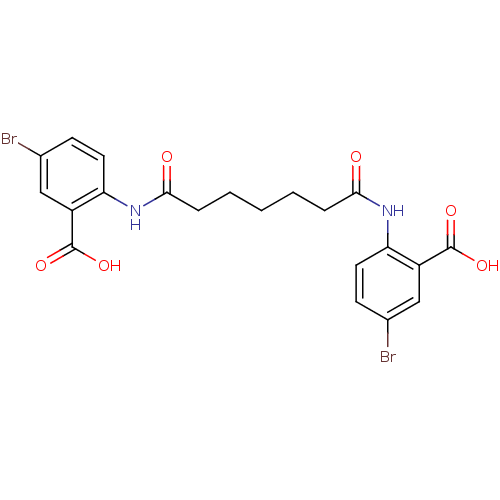 (5-bromo-2-[5-(4-bromo-2-carboxyphenylcarbamoyl)pen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071768 (2-({6-[(2-carboxy-4-iodophenyl)amino]-6-oxohexanoy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071765 (2-[4-(2-carboxy-4-methoxyphenylcarbamoyl)butylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071766 (5-bromo-2-[5-(4-bromo-2-carboxyphenylcarbamoyl)pen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071771 (5-bromo-2-{[(5-{[(4-bromo-2-carboxyphenyl)amino]ca...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071767 (2-({6-[(2-carboxy-4-hydroxyphenyl)amino]-6-oxohexa...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071779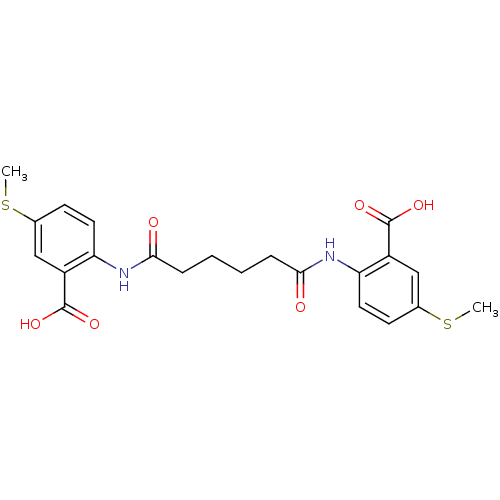 (2-[4-(2-carboxy-4-methylsulfanylphenylcarbamoyl)bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071762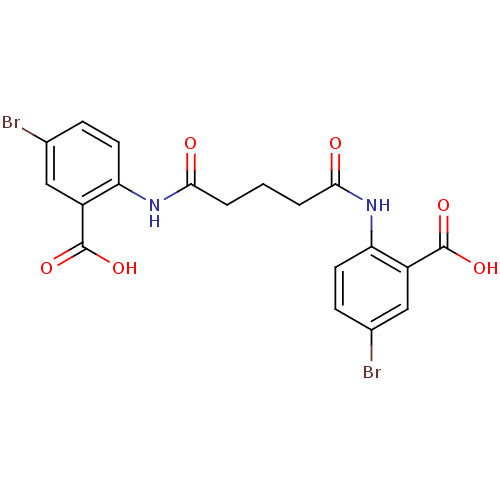 (5-bromo-2-({5-[(4-bromo-2-carboxyphenyl)amino]-5-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071761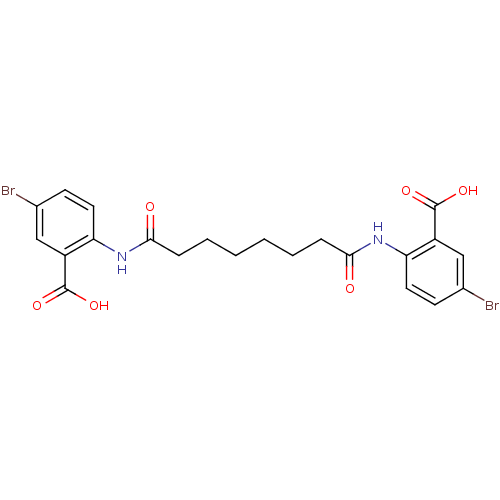 (5-bromo-2-[6-(4-bromo-2-carboxyphenylcarbamoyl)hex...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of HIV-2 reverse transcriptase using rC.dG and [3H]-dGTP as substrates at 100 microg/ml | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071778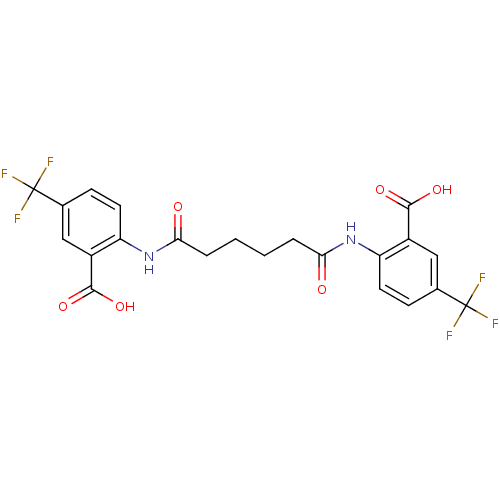 (2-[4-(2-carboxy-4-trifluoromethylphenylcarbamoyl)b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071768 (2-({6-[(2-carboxy-4-iodophenyl)amino]-6-oxohexanoy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071774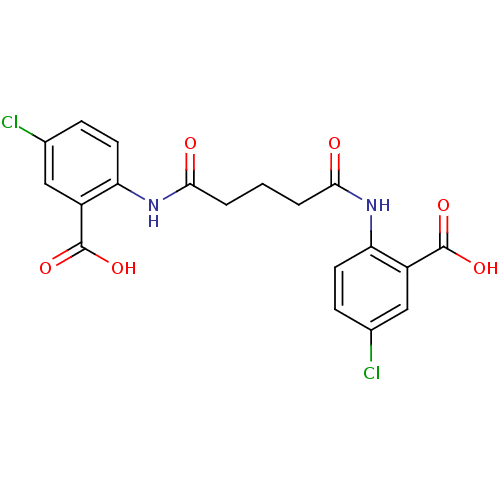 (2-({5-[(2-carboxy-4-chlorophenyl)amino]-5-oxopenta...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071776 (2-({6-[(2-carboxy-4-chlorophenyl)amino]-6-oxohexan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071763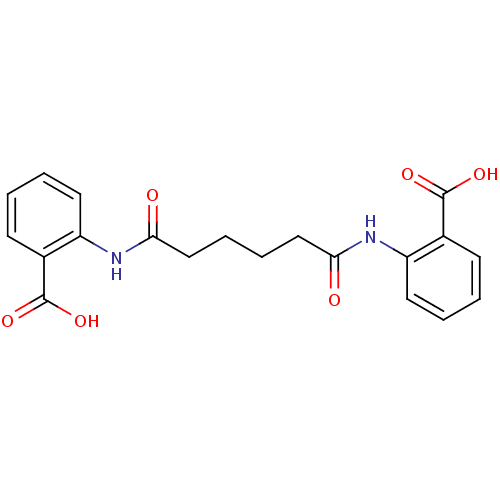 (2-({6-[(2-carboxyphenyl)amino]-6-oxohexanoyl}amino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071773 (2-[5-(2-carboxy-4-chlorophenylcarbamoyl)pentylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071773 (2-[5-(2-carboxy-4-chlorophenylcarbamoyl)pentylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071763 (2-({6-[(2-carboxyphenyl)amino]-6-oxohexanoyl}amino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071770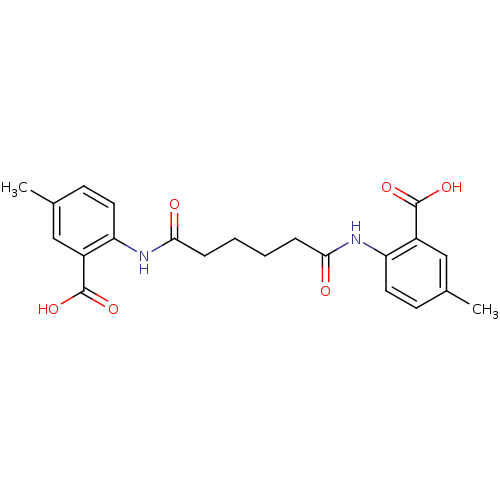 (2-[4-(2-carboxy-4-methylphenylcarbamoyl)butylcarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071774 (2-({5-[(2-carboxy-4-chlorophenyl)amino]-5-oxopenta...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071762 (5-bromo-2-({5-[(4-bromo-2-carboxyphenyl)amino]-5-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071765 (2-[4-(2-carboxy-4-methoxyphenylcarbamoyl)butylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071778 (2-[4-(2-carboxy-4-trifluoromethylphenylcarbamoyl)b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071770 (2-[4-(2-carboxy-4-methylphenylcarbamoyl)butylcarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071780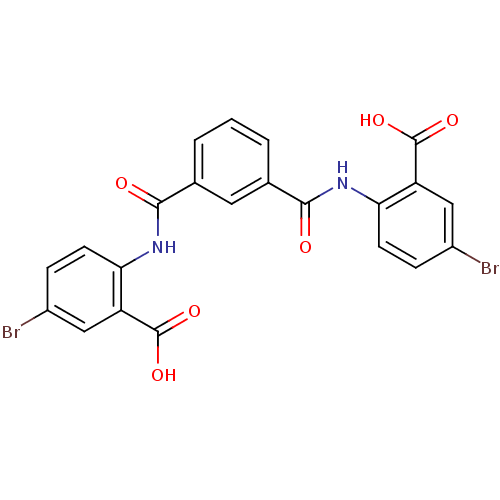 (5-bromo-2-[(3-{[(4-bromo-2-carboxyphenyl)amino]car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071760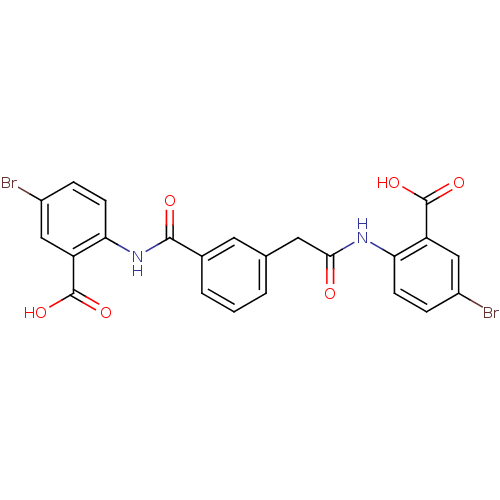 (5-bromo-2-{[(3-{[(4-bromo-2-carboxyphenyl)amino]ca...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071764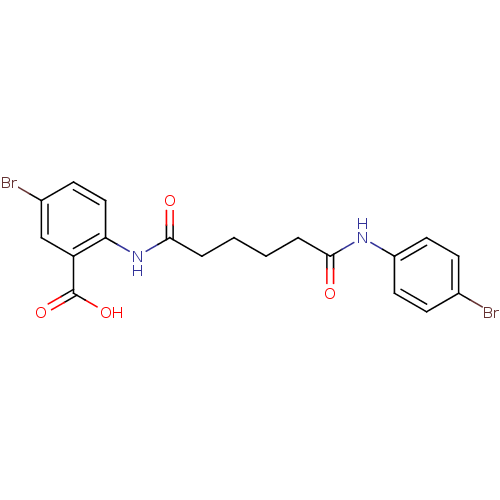 (5-Bromo-2-[5-(4-bromo-phenylcarbamoyl)-pentanoylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071761 (5-bromo-2-[6-(4-bromo-2-carboxyphenylcarbamoyl)hex...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of HIV-1 reverse transcriptase using rC.dG and [3H]-dGTP as substrates at 100 ug/mL | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071779 (2-[4-(2-carboxy-4-methylsulfanylphenylcarbamoyl)bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071780 (5-bromo-2-[(3-{[(4-bromo-2-carboxyphenyl)amino]car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071760 (5-bromo-2-{[(3-{[(4-bromo-2-carboxyphenyl)amino]ca...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071764 (5-Bromo-2-[5-(4-bromo-phenylcarbamoyl)-pentanoylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071771 (5-bromo-2-{[(5-{[(4-bromo-2-carboxyphenyl)amino]ca...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071772 (5-bromo-2-[(2-{[(4-bromo-2-carboxyphenyl)amino]car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071772 (5-bromo-2-[(2-{[(4-bromo-2-carboxyphenyl)amino]car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071759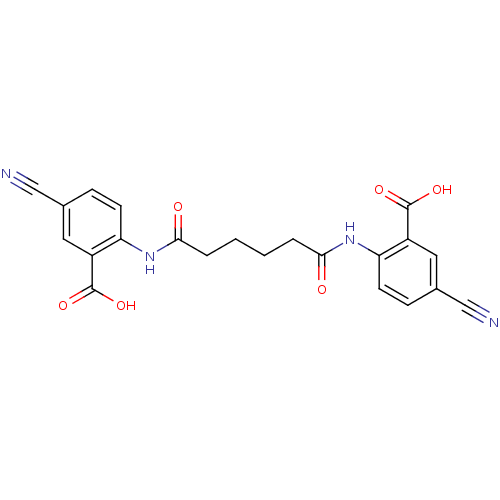 (2-({6-[(2-carboxy-4-cyanophenyl)amino]-6-oxohexano...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071775 (2-[4-(2-carboxy-4-methylcarboxamidophenylcarbamoyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of HIV-1 reverse transcriptase using rC.dG and [3H]-dGTP as substrates at 100 microg/ml | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071759 (2-({6-[(2-carboxy-4-cyanophenyl)amino]-6-oxohexano...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of HIV-2 reverse transcriptase using rC.dG and [3H]-dGTP as substrates at 100 ug/mL | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071775 (2-[4-(2-carboxy-4-methylcarboxamidophenylcarbamoyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071769 (2-({6-[(2-carboxy-4-fluorophenyl)amino]-6-oxohexan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071769 (2-({6-[(2-carboxy-4-fluorophenyl)amino]-6-oxohexan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071767 (2-({6-[(2-carboxy-4-hydroxyphenyl)amino]-6-oxohexa...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||