

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.270 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18699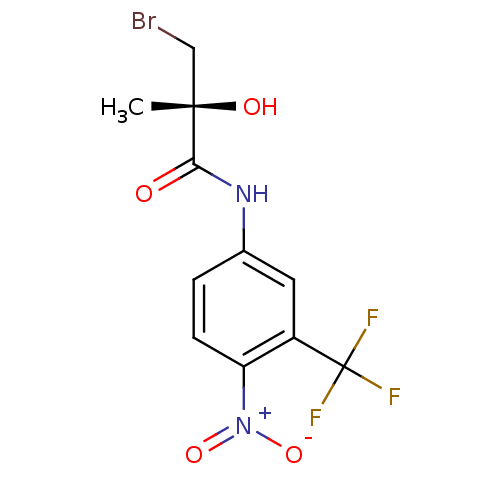 ((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215713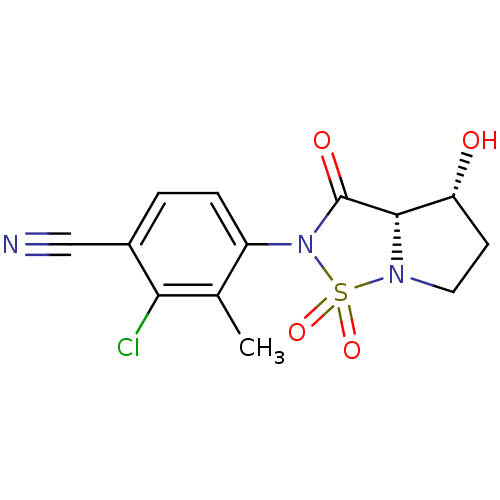 (2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM26260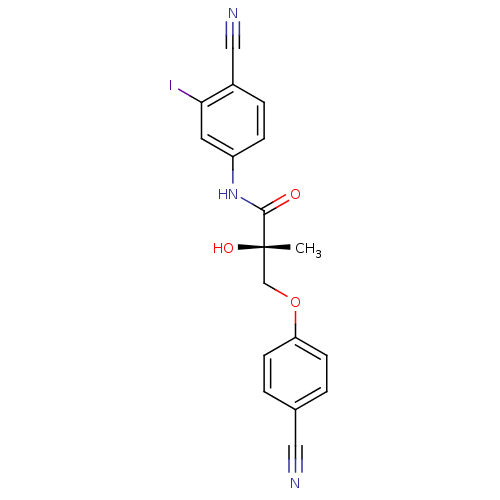 ((2S)-N-(4-cyano-3-iodophenyl)-3-(4-cyanophenoxy)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 0.540 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18177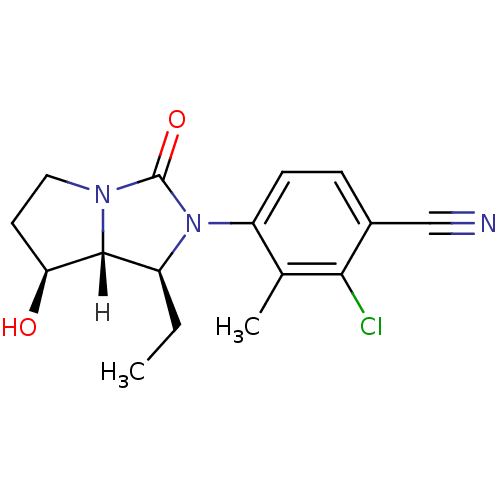 (4-[(1S,7S,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50258751 ((R)-2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-1,1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM26262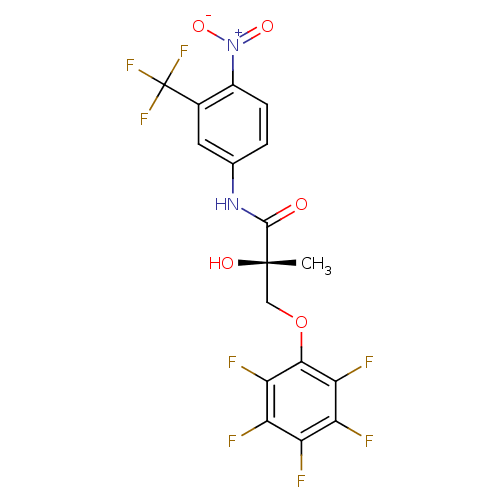 ((2S)-2-hydroxy-2-methyl-N-[4-nitro-3-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.40 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18522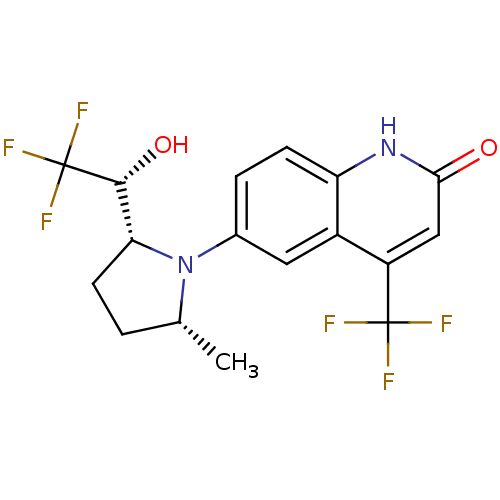 (6-(1-Pyrrolidine)quinolin-2(1H)-one, 6a | 6-[(2R,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18524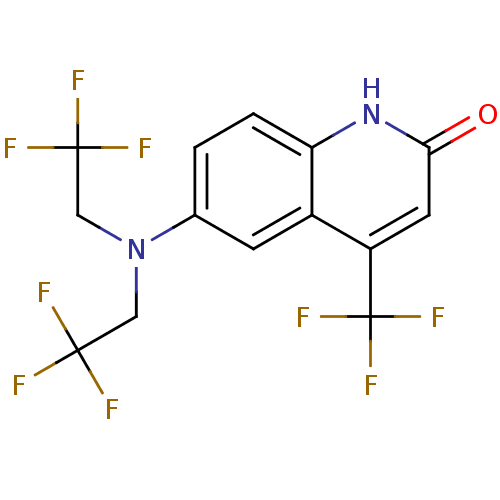 (6-[bis(2,2,2-trifluoroethyl)amino]-4-(trifluoromet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18685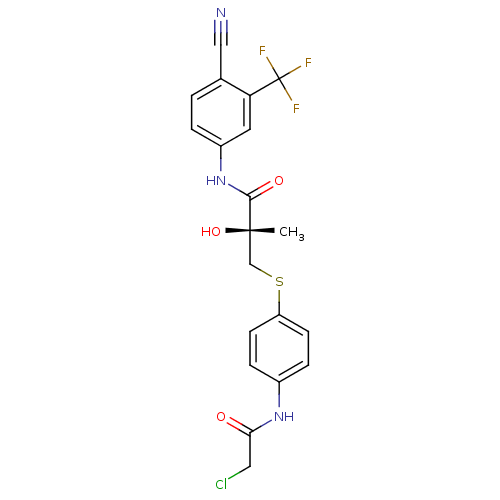 ((2R)-3-{[4-(2-chloroacetamido)phenyl]sulfanyl}-N-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM26261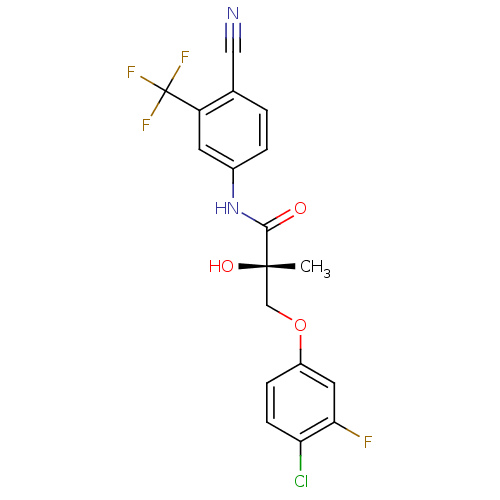 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.70 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM26261 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18173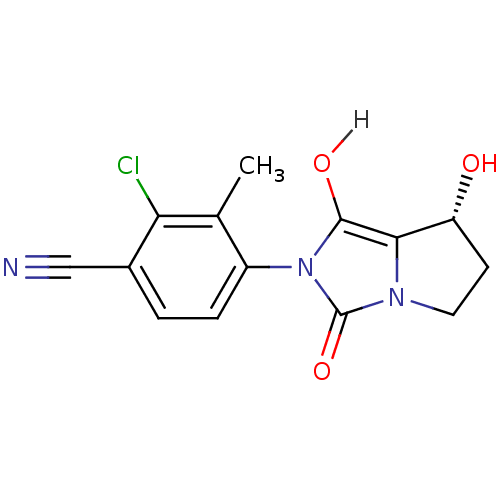 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM26259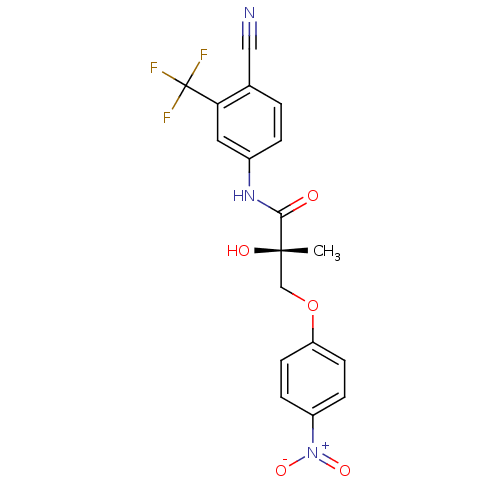 ((2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 2.5 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18665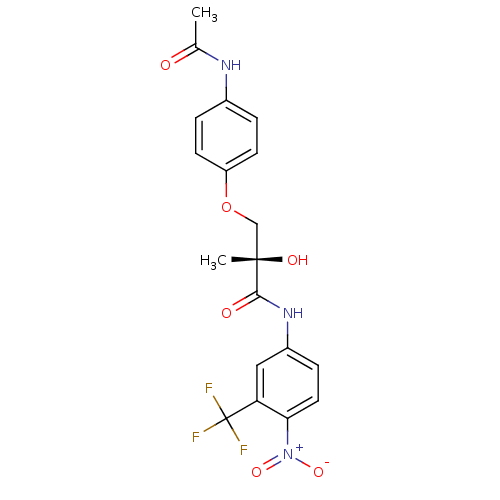 ((2S)-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.98 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18665 ((2S)-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18665 ((2S)-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 4 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18675 ((2S)-2-hydroxy-3-(4-isothiocyanatophenoxy)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.62 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18661 ((2R)-3-[(4-acetamidophenyl)sulfanyl]-2-hydroxy-2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.90 | -44.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50258791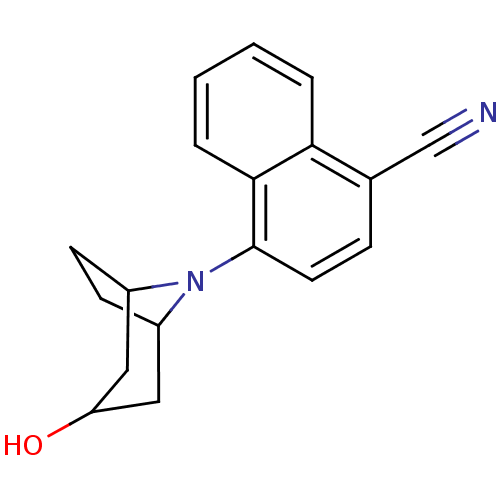 (4-(3-exo-Hydroxy-8-azabicyclo[3.2.1]oct-8-yl)napht...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18667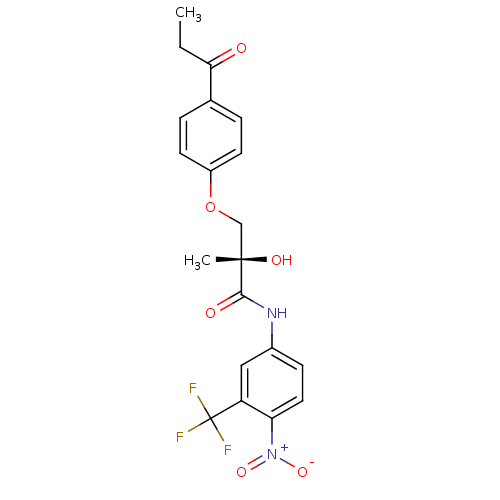 ((2S)-2-hydroxy-2-methyl-N-[4-nitro-3-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.07 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18663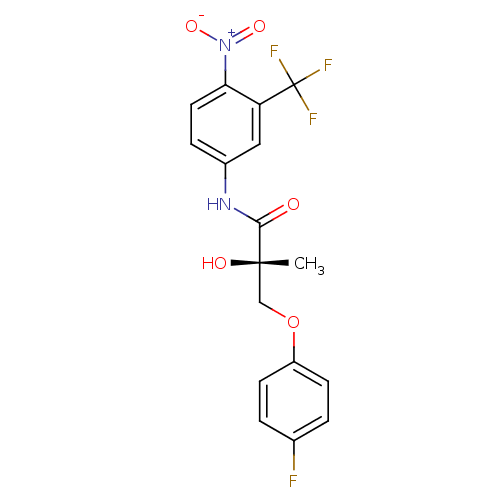 ((2S)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-N-[4-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18663 ((2S)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-N-[4-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6.10 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18663 ((2S)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-N-[4-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6.11 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18216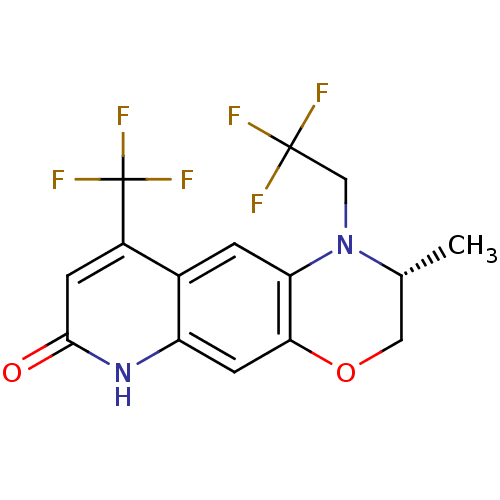 ((2R)-2-methyl-1-(2,2,2-trifluoroethyl)-9-(trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18676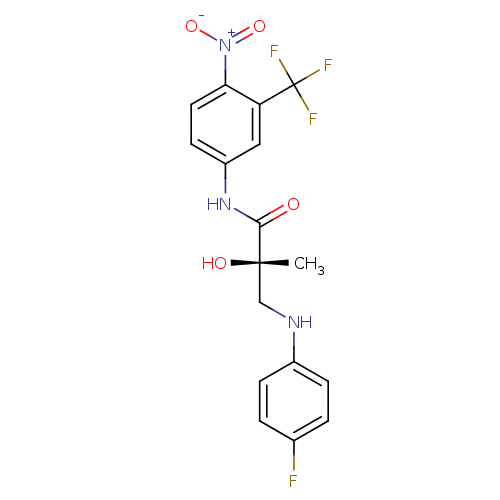 ((2S)-3-[(4-fluorophenyl)amino]-2-hydroxy-2-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7.96 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18668 ((2S)-3-(4-chlorophenoxy)-2-hydroxy-2-methyl-N-[4-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 9.56 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18678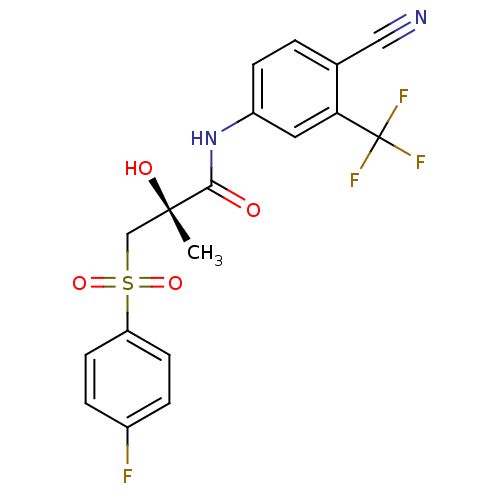 ((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 11 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18669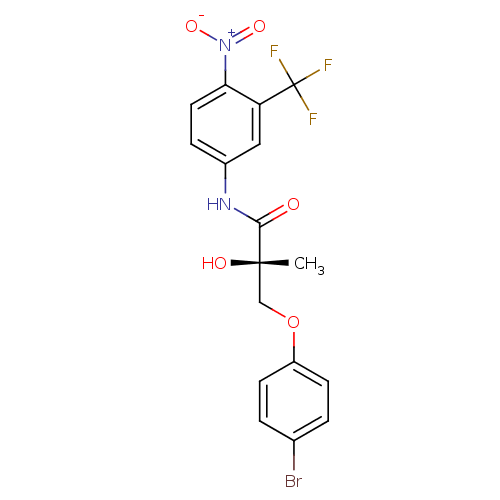 ((2S)-3-(4-bromophenoxy)-2-hydroxy-2-methyl-N-[4-ni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11.6 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18672 ((2S)-2-hydroxy-3-(4-methoxyphenoxy)-2-methyl-N-[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13.7 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50258752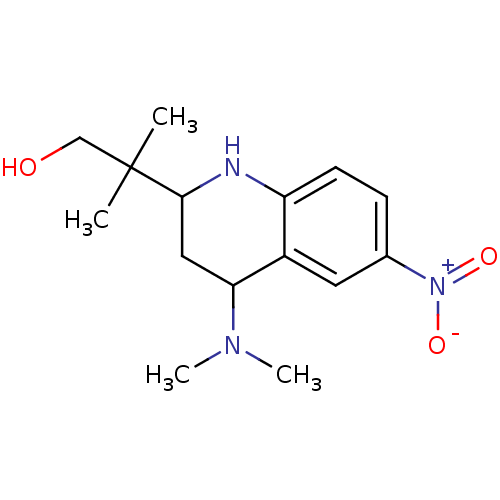 (2-(4-(dimethylamino)-6-nitro-1,2,3,4-tetrahydroqui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18674 ((2S)-2-hydroxy-3-[4-(1H-indol-5-yl)phenoxy]-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17.0 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 5 [180-384] (Homo sapiens (Human)) | BDBM50336799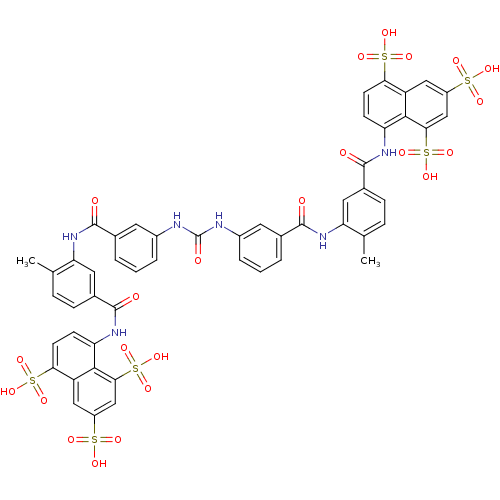 (5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | -43.4 | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Texas Wesleyan University | Assay Description For the 96-well plate validation assay, sodium orthovanadate (Sigma Aldrich) was utilized as a positive control for inhibition [Swarup et al., Bioche... | BMC Biochem 16: 19 (2015) Article DOI: 10.1186/s12858-015-0048-3 BindingDB Entry DOI: 10.7270/Q26972FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18670 ((2S)-2-hydroxy-3-(4-iodophenoxy)-2-methyl-N-[4-nit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30.0 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18671 ((2S)-2-hydroxy-2-methyl-3-(4-methylphenoxy)-N-[4-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 34.8 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18666 ((2S)-3-(4-acetylphenoxy)-2-hydroxy-2-methyl-N-[4-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 36.6 | -39.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18677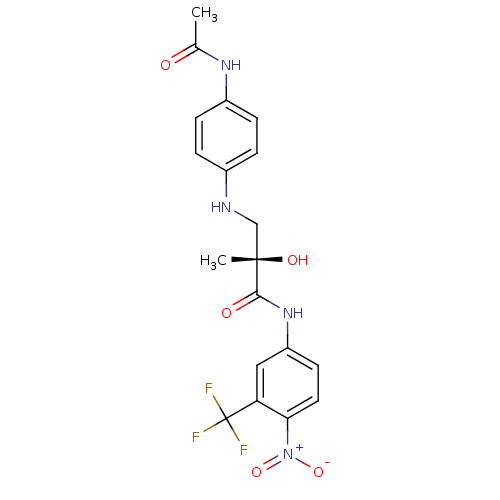 ((2S)-3-[(4-acetamidophenyl)amino]-2-hydroxy-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 128 | -36.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18664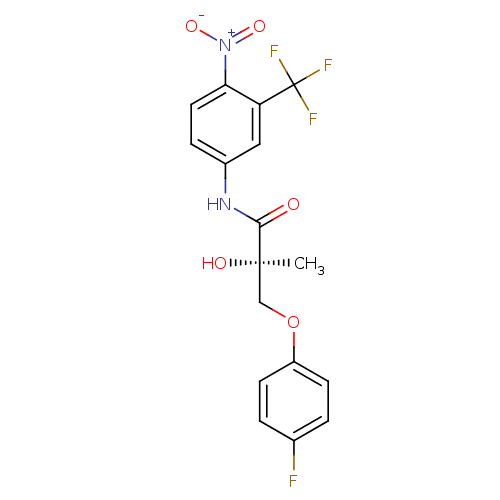 ((2R)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-N-[4-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 225 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18673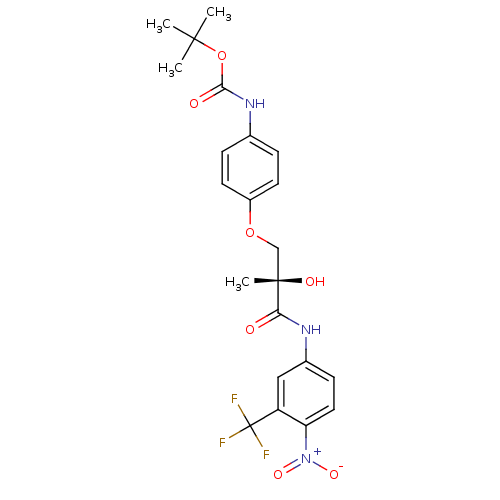 (Nonsteroidal AR Ligand, S-14 | tert-butyl N-{4-[(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 336 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50241192 ((S)-3-methyl-5-trifluoromethyl-3,4-dihydro-2H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50345228 ((R)-3-(2-benzylphenylsulfonyl)-N-(4-cyano-3-(trifl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Curated by ChEMBL | Assay Description Binding affinity to wild type androgen receptor by competitive binding assay | J Med Chem 54: 3973-6 (2011) Article DOI: 10.1021/jm2000097 BindingDB Entry DOI: 10.7270/Q23F4PZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50345227 ((R)-N-(4-cyano-3-(trifluoromethyl)phenyl)-2-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Curated by ChEMBL | Assay Description Binding affinity to wild type androgen receptor by competitive binding assay | J Med Chem 54: 3973-6 (2011) Article DOI: 10.1021/jm2000097 BindingDB Entry DOI: 10.7270/Q23F4PZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50345229 ((R)-3-(biphenyl-2-ylsulfonyl)-N-(4-cyano-3-(triflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Curated by ChEMBL | Assay Description Binding affinity to wild type androgen receptor by competitive binding assay | J Med Chem 54: 3973-6 (2011) Article DOI: 10.1021/jm2000097 BindingDB Entry DOI: 10.7270/Q23F4PZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM231697 (RR601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Concordia University of Wisconsin | Assay Description Assays with and without inhibitor were performed in Corning 96-well clear bottom plates having a nonbinding surface, with a total assay volume of 200... | BMC Biochem 18: 10 (2017) Article DOI: 10.1186/s12858-017-0083-3 BindingDB Entry DOI: 10.7270/Q2JW8CSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 5 (Homo sapiens (Human)) | BDBM231694 (1-Amin-5-Naphthol-7-Sulfonic acid | NCI2602 | RR53...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University of Wisconsin | Assay Description This assay was done with full-length protein (containing both domains), andusing pERK as substrate. | BMC Biochem 18: 10 (2017) Article DOI: 10.1186/s12858-017-0083-3 BindingDB Entry DOI: 10.7270/Q2JW8CSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM231697 (RR601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Concordia University of Wisconsin | Assay Description Assays with and without inhibitor were performed in Corning 96-well clear bottom plates having a nonbinding surface, with a total assay volume of 200... | BMC Biochem 18: 10 (2017) Article DOI: 10.1186/s12858-017-0083-3 BindingDB Entry DOI: 10.7270/Q2JW8CSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50345227 ((R)-N-(4-cyano-3-(trifluoromethyl)phenyl)-2-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Curated by ChEMBL | Assay Description Antagonist activity at wild type androgen receptor expressed in african green monkey CV1 cells assessed as inhibition of dihydrotestosterone-induced ... | J Med Chem 54: 3973-6 (2011) Article DOI: 10.1021/jm2000097 BindingDB Entry DOI: 10.7270/Q23F4PZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50345228 ((R)-3-(2-benzylphenylsulfonyl)-N-(4-cyano-3-(trifl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Curated by ChEMBL | Assay Description Antagonist activity at wild type androgen receptor expressed in african green monkey CV1 cells assessed as inhibition of dihydrotestosterone-induced ... | J Med Chem 54: 3973-6 (2011) Article DOI: 10.1021/jm2000097 BindingDB Entry DOI: 10.7270/Q23F4PZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50345229 ((R)-3-(biphenyl-2-ylsulfonyl)-N-(4-cyano-3-(triflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Curated by ChEMBL | Assay Description Antagonist activity at wild type androgen receptor expressed in african green monkey CV1 cells assessed as inhibition of dihydrotestosterone-induced ... | J Med Chem 54: 3973-6 (2011) Article DOI: 10.1021/jm2000097 BindingDB Entry DOI: 10.7270/Q23F4PZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 5 (Homo sapiens (Human)) | BDBM231694 (1-Amin-5-Naphthol-7-Sulfonic acid | NCI2602 | RR53...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Concordia University of Wisconsin | Assay Description Upon the addition of 4 μL of these respective inhibitor stock solutions to the assay buffer, the resulting range in assay inhibitor concentratio... | BMC Biochem 18: 10 (2017) Article DOI: 10.1186/s12858-017-0083-3 BindingDB Entry DOI: 10.7270/Q2JW8CSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 72 total ) | Next | Last >> |