 Found 469 hits with Last Name = 'bolton' and Initial = 'sa'
Found 469 hits with Last Name = 'bolton' and Initial = 'sa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase delta
(Homo sapiens (Human)) | BDBM50217812

(CHEMBL322526)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6@@H](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]-1=O)-[#6](-[#7])=O Show InChI InChI=1S/C18H31N7O5/c1-18(2,3)30-17(29)24-9-7-23(8-10-24)16(28)25-12(13(19)26)11(14(25)27)5-4-6-22-15(20)21/h11-12H,4-10H2,1-3H3,(H2,19,26)(H4,20,21,22)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217626
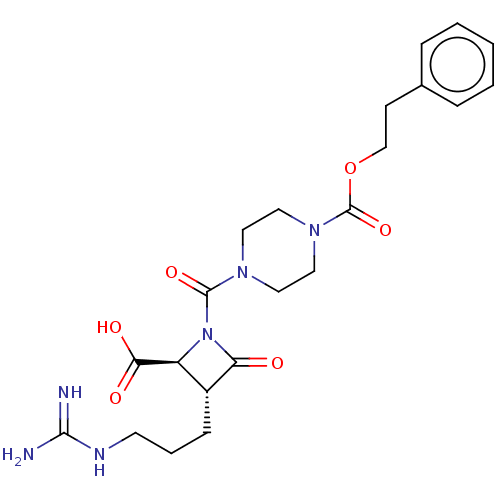
(CHEMBL322538)Show SMILES NC(=N)NCCC[C@@H]1[C@H](N(C(=O)N2CCN(CC2)C(=O)OCCc2ccccc2)C1=O)C(O)=O Show InChI InChI=1S/C22H30N6O6/c23-20(24)25-9-4-7-16-17(19(30)31)28(18(16)29)21(32)26-10-12-27(13-11-26)22(33)34-14-8-15-5-2-1-3-6-15/h1-3,5-6,16-17H,4,7-14H2,(H,30,31)(H4,23,24,25)/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217627
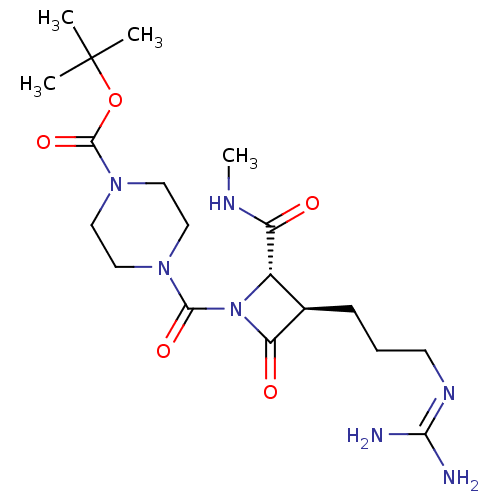
(CHEMBL110061)Show SMILES [#6]-[#7]-[#6](=O)-[#6@@H]-1-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#8]C([#6])([#6])[#6] Show InChI InChI=1S/C19H33N7O5/c1-19(2,3)31-18(30)25-10-8-24(9-11-25)17(29)26-13(14(27)22-4)12(15(26)28)6-5-7-23-16(20)21/h12-13H,5-11H2,1-4H3,(H,22,27)(H4,20,21,23)/t12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144535
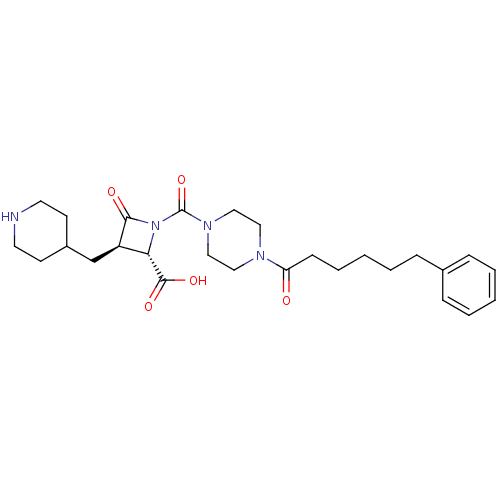
((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C27H38N4O5/c32-23(10-6-2-5-9-20-7-3-1-4-8-20)29-15-17-30(18-16-29)27(36)31-24(26(34)35)22(25(31)33)19-21-11-13-28-14-12-21/h1,3-4,7-8,21-22,24,28H,2,5-6,9-19H2,(H,34,35)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217801
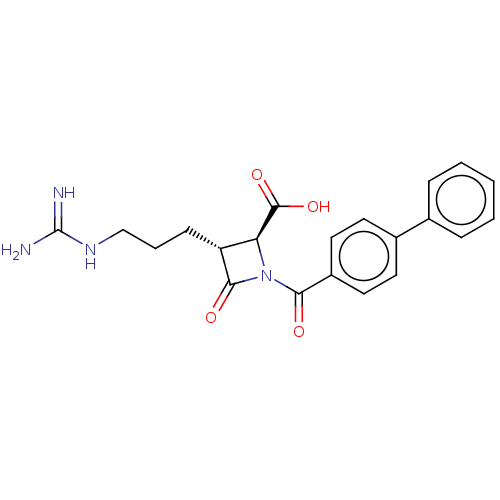
(CHEMBL111250)Show SMILES NC(=N)NCCC[C@@H]1[C@H](N(C(=O)c2ccc(cc2)-c2ccccc2)C1=O)C(O)=O Show InChI InChI=1S/C21H22N4O4/c22-21(23)24-12-4-7-16-17(20(28)29)25(19(16)27)18(26)15-10-8-14(9-11-15)13-5-2-1-3-6-13/h1-3,5-6,8-11,16-17H,4,7,12H2,(H,28,29)(H4,22,23,24)/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217622
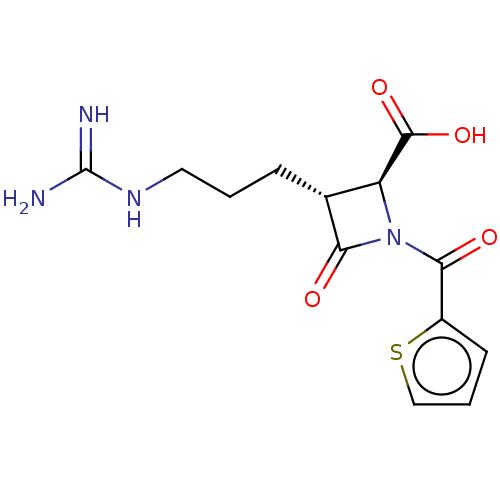
(CHEMBL443539)Show SMILES NC(=N)NCCC[C@@H]1[C@H](N(C(=O)c2cccs2)C1=O)C(O)=O Show InChI InChI=1S/C13H16N4O4S/c14-13(15)16-5-1-3-7-9(12(20)21)17(10(7)18)11(19)8-4-2-6-22-8/h2,4,6-7,9H,1,3,5H2,(H,20,21)(H4,14,15,16)/t7-,9+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217599
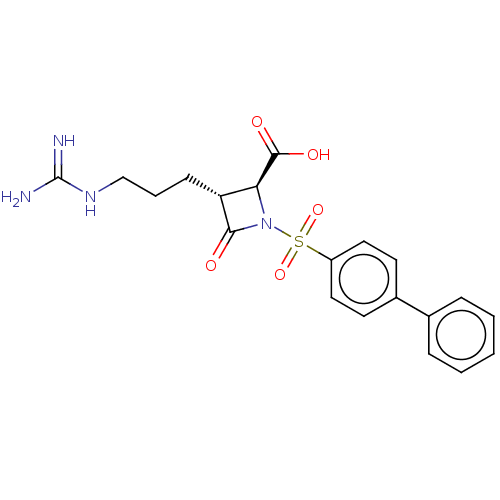
(CHEMBL109888)Show SMILES NC(=N)NCCC[C@@H]1[C@H](N(C1=O)S(=O)(=O)c1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C20H22N4O5S/c21-20(22)23-12-4-7-16-17(19(26)27)24(18(16)25)30(28,29)15-10-8-14(9-11-15)13-5-2-1-3-6-13/h1-3,5-6,8-11,16-17H,4,7,12H2,(H,26,27)(H4,21,22,23)/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144582

((R)-1-[4-(6-Naphthalen-2-yl-hexanoyl)-piperazine-1...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccc2ccccc2c1 Show InChI InChI=1S/C31H40N4O5/c36-27(9-3-1-2-6-22-10-11-24-7-4-5-8-25(24)20-22)33-16-18-34(19-17-33)31(40)35-28(30(38)39)26(29(35)37)21-23-12-14-32-15-13-23/h4-5,7-8,10-11,20,23,26,28,32H,1-3,6,9,12-19,21H2,(H,38,39)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144535

((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C27H38N4O5/c32-23(10-6-2-5-9-20-7-3-1-4-8-20)29-15-17-30(18-16-29)27(36)31-24(26(34)35)22(25(31)33)19-21-11-13-28-14-12-21/h1,3-4,7-8,21-22,24,28H,2,5-6,9-19H2,(H,34,35)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144555
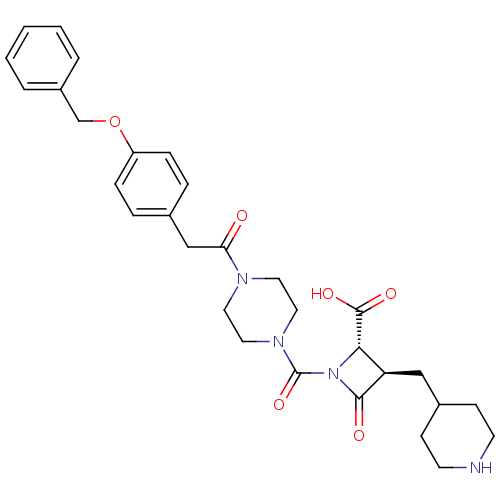
((R)-1-{4-[2-(4-Benzyloxy-phenyl)-acetyl]-piperazin...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C30H36N4O6/c35-26(19-21-6-8-24(9-7-21)40-20-23-4-2-1-3-5-23)32-14-16-33(17-15-32)30(39)34-27(29(37)38)25(28(34)36)18-22-10-12-31-13-11-22/h1-9,22,25,27,31H,10-20H2,(H,37,38)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144586
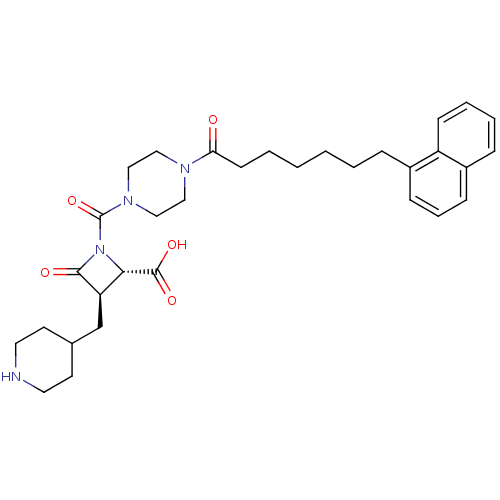
((R)-1-[4-(7-Naphthalen-1-yl-heptanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCCc1cccc2ccccc12 Show InChI InChI=1S/C32H42N4O5/c37-28(13-4-2-1-3-8-24-10-7-11-25-9-5-6-12-26(24)25)34-18-20-35(21-19-34)32(41)36-29(31(39)40)27(30(36)38)22-23-14-16-33-17-15-23/h5-7,9-12,23,27,29,33H,1-4,8,13-22H2,(H,39,40)/t27-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144577

((R)-1-[4-(6-Naphthalen-1-yl-hexanoyl)-piperazine-1...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1cccc2ccccc12 Show InChI InChI=1S/C31H40N4O5/c36-27(12-3-1-2-7-23-9-6-10-24-8-4-5-11-25(23)24)33-17-19-34(20-18-33)31(40)35-28(30(38)39)26(29(35)37)21-22-13-15-32-16-14-22/h4-6,8-11,22,26,28,32H,1-3,7,12-21H2,(H,38,39)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217817
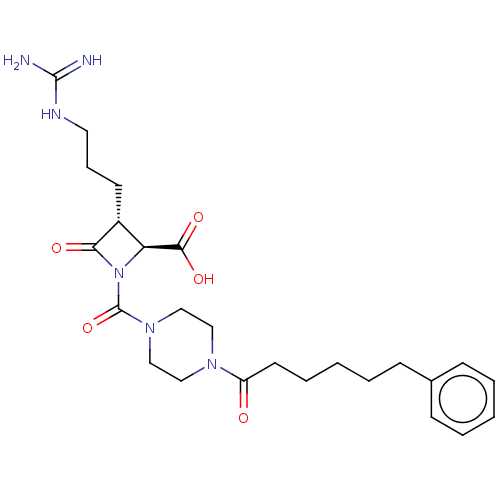
(CHEMBL326209)Show SMILES NC(=N)NCCC[C@@H]1[C@H](N(C(=O)N2CCN(CC2)C(=O)CCCCCc2ccccc2)C1=O)C(O)=O Show InChI InChI=1S/C25H36N6O5/c26-24(27)28-13-7-11-19-21(23(34)35)31(22(19)33)25(36)30-16-14-29(15-17-30)20(32)12-6-2-5-10-18-8-3-1-4-9-18/h1,3-4,8-9,19,21H,2,5-7,10-17H2,(H,34,35)(H4,26,27,28)/t19-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217824
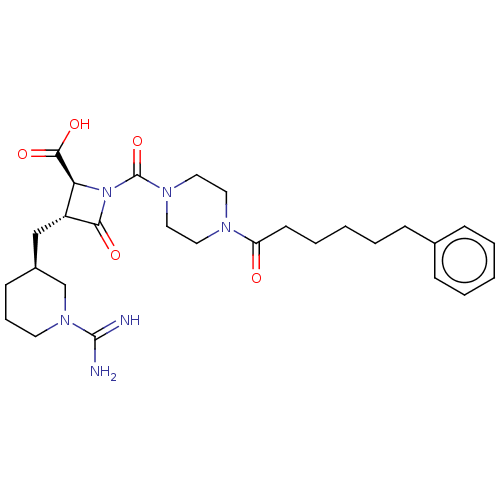
(BMS-363130 | CHEMBL70738)Show SMILES [H][C@@]1(C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)CCCCCc3ccccc3)C2=O)C(O)=O)CCCN(C1)C(N)=N Show InChI InChI=1S/C28H40N6O5/c29-27(30)33-13-7-11-21(19-33)18-22-24(26(37)38)34(25(22)36)28(39)32-16-14-31(15-17-32)23(35)12-6-2-5-10-20-8-3-1-4-9-20/h1,3-4,8-9,21-22,24H,2,5-7,10-19H2,(H3,29,30)(H,37,38)/t21-,22+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50221046
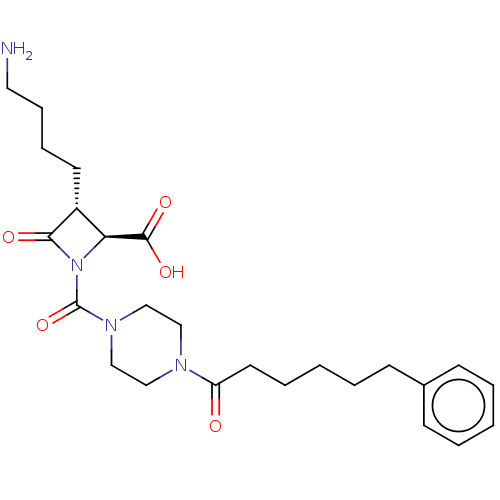
(CHEMBL72282)Show SMILES NCCCC[C@@H]1[C@H](N(C(=O)N2CCN(CC2)C(=O)CCCCCc2ccccc2)C1=O)C(O)=O Show InChI InChI=1S/C25H36N4O5/c26-14-8-7-12-20-22(24(32)33)29(23(20)31)25(34)28-17-15-27(16-18-28)21(30)13-6-2-5-11-19-9-3-1-4-10-19/h1,3-4,9-10,20,22H,2,5-8,11-18,26H2,(H,32,33)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217822
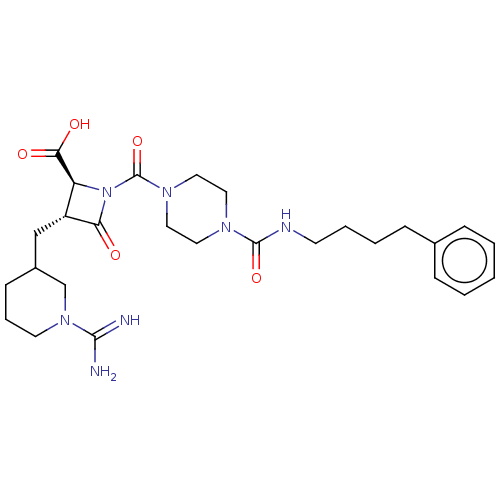
(CHEMBL111173)Show SMILES NC(=N)N1CCCC(C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)NCCCCc3ccccc3)C2=O)C(O)=O)C1 Show InChI InChI=1S/C27H39N7O5/c28-25(29)33-12-6-10-20(18-33)17-21-22(24(36)37)34(23(21)35)27(39)32-15-13-31(14-16-32)26(38)30-11-5-4-9-19-7-2-1-3-8-19/h1-3,7-8,20-22H,4-6,9-18H2,(H3,28,29)(H,30,38)(H,36,37)/t20?,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217823
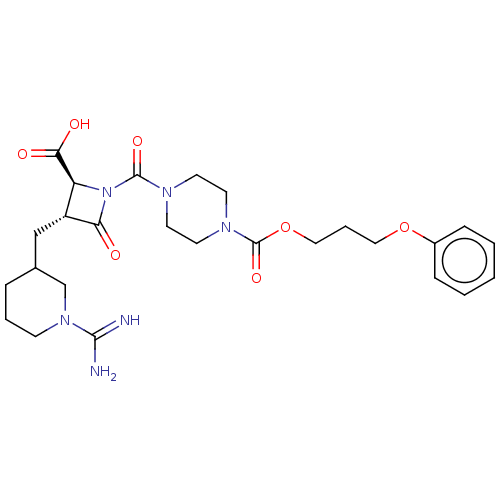
(CHEMBL111630)Show SMILES NC(=N)N1CCCC(C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)OCCCOc3ccccc3)C2=O)C(O)=O)C1 Show InChI InChI=1S/C26H36N6O7/c27-24(28)31-9-4-6-18(17-31)16-20-21(23(34)35)32(22(20)33)25(36)29-10-12-30(13-11-29)26(37)39-15-5-14-38-19-7-2-1-3-8-19/h1-3,7-8,18,20-21H,4-6,9-17H2,(H3,27,28)(H,34,35)/t18?,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50120387
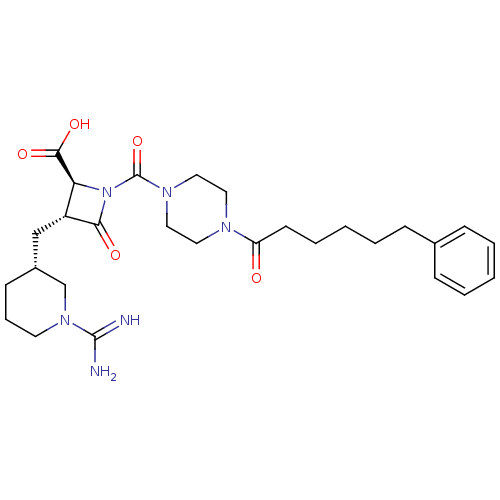
((2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethy...)Show SMILES NC(=N)N1CCC[C@H](C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)CCCCCc3ccccc3)C2=O)C(O)=O)C1 Show InChI InChI=1S/C28H40N6O5/c29-27(30)33-13-7-11-21(19-33)18-22-24(26(37)38)34(25(22)36)28(39)32-16-14-31(15-17-32)23(35)12-6-2-5-10-20-8-3-1-4-9-20/h1,3-4,8-9,21-22,24H,2,5-7,10-19H2,(H3,29,30)(H,37,38)/t21-,22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217818
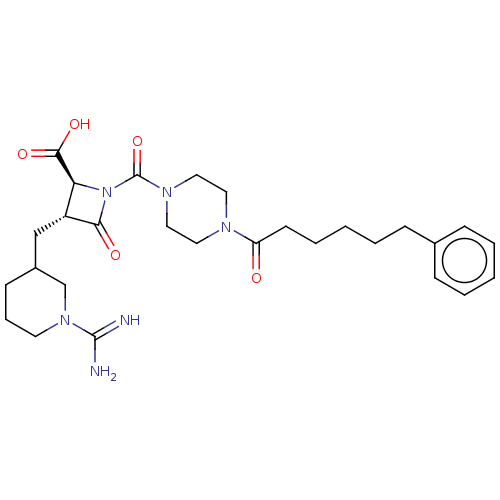
(CHEMBL440515)Show SMILES NC(=N)N1CCCC(C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)CCCCCc3ccccc3)C2=O)C(O)=O)C1 Show InChI InChI=1S/C28H40N6O5/c29-27(30)33-13-7-11-21(19-33)18-22-24(26(37)38)34(25(22)36)28(39)32-16-14-31(15-17-32)23(35)12-6-2-5-10-20-8-3-1-4-9-20/h1,3-4,8-9,21-22,24H,2,5-7,10-19H2,(H3,29,30)(H,37,38)/t21?,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50120387

((2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethy...)Show SMILES NC(=N)N1CCC[C@H](C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)CCCCCc3ccccc3)C2=O)C(O)=O)C1 Show InChI InChI=1S/C28H40N6O5/c29-27(30)33-13-7-11-21(19-33)18-22-24(26(37)38)34(25(22)36)28(39)32-16-14-31(15-17-32)23(35)12-6-2-5-10-20-8-3-1-4-9-20/h1,3-4,8-9,21-22,24H,2,5-7,10-19H2,(H3,29,30)(H,37,38)/t21-,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217824

(BMS-363130 | CHEMBL70738)Show SMILES [H][C@@]1(C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)CCCCCc3ccccc3)C2=O)C(O)=O)CCCN(C1)C(N)=N Show InChI InChI=1S/C28H40N6O5/c29-27(30)33-13-7-11-21(19-33)18-22-24(26(37)38)34(25(22)36)28(39)32-16-14-31(15-17-32)23(35)12-6-2-5-10-20-8-3-1-4-9-20/h1,3-4,8-9,21-22,24H,2,5-7,10-19H2,(H3,29,30)(H,37,38)/t21-,22+,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50120387

((2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethy...)Show SMILES NC(=N)N1CCC[C@H](C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)CCCCCc3ccccc3)C2=O)C(O)=O)C1 Show InChI InChI=1S/C28H40N6O5/c29-27(30)33-13-7-11-21(19-33)18-22-24(26(37)38)34(25(22)36)28(39)32-16-14-31(15-17-32)23(35)12-6-2-5-10-20-8-3-1-4-9-20/h1,3-4,8-9,21-22,24H,2,5-7,10-19H2,(H3,29,30)(H,37,38)/t21-,22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144532
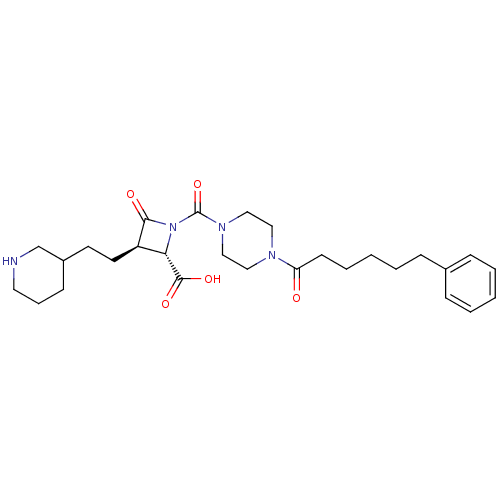
((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CCC2CCCNC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C28H40N4O5/c33-24(12-6-2-5-10-21-8-3-1-4-9-21)30-16-18-31(19-17-30)28(37)32-25(27(35)36)23(26(32)34)14-13-22-11-7-15-29-20-22/h1,3-4,8-9,22-23,25,29H,2,5-7,10-20H2,(H,35,36)/t22?,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217819
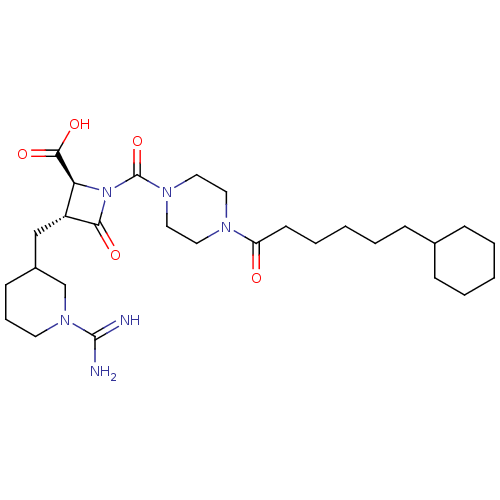
(CHEMBL109504)Show SMILES NC(=N)N1CCCC(C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)CCCCCC3CCCCC3)C2=O)C(O)=O)C1 Show InChI InChI=1S/C28H46N6O5/c29-27(30)33-13-7-11-21(19-33)18-22-24(26(37)38)34(25(22)36)28(39)32-16-14-31(15-17-32)23(35)12-6-2-5-10-20-8-3-1-4-9-20/h20-22,24H,1-19H2,(H3,29,30)(H,37,38)/t21?,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217628
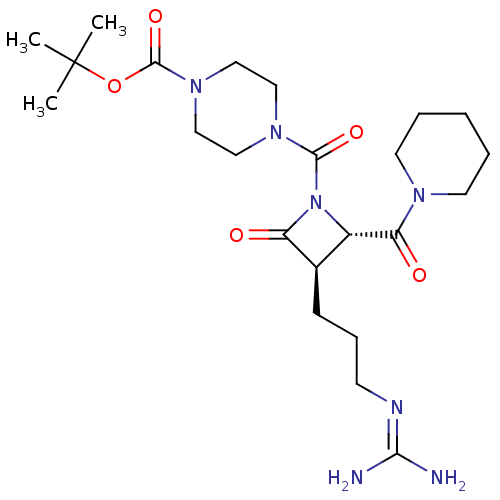
(CHEMBL107493)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6@@H](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C23H39N7O5/c1-23(2,3)35-22(34)29-14-12-28(13-15-29)21(33)30-17(19(32)27-10-5-4-6-11-27)16(18(30)31)8-7-9-26-20(24)25/h16-17H,4-15H2,1-3H3,(H4,24,25,26)/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144558
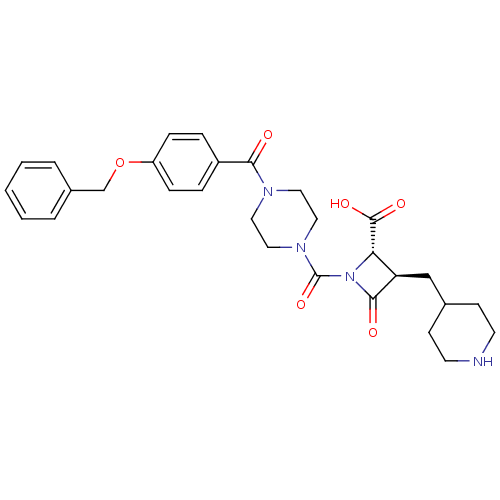
((R)-1-[4-(4-Benzyloxy-benzoyl)-piperazine-1-carbon...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C29H34N4O6/c34-26(22-6-8-23(9-7-22)39-19-21-4-2-1-3-5-21)31-14-16-32(17-15-31)29(38)33-25(28(36)37)24(27(33)35)18-20-10-12-30-13-11-20/h1-9,20,24-25,30H,10-19H2,(H,36,37)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50120368
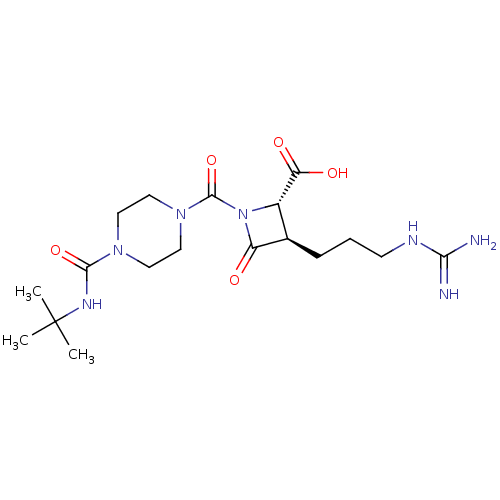
((2S,3R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carb...)Show SMILES CC(C)(C)NC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CCCNC(N)=N)C1=O)C(O)=O Show InChI InChI=1S/C18H31N7O5/c1-18(2,3)22-16(29)23-7-9-24(10-8-23)17(30)25-12(14(27)28)11(13(25)26)5-4-6-21-15(19)20/h11-12H,4-10H2,1-3H3,(H,22,29)(H,27,28)(H4,19,20,21)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50120368

((2S,3R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carb...)Show SMILES CC(C)(C)NC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CCCNC(N)=N)C1=O)C(O)=O Show InChI InChI=1S/C18H31N7O5/c1-18(2,3)22-16(29)23-7-9-24(10-8-23)17(30)25-12(14(27)28)11(13(25)26)5-4-6-21-15(19)20/h11-12H,4-10H2,1-3H3,(H,22,29)(H,27,28)(H4,19,20,21)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217624
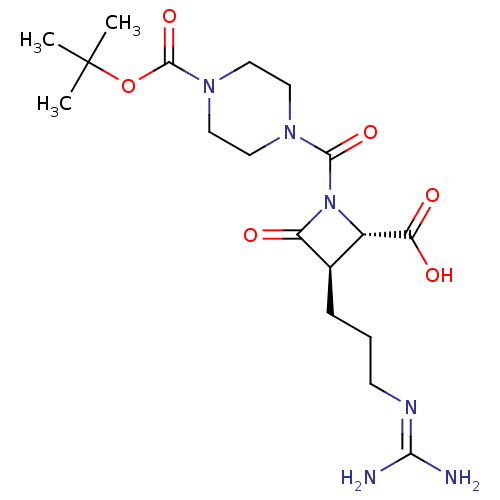
(CHEMBL321622)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6@@H](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]-1=O)-[#6](-[#8])=O Show InChI InChI=1S/C18H30N6O6/c1-18(2,3)30-17(29)23-9-7-22(8-10-23)16(28)24-12(14(26)27)11(13(24)25)5-4-6-21-15(19)20/h11-12H,4-10H2,1-3H3,(H,26,27)(H4,19,20,21)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217813
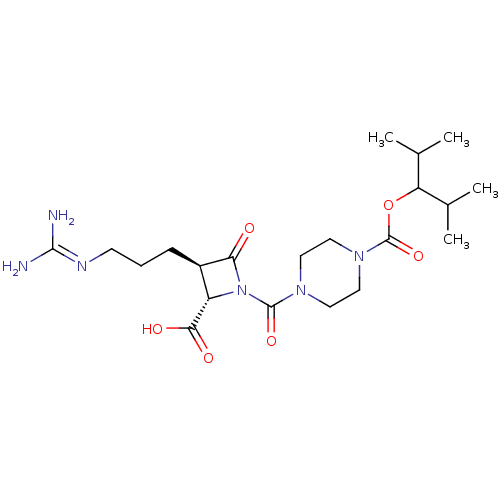
(CHEMBL302058)Show SMILES [#6]-[#6](-[#6])-[#6](-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6@@H](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]-1=O)-[#6](-[#8])=O)-[#6](-[#6])-[#6] Show InChI InChI=1S/C21H36N6O6/c1-12(2)16(13(3)4)33-21(32)26-10-8-25(9-11-26)20(31)27-15(18(29)30)14(17(27)28)6-5-7-24-19(22)23/h12-16H,5-11H2,1-4H3,(H,29,30)(H4,22,23,24)/t14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50120368

((2S,3R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carb...)Show SMILES CC(C)(C)NC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CCCNC(N)=N)C1=O)C(O)=O Show InChI InChI=1S/C18H31N7O5/c1-18(2,3)22-16(29)23-7-9-24(10-8-23)17(30)25-12(14(27)28)11(13(25)26)5-4-6-21-15(19)20/h11-12H,4-10H2,1-3H3,(H,22,29)(H,27,28)(H4,19,20,21)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220841
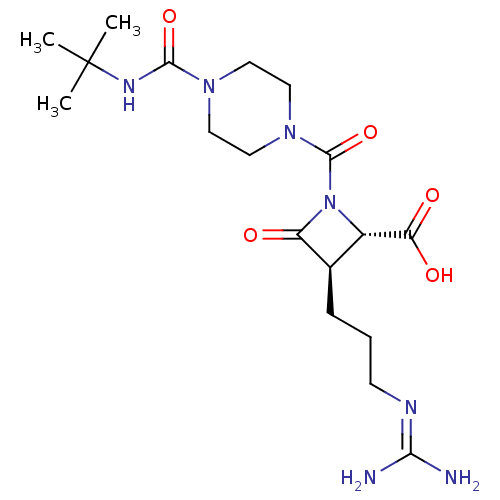
(BMS-262084 | CHEMBL71037)Show SMILES [#6]C([#6])([#6])[#7]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6@@H](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]-1=O)-[#6](-[#8])=O Show InChI InChI=1S/C18H31N7O5/c1-18(2,3)22-16(29)23-7-9-24(10-8-23)17(30)25-12(14(27)28)11(13(25)26)5-4-6-21-15(19)20/h11-12H,4-10H2,1-3H3,(H,22,29)(H,27,28)(H4,19,20,21)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217813

(CHEMBL302058)Show SMILES [#6]-[#6](-[#6])-[#6](-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6@@H](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]-1=O)-[#6](-[#8])=O)-[#6](-[#6])-[#6] Show InChI InChI=1S/C21H36N6O6/c1-12(2)16(13(3)4)33-21(32)26-10-8-25(9-11-26)20(31)27-15(18(29)30)14(17(27)28)6-5-7-24-19(22)23/h12-16H,5-11H2,1-4H3,(H,29,30)(H4,22,23,24)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217820
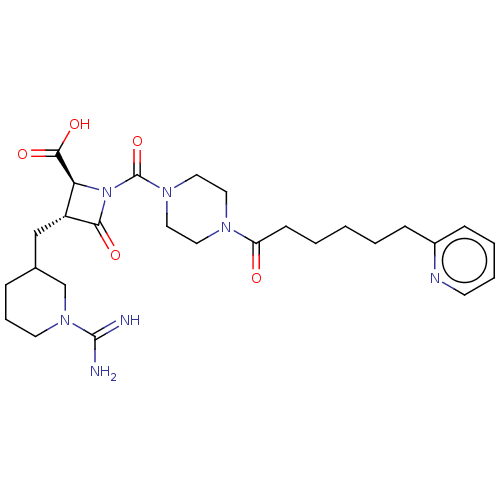
(CHEMBL447534)Show SMILES NC(=N)N1CCCC(C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)CCCCCc3ccccn3)C2=O)C(O)=O)C1 Show InChI InChI=1S/C27H39N7O5/c28-26(29)33-12-6-7-19(18-33)17-21-23(25(37)38)34(24(21)36)27(39)32-15-13-31(14-16-32)22(35)10-3-1-2-8-20-9-4-5-11-30-20/h4-5,9,11,19,21,23H,1-3,6-8,10,12-18H2,(H3,28,29)(H,37,38)/t19?,21-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144557
((R)-4-Oxo-1-[4-(8-phenyl-octanoyl)-piperazine-1-ca...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCCCc1ccccc1 Show InChI InChI=1S/C29H42N4O5/c34-25(12-8-3-1-2-5-9-22-10-6-4-7-11-22)31-17-19-32(20-18-31)29(38)33-26(28(36)37)24(27(33)35)21-23-13-15-30-16-14-23/h4,6-7,10-11,23-24,26,30H,1-3,5,8-9,12-21H2,(H,36,37)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144580
((R)-1-(4-{4-[3-(4-Chloro-phenyl)-[1,2,4]oxadiazol-...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCc1nc(no1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H33ClN6O6/c28-19-6-4-18(5-7-19)24-30-21(40-31-24)2-1-3-22(35)32-12-14-33(15-13-32)27(39)34-23(26(37)38)20(25(34)36)16-17-8-10-29-11-9-17/h4-7,17,20,23,29H,1-3,8-16H2,(H,37,38)/t20-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144569

((R)-1-[4-(2-Biphenyl-4-yl-acetyl)-piperazine-1-car...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C29H34N4O5/c34-25(19-20-6-8-23(9-7-20)22-4-2-1-3-5-22)31-14-16-32(17-15-31)29(38)33-26(28(36)37)24(27(33)35)18-21-10-12-30-13-11-21/h1-9,21,24,26,30H,10-19H2,(H,36,37)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144575

((R)-4-Oxo-1-{4-[2-(4-phenoxy-phenyl)-acetyl]-piper...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)Cc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C29H34N4O6/c34-25(19-20-6-8-23(9-7-20)39-22-4-2-1-3-5-22)31-14-16-32(17-15-31)29(38)33-26(28(36)37)24(27(33)35)18-21-10-12-30-13-11-21/h1-9,21,24,26,30H,10-19H2,(H,36,37)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144588
((R)-4-Oxo-1-[4-(7-phenyl-heptanoyl)-piperazine-1-c...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCCc1ccccc1 Show InChI InChI=1S/C28H40N4O5/c33-24(11-7-2-1-4-8-21-9-5-3-6-10-21)30-16-18-31(19-17-30)28(37)32-25(27(35)36)23(26(32)34)20-22-12-14-29-15-13-22/h3,5-6,9-10,22-23,25,29H,1-2,4,7-8,11-20H2,(H,35,36)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144561
((R)-4-Oxo-1-{4-[3-(3-oxo-4-p-tolyl-3H-pyrrol-2-yl)...)Show SMILES Cc1ccc(cc1)-c1c[nH]c(C=CC(=O)N2CCN(CC2)C(=O)N2[C@@H]([C@@H](CC3CCNCC3)C2=O)C(O)=O)c1O |w:11.11| Show InChI InChI=1S/C29H35N5O6/c1-18-2-4-20(5-3-18)22-17-31-23(26(22)36)6-7-24(35)32-12-14-33(15-13-32)29(40)34-25(28(38)39)21(27(34)37)16-19-8-10-30-11-9-19/h2-7,17,19,21,25,30-31,36H,8-16H2,1H3,(H,38,39)/t21-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM13592
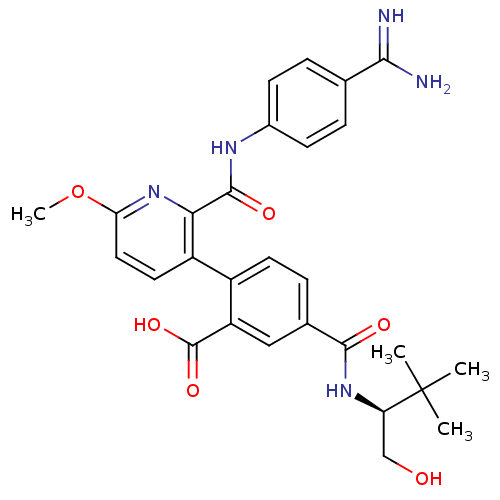
(2-{2-[(4-carbamimidoylphenyl)carbamoyl]-6-methoxyp...)Show SMILES COc1ccc(-c2ccc(cc2C(O)=O)C(=O)N[C@H](CO)C(C)(C)C)c(n1)C(=O)Nc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C28H31N5O6/c1-28(2,3)21(14-34)32-25(35)16-7-10-18(20(13-16)27(37)38)19-11-12-22(39-4)33-23(19)26(36)31-17-8-5-15(6-9-17)24(29)30/h5-13,21,34H,14H2,1-4H3,(H3,29,30)(H,31,36)(H,32,35)(H,37,38)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217623
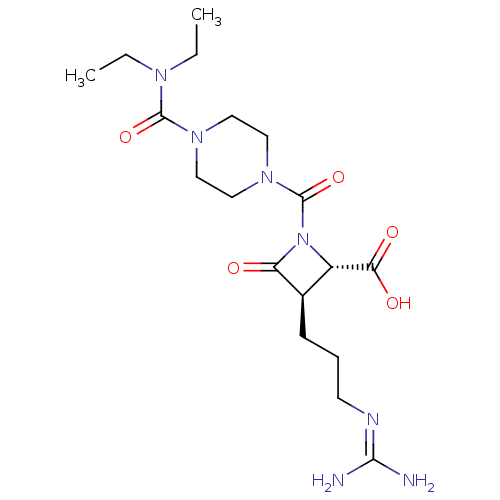
(CHEMBL109947)Show SMILES [#6]-[#6]-[#7](-[#6]-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6@@H](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]-1=O)-[#6](-[#8])=O Show InChI InChI=1S/C18H31N7O5/c1-3-22(4-2)17(29)23-8-10-24(11-9-23)18(30)25-13(15(27)28)12(14(25)26)6-5-7-21-16(19)20/h12-13H,3-11H2,1-2H3,(H,27,28)(H4,19,20,21)/t12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217805
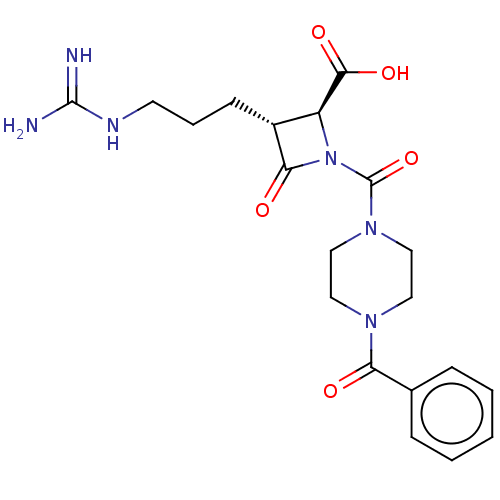
(CHEMBL111548)Show SMILES NC(=N)NCCC[C@@H]1[C@H](N(C(=O)N2CCN(CC2)C(=O)c2ccccc2)C1=O)C(O)=O Show InChI InChI=1S/C20H26N6O5/c21-19(22)23-8-4-7-14-15(18(29)30)26(17(14)28)20(31)25-11-9-24(10-12-25)16(27)13-5-2-1-3-6-13/h1-3,5-6,14-15H,4,7-12H2,(H,29,30)(H4,21,22,23)/t14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144579
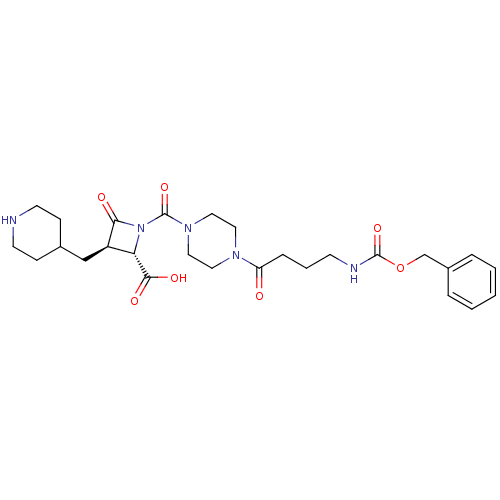
((R)-1-[4-(4-Benzyloxycarbonylamino-butyryl)-pipera...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCNC(=O)OCc1ccccc1 Show InChI InChI=1S/C27H37N5O7/c33-22(7-4-10-29-26(37)39-18-20-5-2-1-3-6-20)30-13-15-31(16-14-30)27(38)32-23(25(35)36)21(24(32)34)17-19-8-11-28-12-9-19/h1-3,5-6,19,21,23,28H,4,7-18H2,(H,29,37)(H,35,36)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144591
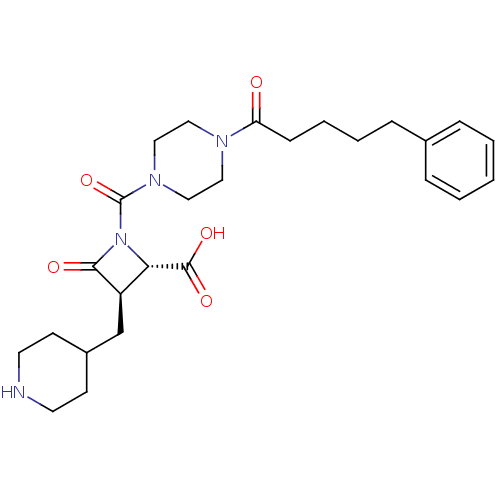
((R)-4-Oxo-1-[4-(5-phenyl-pentanoyl)-piperazine-1-c...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCc1ccccc1 Show InChI InChI=1S/C26H36N4O5/c31-22(9-5-4-8-19-6-2-1-3-7-19)28-14-16-29(17-15-28)26(35)30-23(25(33)34)21(24(30)32)18-20-10-12-27-13-11-20/h1-3,6-7,20-21,23,27H,4-5,8-18H2,(H,33,34)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144554

((R)-1-[4-(Biphenyl-4-carbonyl)-piperazine-1-carbon...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C28H32N4O5/c33-25(22-8-6-21(7-9-22)20-4-2-1-3-5-20)30-14-16-31(17-15-30)28(37)32-24(27(35)36)23(26(32)34)18-19-10-12-29-13-11-19/h1-9,19,23-24,29H,10-18H2,(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217815
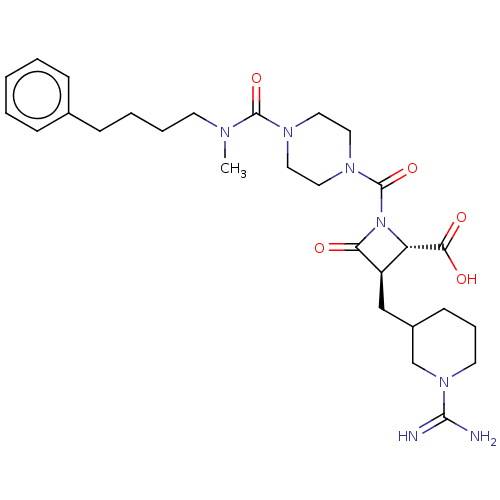
(CHEMBL113591)Show SMILES CN(CCCCc1ccccc1)C(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCCN(C2)C(N)=N)C1=O)C(O)=O Show InChI InChI=1S/C28H41N7O5/c1-31(12-6-5-10-20-8-3-2-4-9-20)27(39)32-14-16-33(17-15-32)28(40)35-23(25(37)38)22(24(35)36)18-21-11-7-13-34(19-21)26(29)30/h2-4,8-9,21-23H,5-7,10-19H2,1H3,(H3,29,30)(H,37,38)/t21?,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human tryptase |
Bioorg Med Chem Lett 12: 3235-8 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9DZP |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217601
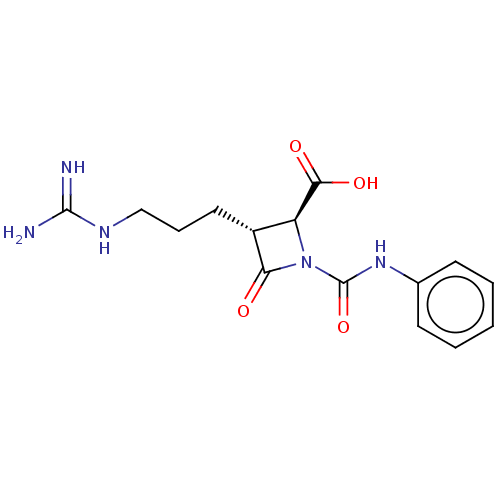
(CHEMBL320744)Show SMILES NC(=N)NCCC[C@@H]1[C@H](N(C(=O)Nc2ccccc2)C1=O)C(O)=O Show InChI InChI=1S/C15H19N5O4/c16-14(17)18-8-4-7-10-11(13(22)23)20(12(10)21)15(24)19-9-5-2-1-3-6-9/h1-3,5-6,10-11H,4,7-8H2,(H,19,24)(H,22,23)(H4,16,17,18)/t10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase delta
(Homo sapiens (Human)) | BDBM50217601

(CHEMBL320744)Show SMILES NC(=N)NCCC[C@@H]1[C@H](N(C(=O)Nc2ccccc2)C1=O)C(O)=O Show InChI InChI=1S/C15H19N5O4/c16-14(17)18-8-4-7-10-11(13(22)23)20(12(10)21)15(24)19-9-5-2-1-3-6-9/h1-3,5-6,10-11H,4,7-8H2,(H,19,24)(H,22,23)(H4,16,17,18)/t10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tryptase. |
Bioorg Med Chem Lett 12: 3229-33 (2002)
BindingDB Entry DOI: 10.7270/Q2QC05PZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144564
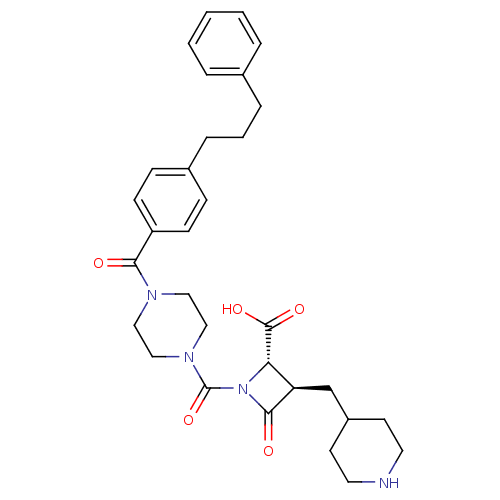
((R)-4-Oxo-1-{4-[4-(3-phenyl-propyl)-benzoyl]-piper...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)c1ccc(CCCc2ccccc2)cc1 Show InChI InChI=1S/C31H38N4O5/c36-28(25-11-9-23(10-12-25)8-4-7-22-5-2-1-3-6-22)33-17-19-34(20-18-33)31(40)35-27(30(38)39)26(29(35)37)21-24-13-15-32-16-14-24/h1-3,5-6,9-12,24,26-27,32H,4,7-8,13-21H2,(H,38,39)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase |
Bioorg Med Chem Lett 14: 2233-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.012
BindingDB Entry DOI: 10.7270/Q2SB4697 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data