 Found 83 hits with Last Name = 'borg' and Initial = 's'
Found 83 hits with Last Name = 'borg' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity towards rat Opioid receptor mu 1 by displacing [3H]DAMGO. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50059841

((S)-1-[(S)-2-[2-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C40H50N8O10/c1-23(44-37(55)29(41)18-25-9-13-27(50)14-10-25)36(54)46-30(19-24-6-3-2-4-7-24)38(56)43-21-34(52)45-31(20-26-11-15-28(51)16-12-26)40(58)48-17-5-8-33(48)39(57)47-32(22-49)35(42)53/h2-4,6-7,9-16,23,29-33,49-51H,5,8,17-22,41H2,1H3,(H2,42,53)(H,43,56)(H,44,55)(H,45,52)(H,46,54)(H,47,57)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity towards [3H]DAMGO, in rat Opioid receptor mu 1 |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50081923
((S)-1-[(S)-2-{[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-h...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nc(CN[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)no1 Show InChI InChI=1S/C40H49N9O9/c1-23(44-37(55)29(41)18-25-9-13-27(51)14-10-25)36(54)45-30(19-24-6-3-2-4-7-24)39-47-34(48-58-39)21-43-31(20-26-11-15-28(52)16-12-26)40(57)49-17-5-8-33(49)38(56)46-32(22-50)35(42)53/h2-4,6-7,9-16,23,29-33,43,50-52H,5,8,17-22,41H2,1H3,(H2,42,53)(H,44,55)(H,45,54)(H,46,56)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity towards rat Opioid receptor mu 1 by displacing [3H]DAMGO. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50081924

((S)-1-[(S)-2-[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nc(N[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)no1 Show InChI InChI=1S/C39H47N9O9/c1-22(42-35(54)28(40)18-24-9-13-26(50)14-10-24)34(53)43-29(19-23-6-3-2-4-7-23)37-46-39(47-57-37)45-30(20-25-11-15-27(51)16-12-25)38(56)48-17-5-8-32(48)36(55)44-31(21-49)33(41)52/h2-4,6-7,9-16,22,28-32,49-51H,5,8,17-21,40H2,1H3,(H2,41,52)(H,42,54)(H,43,53)(H,44,55)(H,45,47)/t22-,28+,29+,30+,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity towards rat Opioid receptor mu 1 by displacing [3H]DAMGO. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50081925
((S)-1-[(S)-2-[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nnc(N[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)o1 Show InChI InChI=1S/C39H47N9O9/c1-22(42-35(54)28(40)18-24-9-13-26(50)14-10-24)34(53)43-29(19-23-6-3-2-4-7-23)37-46-47-39(57-37)45-30(20-25-11-15-27(51)16-12-25)38(56)48-17-5-8-32(48)36(55)44-31(21-49)33(41)52/h2-4,6-7,9-16,22,28-32,49-51H,5,8,17-21,40H2,1H3,(H2,41,52)(H,42,54)(H,43,53)(H,44,55)(H,45,47)/t22-,28+,29+,30+,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity towards rat Opioid receptor mu 1 by displacing [3H]DAMGO. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306888

(4,6-dimethoxy-3-methyl-1H-pyrazolo[3,4-b]quinoline...)Show InChI InChI=1S/C13H13N3O2/c1-7-11-12(18-3)9-6-8(17-2)4-5-10(9)14-13(11)16-15-7/h4-6H,1-3H3,(H,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50081929

((S)-1-[(S)-2-{[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-h...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nnc(CN[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)[nH]1 Show InChI InChI=1S/C40H50N10O8/c1-23(44-38(56)29(41)18-25-9-13-27(52)14-10-25)37(55)45-30(19-24-6-3-2-4-7-24)36-47-34(48-49-36)21-43-31(20-26-11-15-28(53)16-12-26)40(58)50-17-5-8-33(50)39(57)46-32(22-51)35(42)54/h2-4,6-7,9-16,23,29-33,43,51-53H,5,8,17-22,41H2,1H3,(H2,42,54)(H,44,56)(H,45,55)(H,46,57)(H,47,48,49)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity towards rat Opioid receptor mu 1 by displacing [3H]DAMGO. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50081927

((S)-1-[(S)-2-{[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-h...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nnc(CN[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)o1 Show InChI InChI=1S/C40H49N9O9/c1-23(44-37(55)29(41)18-25-9-13-27(51)14-10-25)36(54)45-30(19-24-6-3-2-4-7-24)39-48-47-34(58-39)21-43-31(20-26-11-15-28(52)16-12-26)40(57)49-17-5-8-33(49)38(56)46-32(22-50)35(42)53/h2-4,6-7,9-16,23,29-33,43,50-52H,5,8,17-22,41H2,1H3,(H2,42,53)(H,44,55)(H,45,54)(H,46,56)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity towards rat Opioid receptor mu 1 by displacing [3H]DAMGO. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306874
(8-Hydroxy-6H-benzo[f][1,3]dioxolo[4',5':4,5]benzo[...)Show InChI InChI=1S/C16H9NO4/c18-11-3-1-2-7-8(11)4-10-13-9(16(19)17-10)5-12-15(14(7)13)21-6-20-12/h1-5,18H,6H2,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50197834
(2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...)Show InChI InChI=1S/C16H11NO4/c1-21-15-12(19)6-10-13-11(17-16(10)20)4-7-2-3-8(18)5-9(7)14(13)15/h2-6,18-19H,1H3,(H,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50197834

(2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...)Show InChI InChI=1S/C16H11NO4/c1-21-15-12(19)6-10-13-11(17-16(10)20)4-7-2-3-8(18)5-9(7)14(13)15/h2-6,18-19H,1H3,(H,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1344-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.018
BindingDB Entry DOI: 10.7270/Q2BZ664X |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50306888

(4,6-dimethoxy-3-methyl-1H-pyrazolo[3,4-b]quinoline...)Show InChI InChI=1S/C13H13N3O2/c1-7-11-12(18-3)9-6-8(17-2)4-5-10(9)14-13(11)16-15-7/h4-6H,1-3H3,(H,14,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50197834

(2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...)Show InChI InChI=1S/C16H11NO4/c1-21-15-12(19)6-10-13-11(17-16(10)20)4-7-2-3-8(18)5-9(7)14(13)15/h2-6,18-19H,1H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50197834

(2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...)Show InChI InChI=1S/C16H11NO4/c1-21-15-12(19)6-10-13-11(17-16(10)20)4-7-2-3-8(18)5-9(7)14(13)15/h2-6,18-19H,1H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
Bioorg Med Chem Lett 20: 1344-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.018
BindingDB Entry DOI: 10.7270/Q2BZ664X |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50306874

(8-Hydroxy-6H-benzo[f][1,3]dioxolo[4',5':4,5]benzo[...)Show InChI InChI=1S/C16H9NO4/c18-11-3-1-2-7-8(11)4-10-13-9(16(19)17-10)5-12-15(14(7)13)21-6-20-12/h1-5,18H,6H2,(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081935
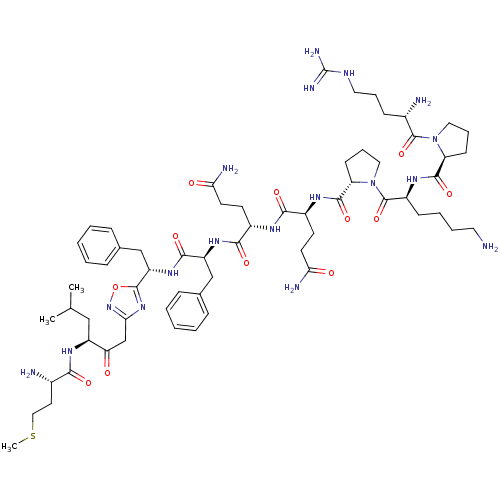
((alpha)-H-Arg-Pro-Lys-pro-Gln-Gln-Phe-{2-[5-((S)-1...)Show SMILES CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)Cc1noc(n1)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCNC(N)=N Show InChI InChI=1S/C63H96N18O12S/c1-37(2)33-45(75-54(85)40(65)27-32-94-3)50(82)36-53-78-60(93-79-53)47(35-39-17-8-5-9-18-39)77-57(88)46(34-38-15-6-4-7-16-38)76-56(87)42(23-25-51(67)83)72-55(86)43(24-26-52(68)84)73-58(89)49-22-14-31-81(49)62(92)44(20-10-11-28-64)74-59(90)48-21-13-30-80(48)61(91)41(66)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-66H2,1-3H3,(H2,67,83)(H2,68,84)(H,72,86)(H,73,89)(H,74,90)(H,75,85)(H,76,87)(H,77,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 307 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50081926
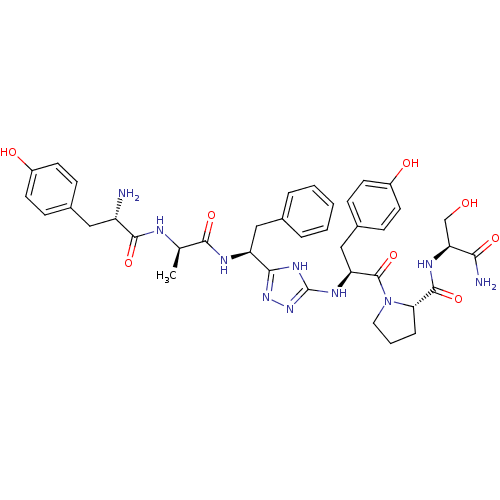
((S)-1-[(S)-2-[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nnc(N[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)[nH]1 Show InChI InChI=1S/C39H48N10O8/c1-22(42-36(55)28(40)18-24-9-13-26(51)14-10-24)35(54)43-29(19-23-6-3-2-4-7-23)34-46-39(48-47-34)45-30(20-25-11-15-27(52)16-12-25)38(57)49-17-5-8-32(49)37(56)44-31(21-50)33(41)53/h2-4,6-7,9-16,22,28-32,50-52H,5,8,17-21,40H2,1H3,(H2,41,53)(H,42,55)(H,43,54)(H,44,56)(H2,45,46,47,48)/t22-,28+,29+,30+,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity towards rat Opioid receptor mu 1 by displacing [3H]DAMGO. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081936
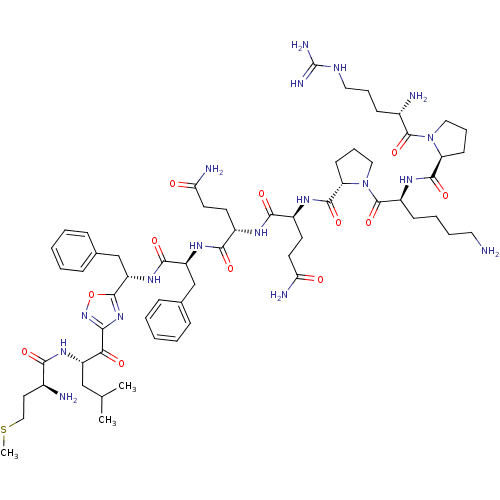
(CHEMBL263584 | H-Arg-Pro-Lys-pro-Gln-Gln-Phe-{5-[(...)Show SMILES CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)c1noc(n1)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCNC(N)=N Show InChI InChI=1S/C62H94N18O12S/c1-36(2)33-44(74-53(84)39(64)27-32-93-3)51(83)52-77-59(92-78-52)46(35-38-17-8-5-9-18-38)76-56(87)45(34-37-15-6-4-7-16-37)75-55(86)41(23-25-49(66)81)71-54(85)42(24-26-50(67)82)72-57(88)48-22-14-31-80(48)61(91)43(20-10-11-28-63)73-58(89)47-21-13-30-79(47)60(90)40(65)19-12-29-70-62(68)69/h4-9,15-18,36,39-48H,10-14,19-35,63-65H2,1-3H3,(H2,66,81)(H2,67,82)(H,71,85)(H,72,88)(H,73,89)(H,74,84)(H,75,86)(H,76,87)(H4,68,69,70)/t39-,40-,41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081924

((S)-1-[(S)-2-[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nc(N[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)no1 Show InChI InChI=1S/C39H47N9O9/c1-22(42-35(54)28(40)18-24-9-13-26(50)14-10-24)34(53)43-29(19-23-6-3-2-4-7-23)37-46-39(47-57-37)45-30(20-25-11-15-27(51)16-12-25)38(56)48-17-5-8-32(48)36(55)44-31(21-49)33(41)52/h2-4,6-7,9-16,22,28-32,49-51H,5,8,17-21,40H2,1H3,(H2,41,52)(H,42,54)(H,43,53)(H,44,55)(H,45,47)/t22-,28+,29+,30+,31+,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081927

((S)-1-[(S)-2-{[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-h...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nnc(CN[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)o1 Show InChI InChI=1S/C40H49N9O9/c1-23(44-37(55)29(41)18-25-9-13-27(51)14-10-25)36(54)45-30(19-24-6-3-2-4-7-24)39-48-47-34(58-39)21-43-31(20-26-11-15-28(52)16-12-26)40(57)49-17-5-8-33(49)38(56)46-32(22-50)35(42)53/h2-4,6-7,9-16,23,29-33,43,50-52H,5,8,17-22,41H2,1H3,(H2,42,53)(H,44,55)(H,45,54)(H,46,56)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081935

((alpha)-H-Arg-Pro-Lys-pro-Gln-Gln-Phe-{2-[5-((S)-1...)Show SMILES CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)Cc1noc(n1)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCNC(N)=N Show InChI InChI=1S/C63H96N18O12S/c1-37(2)33-45(75-54(85)40(65)27-32-94-3)50(82)36-53-78-60(93-79-53)47(35-39-17-8-5-9-18-39)77-57(88)46(34-38-15-6-4-7-16-38)76-56(87)42(23-25-51(67)83)72-55(86)43(24-26-52(68)84)73-58(89)49-22-14-31-81(49)62(92)44(20-10-11-28-64)74-59(90)48-21-13-30-80(48)61(91)41(66)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-66H2,1-3H3,(H2,67,83)(H2,68,84)(H,72,86)(H,73,89)(H,74,90)(H,75,85)(H,76,87)(H,77,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081926

((S)-1-[(S)-2-[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nnc(N[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)[nH]1 Show InChI InChI=1S/C39H48N10O8/c1-22(42-36(55)28(40)18-24-9-13-26(51)14-10-24)35(54)43-29(19-23-6-3-2-4-7-23)34-46-39(48-47-34)45-30(20-25-11-15-27(52)16-12-25)38(57)49-17-5-8-32(49)37(56)44-31(21-50)33(41)53/h2-4,6-7,9-16,22,28-32,50-52H,5,8,17-21,40H2,1H3,(H2,41,53)(H,42,55)(H,43,54)(H,44,56)(H2,45,46,47,48)/t22-,28+,29+,30+,31+,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081932
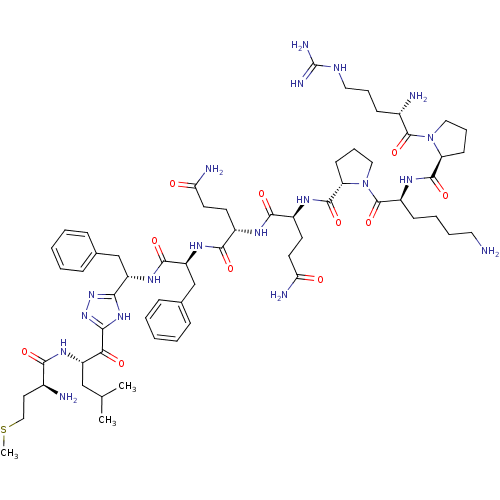
(CHEMBL410170 | H-Arg-Pro-Lys-pro-Gln-Gln-Phe-{5-[(...)Show SMILES CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)c1nnc([nH]1)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCNC(N)=N Show InChI InChI=1S/C62H95N19O11S/c1-36(2)33-44(74-54(85)39(64)27-32-93-3)51(84)53-77-52(78-79-53)45(34-37-15-6-4-7-16-37)75-57(88)46(35-38-17-8-5-9-18-38)76-56(87)41(23-25-49(66)82)71-55(86)42(24-26-50(67)83)72-58(89)48-22-14-31-81(48)61(92)43(20-10-11-28-63)73-59(90)47-21-13-30-80(47)60(91)40(65)19-12-29-70-62(68)69/h4-9,15-18,36,39-48H,10-14,19-35,63-65H2,1-3H3,(H2,66,82)(H2,67,83)(H,71,86)(H,72,89)(H,73,90)(H,74,85)(H,75,88)(H,76,87)(H4,68,69,70)(H,77,78,79)/t39-,40-,41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081931
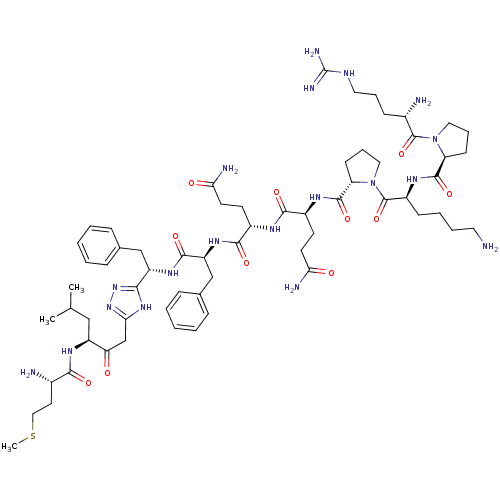
(CHEMBL402884 | H-Arg-Pro-Lys-pro-Gln-Gln-Phe-{2-[5...)Show SMILES CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)Cc1nnc([nH]1)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCNC(N)=N Show InChI InChI=1S/C63H97N19O11S/c1-37(2)33-45(75-55(86)40(65)27-32-94-3)50(83)36-53-78-54(80-79-53)46(34-38-15-6-4-7-16-38)76-58(89)47(35-39-17-8-5-9-18-39)77-57(88)42(23-25-51(67)84)72-56(87)43(24-26-52(68)85)73-59(90)49-22-14-31-82(49)62(93)44(20-10-11-28-64)74-60(91)48-21-13-30-81(48)61(92)41(66)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-66H2,1-3H3,(H2,67,84)(H2,68,85)(H,72,87)(H,73,90)(H,74,91)(H,75,86)(H,76,89)(H,77,88)(H4,69,70,71)(H,78,79,80)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081930

(CHEMBL268138 | H-Arg-Pro-Lys-pro-Gln-Gln-Phe-{5-[(...)Show SMILES CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)c1nnc(o1)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCNC(N)=N Show InChI InChI=1S/C62H94N18O12S/c1-36(2)33-44(74-52(84)39(64)27-32-93-3)51(83)59-78-77-58(92-59)46(35-38-17-8-5-9-18-38)76-55(87)45(34-37-15-6-4-7-16-37)75-54(86)41(23-25-49(66)81)71-53(85)42(24-26-50(67)82)72-56(88)48-22-14-31-80(48)61(91)43(20-10-11-28-63)73-57(89)47-21-13-30-79(47)60(90)40(65)19-12-29-70-62(68)69/h4-9,15-18,36,39-48H,10-14,19-35,63-65H2,1-3H3,(H2,66,81)(H2,67,82)(H,71,85)(H,72,88)(H,73,89)(H,74,84)(H,75,86)(H,76,87)(H4,68,69,70)/t39-,40-,41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081929

((S)-1-[(S)-2-{[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-h...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nnc(CN[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)[nH]1 Show InChI InChI=1S/C40H50N10O8/c1-23(44-38(56)29(41)18-25-9-13-27(52)14-10-25)37(55)45-30(19-24-6-3-2-4-7-24)36-47-34(48-49-36)21-43-31(20-26-11-15-28(53)16-12-26)40(58)50-17-5-8-33(50)39(57)46-32(22-51)35(42)54/h2-4,6-7,9-16,23,29-33,43,51-53H,5,8,17-22,41H2,1H3,(H2,42,54)(H,44,56)(H,45,55)(H,46,57)(H,47,48,49)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081928
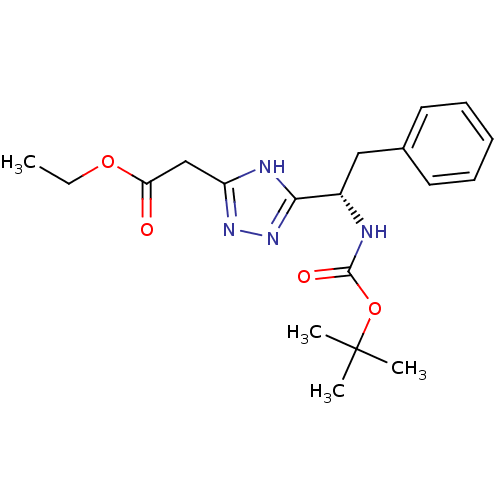
(CHEMBL136875 | [5-((S)-1-tert-Butoxycarbonylamino-...)Show SMILES CCOC(=O)Cc1nnc([nH]1)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C19H26N4O4/c1-5-26-16(24)12-15-21-17(23-22-15)14(11-13-9-7-6-8-10-13)20-18(25)27-19(2,3)4/h6-10,14H,5,11-12H2,1-4H3,(H,20,25)(H,21,22,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081925

((S)-1-[(S)-2-[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nnc(N[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)o1 Show InChI InChI=1S/C39H47N9O9/c1-22(42-35(54)28(40)18-24-9-13-26(50)14-10-24)34(53)43-29(19-23-6-3-2-4-7-23)37-46-47-39(57-37)45-30(20-25-11-15-27(51)16-12-25)38(56)48-17-5-8-32(48)36(55)44-31(21-49)33(41)52/h2-4,6-7,9-16,22,28-32,49-51H,5,8,17-21,40H2,1H3,(H2,41,52)(H,42,54)(H,43,53)(H,44,55)(H,45,47)/t22-,28+,29+,30+,31+,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50059841

((S)-1-[(S)-2-[2-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C40H50N8O10/c1-23(44-37(55)29(41)18-25-9-13-27(50)14-10-25)36(54)46-30(19-24-6-3-2-4-7-24)38(56)43-21-34(52)45-31(20-26-11-15-28(51)16-12-26)40(58)48-17-5-8-33(48)39(57)47-32(22-49)35(42)53/h2-4,6-7,9-16,23,29-33,49-51H,5,8,17-22,41H2,1H3,(H2,42,53)(H,43,56)(H,44,55)(H,45,52)(H,46,54)(H,47,57)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081923

((S)-1-[(S)-2-{[5-((S)-1-{(R)-2-[(S)-2-Amino-3-(4-h...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)c1nc(CN[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CO)C(N)=O)no1 Show InChI InChI=1S/C40H49N9O9/c1-23(44-37(55)29(41)18-25-9-13-27(51)14-10-25)36(54)45-30(19-24-6-3-2-4-7-24)39-47-34(48-58-39)21-43-31(20-26-11-15-28(52)16-12-26)40(57)49-17-5-8-33(49)38(56)46-32(22-50)35(42)53/h2-4,6-7,9-16,23,29-33,43,50-52H,5,8,17-22,41H2,1H3,(H2,42,53)(H,44,55)(H,45,54)(H,46,56)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50081933

(CHEMBL138013 | {(S)-1-[3-(2-Hydroxy-ethyl)-[1,2,4]...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)c1nc(CCO)no1 Show InChI InChI=1S/C17H23N3O4/c1-17(2,3)23-16(22)18-13(11-12-7-5-4-6-8-12)15-19-14(9-10-21)20-24-15/h4-8,13,21H,9-11H2,1-3H3,(H,18,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for rat Tachykinin receptor 1 determined in displacement screening by using radioligand 3,4-[3H]-(L-Pro e2) SP. |
J Med Chem 42: 4331-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SJ1MBK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306869

(8-Methoxy-6H-benzo[f][1,3]dioxolo[4',5':4,5]benzo[...)Show InChI InChI=1S/C17H11NO4/c1-20-12-4-2-3-8-9(12)5-11-14-10(17(19)18-11)6-13-16(15(8)14)22-7-21-13/h2-6H,7H2,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50197834

(2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...)Show InChI InChI=1S/C16H11NO4/c1-21-15-12(19)6-10-13-11(17-16(10)20)4-7-2-3-8(18)5-9(7)14(13)15/h2-6,18-19H,1H3,(H,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 20: 1344-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.018
BindingDB Entry DOI: 10.7270/Q2BZ664X |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50306874

(8-Hydroxy-6H-benzo[f][1,3]dioxolo[4',5':4,5]benzo[...)Show InChI InChI=1S/C16H9NO4/c18-11-3-1-2-7-8(11)4-10-13-9(16(19)17-10)5-12-15(14(7)13)21-6-20-12/h1-5,18H,6H2,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50197834

(2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...)Show InChI InChI=1S/C16H11NO4/c1-21-15-12(19)6-10-13-11(17-16(10)20)4-7-2-3-8(18)5-9(7)14(13)15/h2-6,18-19H,1H3,(H,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50306874

(8-Hydroxy-6H-benzo[f][1,3]dioxolo[4',5':4,5]benzo[...)Show InChI InChI=1S/C16H9NO4/c18-11-3-1-2-7-8(11)4-10-13-9(16(19)17-10)5-12-15(14(7)13)21-6-20-12/h1-5,18H,6H2,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of AUR2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50197834

(2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...)Show InChI InChI=1S/C16H11NO4/c1-21-15-12(19)6-10-13-11(17-16(10)20)4-7-2-3-8(18)5-9(7)14(13)15/h2-6,18-19H,1H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Aurora 2 kinase |
Bioorg Med Chem Lett 20: 1344-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.018
BindingDB Entry DOI: 10.7270/Q2BZ664X |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306877

(10-Hydroxy-6H-benzo[f][1,3]dioxolo[4',5':4,5]benzo...)Show InChI InChI=1S/C16H9NO4/c18-8-2-1-7-3-11-13-10(16(19)17-11)5-12-15(21-6-20-12)14(13)9(7)4-8/h1-5,18H,6H2,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306872
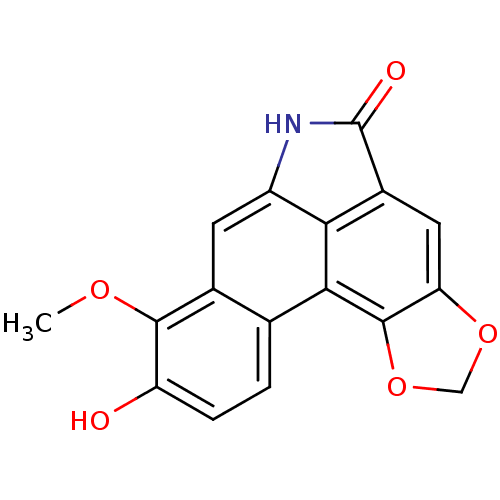
(9-Hydroxy-8-methoxy-6H-benzo[f][1,3]dioxolo[4',5':...)Show InChI InChI=1S/C17H11NO5/c1-21-15-8-4-10-13-9(17(20)18-10)5-12-16(23-6-22-12)14(13)7(8)2-3-11(15)19/h2-5,19H,6H2,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50306888

(4,6-dimethoxy-3-methyl-1H-pyrazolo[3,4-b]quinoline...)Show InChI InChI=1S/C13H13N3O2/c1-7-11-12(18-3)9-6-8(17-2)4-5-10(9)14-13(11)16-15-7/h4-6H,1-3H3,(H,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of AUR2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50197834

(2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...)Show InChI InChI=1S/C16H11NO4/c1-21-15-12(19)6-10-13-11(17-16(10)20)4-7-2-3-8(18)5-9(7)14(13)15/h2-6,18-19H,1H3,(H,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of AUR2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306866
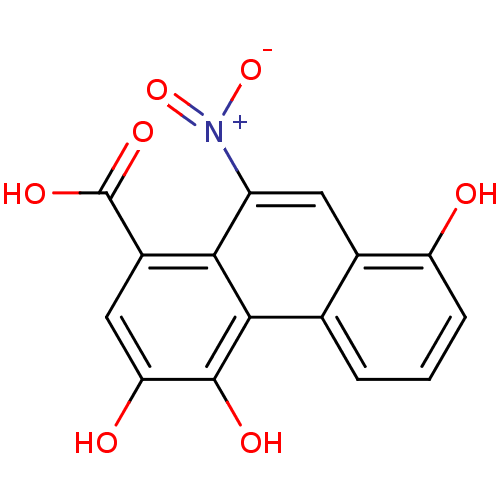
(3,4,8-trihydroxy-10-nitrophenanthrene-1-carboxylic...)Show SMILES OC(=O)c1cc(O)c(O)c2c1c(cc1c(O)cccc21)[N+]([O-])=O Show InChI InChI=1S/C15H9NO7/c17-10-3-1-2-6-7(10)4-9(16(22)23)12-8(15(20)21)5-11(18)14(19)13(6)12/h1-5,17-19H,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50306888

(4,6-dimethoxy-3-methyl-1H-pyrazolo[3,4-b]quinoline...)Show InChI InChI=1S/C13H13N3O2/c1-7-11-12(18-3)9-6-8(17-2)4-5-10(9)14-13(11)16-15-7/h4-6H,1-3H3,(H,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306884
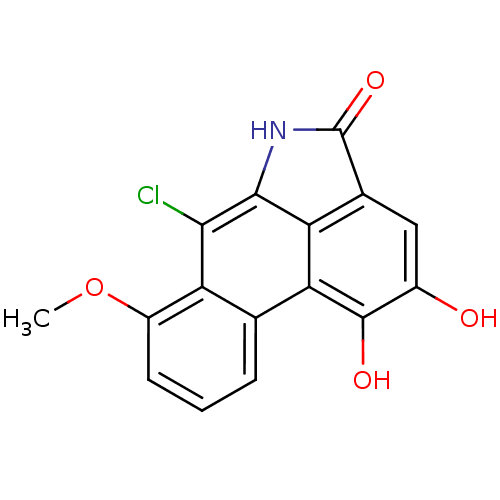
(6-chloro-1,2-dihydroxy-7-methoxydibenzo[cd,f]indol...)Show InChI InChI=1S/C16H10ClNO4/c1-22-9-4-2-3-6-10(9)13(17)14-11-7(16(21)18-14)5-8(19)15(20)12(6)11/h2-5,19-20H,1H3,(H,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306911

(8-((2S,3R,4S,5S,6R)-3,4,5-Trihydroxy-6-hydroxymeth...)Show SMILES OC[C@H]1O[C@@H](Oc2cccc3c2cc2NC(=O)c4cc5OCOc5c3c24)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C22H19NO9/c24-6-14-17(25)18(26)19(27)22(32-14)31-12-3-1-2-8-9(12)4-11-15-10(21(28)23-11)5-13-20(16(8)15)30-7-29-13/h1-5,14,17-19,22,24-27H,6-7H2,(H,23,28)/t14-,17-,18+,19-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1344-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.018
BindingDB Entry DOI: 10.7270/Q2BZ664X |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306888

(4,6-dimethoxy-3-methyl-1H-pyrazolo[3,4-b]quinoline...)Show InChI InChI=1S/C13H13N3O2/c1-7-11-12(18-3)9-6-8(17-2)4-5-10(9)14-13(11)16-15-7/h4-6H,1-3H3,(H,14,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306863

(3,4-dihydroxy-10-nitrophenanthrene-1-carboxylic ac...)Show SMILES OC(=O)c1cc(O)c(O)c2c1c(cc1ccccc21)[N+]([O-])=O Show InChI InChI=1S/C15H9NO6/c17-11-6-9(15(19)20)12-10(16(21)22)5-7-3-1-2-4-8(7)13(12)14(11)18/h1-6,17-18H,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50306870
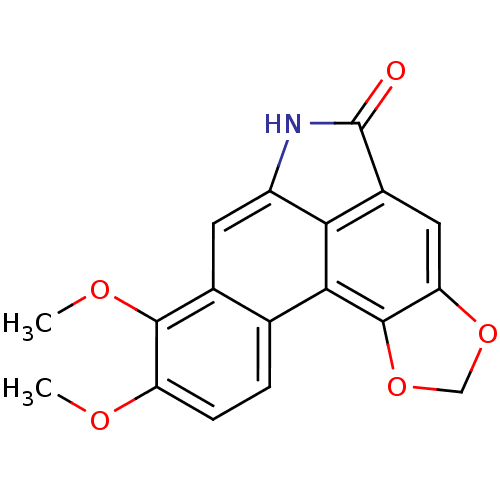
(8,9-Dimethoxy-6H-benzo[f][1,3]dioxolo[4',5':4,5]be...)Show InChI InChI=1S/C18H13NO5/c1-21-12-4-3-8-9(16(12)22-2)5-11-14-10(18(20)19-11)6-13-17(15(8)14)24-7-23-13/h3-6H,7H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 1384-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.007
BindingDB Entry DOI: 10.7270/Q2QF8TTB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data