 Found 430 hits with Last Name = 'catana' and Initial = 'c'
Found 430 hits with Last Name = 'catana' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425862
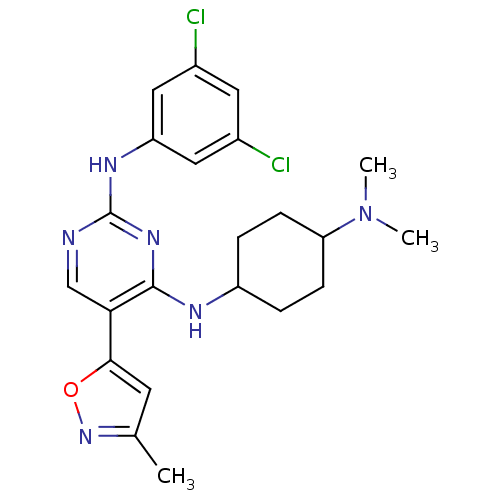
(CHEMBL2312654)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1cc(C)no1 |(9.04,-43.08,;10.39,-42.35,;11.7,-43.16,;10.44,-40.81,;9.13,-40,;9.18,-38.46,;10.53,-37.74,;11.85,-38.53,;11.8,-40.08,;10.57,-36.2,;9.81,-34.87,;8.27,-34.86,;7.51,-33.53,;5.96,-33.52,;5.2,-32.19,;5.97,-30.85,;5.21,-29.52,;5.98,-28.19,;3.67,-29.52,;2.89,-30.86,;1.35,-30.86,;3.66,-32.19,;8.27,-32.2,;9.8,-32.19,;10.58,-33.53,;12.11,-33.53,;12.6,-34.99,;14.14,-34.99,;15.06,-36.23,;14.61,-33.52,;13.35,-32.62,)| Show InChI InChI=1S/C22H26Cl2N6O/c1-13-8-20(31-29-13)19-12-25-22(27-17-10-14(23)9-15(24)11-17)28-21(19)26-16-4-6-18(7-5-16)30(2)3/h8-12,16,18H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425864
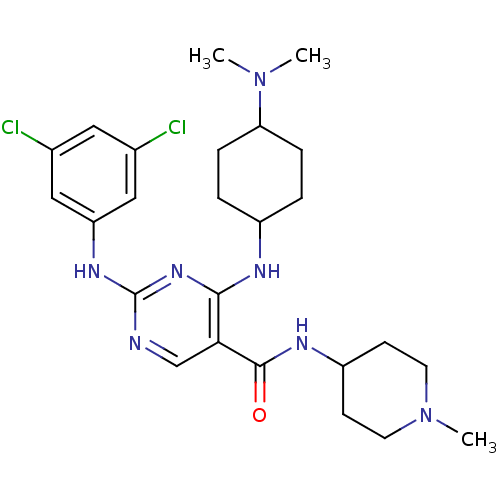
(CHEMBL2312649)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |(47.36,-51.36,;48.9,-51.37,;49.67,-52.7,;49.68,-50.04,;48.92,-48.7,;49.7,-47.37,;51.23,-47.39,;52.01,-48.71,;51.22,-50.05,;52,-46.06,;51.24,-44.72,;49.7,-44.72,;48.94,-43.38,;47.4,-43.38,;46.63,-42.04,;47.41,-40.71,;46.64,-39.37,;47.41,-38.04,;45.1,-39.37,;44.33,-40.71,;42.79,-40.71,;45.1,-42.04,;49.7,-42.05,;51.24,-42.05,;52.01,-43.39,;53.55,-43.39,;54.32,-44.72,;54.32,-42.06,;55.86,-42.06,;56.62,-43.39,;58.16,-43.4,;58.94,-42.07,;60.48,-42.08,;58.17,-40.73,;56.62,-40.72,)| Show InChI InChI=1S/C25H35Cl2N7O/c1-33(2)21-6-4-18(5-7-21)29-23-22(24(35)30-19-8-10-34(3)11-9-19)15-28-25(32-23)31-20-13-16(26)12-17(27)14-20/h12-15,18-19,21H,4-11H2,1-3H3,(H,30,35)(H2,28,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50027970
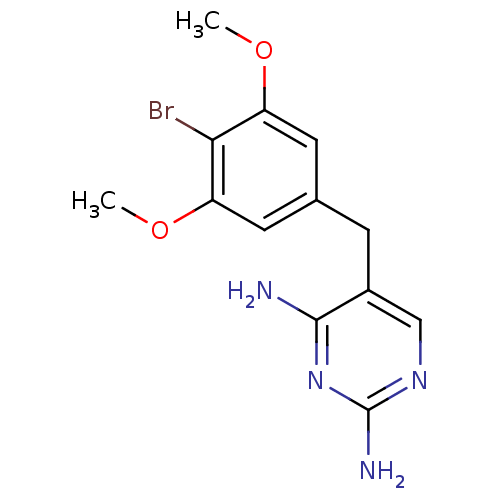
(5-(4-Bromo-3,5-dimethoxy-benzyl)-pyrimidine-2,4-di...)Show InChI InChI=1S/C13H15BrN4O2/c1-19-9-4-7(5-10(20-2)11(9)14)3-8-6-17-13(16)18-12(8)15/h4-6H,3H2,1-2H3,(H4,15,16,17,18) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60612
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Escherichia coli Dihydrofolate reductase. |
J Med Chem 39: 4825-32 (1996)
Article DOI: 10.1021/jm960491r
BindingDB Entry DOI: 10.7270/Q2668GXG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425870
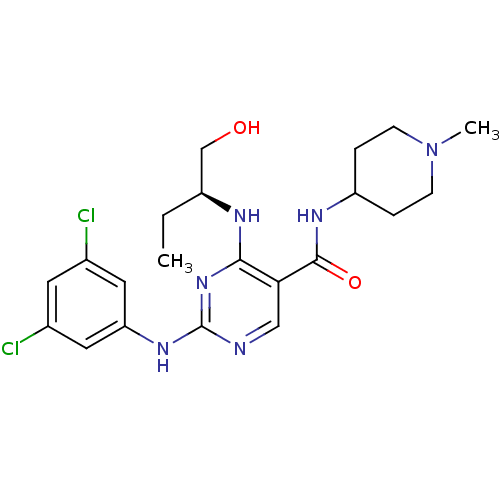
(CHEMBL2311550)Show SMILES CC[C@@H](CO)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |r| Show InChI InChI=1S/C21H28Cl2N6O2/c1-3-15(12-30)25-19-18(20(31)26-16-4-6-29(2)7-5-16)11-24-21(28-19)27-17-9-13(22)8-14(23)10-17/h8-11,15-16,30H,3-7,12H2,1-2H3,(H,26,31)(H2,24,25,27,28)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12103
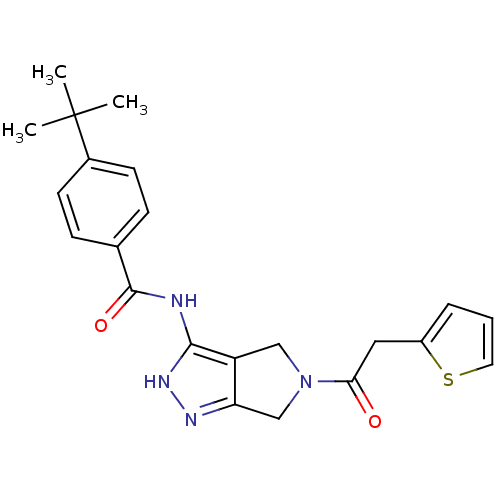
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 11 | 4-te...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)Cc1cccs1 Show InChI InChI=1S/C22H24N4O2S/c1-22(2,3)15-8-6-14(7-9-15)21(28)23-20-17-12-26(13-18(17)24-25-20)19(27)11-16-5-4-10-29-16/h4-10H,11-13H2,1-3H3,(H2,23,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60612
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Escherichia coli Dihydrofolate reductase. |
J Med Chem 39: 4825-32 (1996)
Article DOI: 10.1021/jm960491r
BindingDB Entry DOI: 10.7270/Q2668GXG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425871

(CHEMBL2312651)Show SMILES CN1CCC(CC1)NC(=O)c1cnc(Nc2cc(Cl)cc(Cl)c2)nc1N[C@H]1CCCCNC1=O |r| Show InChI InChI=1S/C23H29Cl2N7O2/c1-32-8-5-16(6-9-32)28-21(33)18-13-27-23(29-17-11-14(24)10-15(25)12-17)31-20(18)30-19-4-2-3-7-26-22(19)34/h10-13,16,19H,2-9H2,1H3,(H,26,34)(H,28,33)(H2,27,29,30,31)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425872

(CHEMBL2312650)Show SMILES CN1CCC(CC1)NC(=O)c1cnc(Nc2cc(Cl)cc(Cl)c2)nc1NC1CCOCC1 Show InChI InChI=1S/C22H28Cl2N6O2/c1-30-6-2-16(3-7-30)27-21(31)19-13-25-22(28-18-11-14(23)10-15(24)12-18)29-20(19)26-17-4-8-32-9-5-17/h10-13,16-17H,2-9H2,1H3,(H,27,31)(H2,25,26,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425863
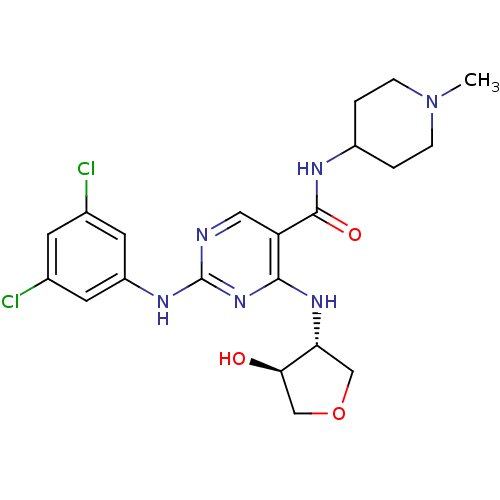
(CHEMBL2312652)Show SMILES CN1CCC(CC1)NC(=O)c1cnc(Nc2cc(Cl)cc(Cl)c2)nc1N[C@@H]1COC[C@H]1O |r| Show InChI InChI=1S/C21H26Cl2N6O3/c1-29-4-2-14(3-5-29)25-20(31)16-9-24-21(26-15-7-12(22)6-13(23)8-15)28-19(16)27-17-10-32-11-18(17)30/h6-9,14,17-18,30H,2-5,10-11H2,1H3,(H,25,31)(H2,24,26,27,28)/t17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50404462

(4-Hydroxytrimethoprim | CHEMBL1181)Show InChI InChI=1S/C13H16N4O3/c1-19-9-4-7(5-10(20-2)11(9)18)3-8-6-16-13(15)17-12(8)14/h4-6,18H,3H2,1-2H3,(H4,14,15,16,17) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60612
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Escherichia coli Dihydrofolate reductase. |
J Med Chem 39: 4825-32 (1996)
Article DOI: 10.1021/jm960491r
BindingDB Entry DOI: 10.7270/Q2668GXG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50360603
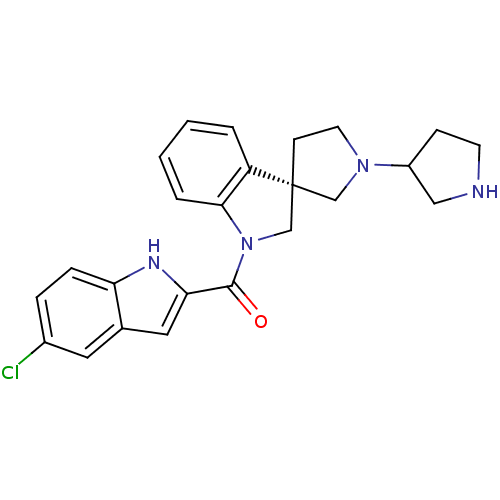
(CHEMBL1933536)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)N1C[C@]2(CCN(C2)C2CCNC2)c2ccccc12 |r| Show InChI InChI=1S/C24H25ClN4O/c25-17-5-6-20-16(11-17)12-21(27-20)23(30)29-15-24(19-3-1-2-4-22(19)29)8-10-28(14-24)18-7-9-26-13-18/h1-6,11-12,18,26-27H,7-10,13-15H2/t18?,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 using dextromethorphan as substrate |
Bioorg Med Chem Lett 22: 190-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.036
BindingDB Entry DOI: 10.7270/Q2CF9QJS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425866
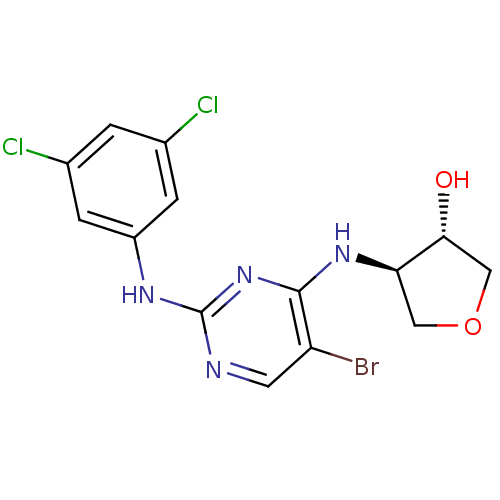
(CHEMBL2312657)Show SMILES O[C@@H]1COC[C@H]1Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1Br |r| Show InChI InChI=1S/C14H13BrCl2N4O2/c15-10-4-18-14(19-9-2-7(16)1-8(17)3-9)21-13(10)20-11-5-23-6-12(11)22/h1-4,11-12,22H,5-6H2,(H2,18,19,20,21)/t11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50425862

(CHEMBL2312654)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1cc(C)no1 |(9.04,-43.08,;10.39,-42.35,;11.7,-43.16,;10.44,-40.81,;9.13,-40,;9.18,-38.46,;10.53,-37.74,;11.85,-38.53,;11.8,-40.08,;10.57,-36.2,;9.81,-34.87,;8.27,-34.86,;7.51,-33.53,;5.96,-33.52,;5.2,-32.19,;5.97,-30.85,;5.21,-29.52,;5.98,-28.19,;3.67,-29.52,;2.89,-30.86,;1.35,-30.86,;3.66,-32.19,;8.27,-32.2,;9.8,-32.19,;10.58,-33.53,;12.11,-33.53,;12.6,-34.99,;14.14,-34.99,;15.06,-36.23,;14.61,-33.52,;13.35,-32.62,)| Show InChI InChI=1S/C22H26Cl2N6O/c1-13-8-20(31-29-13)19-12-25-22(27-17-10-14(23)9-15(24)11-17)28-21(19)26-16-4-6-18(7-5-16)30(2)3/h8-12,16,18H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAP4K4 (unknown origin) in presence of ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50405965
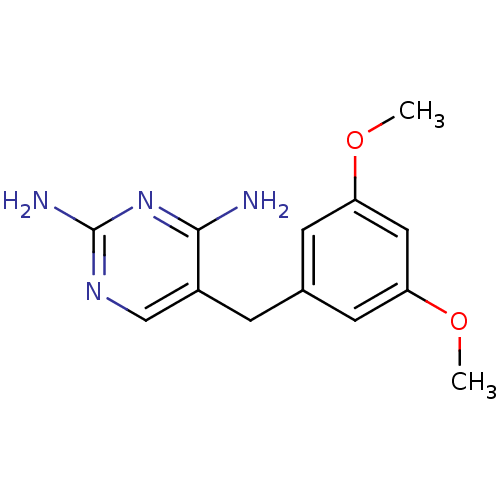
(CHEMBL287241)Show InChI InChI=1S/C13H16N4O2/c1-18-10-4-8(5-11(6-10)19-2)3-9-7-16-13(15)17-12(9)14/h4-7H,3H2,1-2H3,(H4,14,15,16,17) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60612
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Escherichia coli Dihydrofolate reductase. |
J Med Chem 39: 4825-32 (1996)
Article DOI: 10.1021/jm960491r
BindingDB Entry DOI: 10.7270/Q2668GXG |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12110
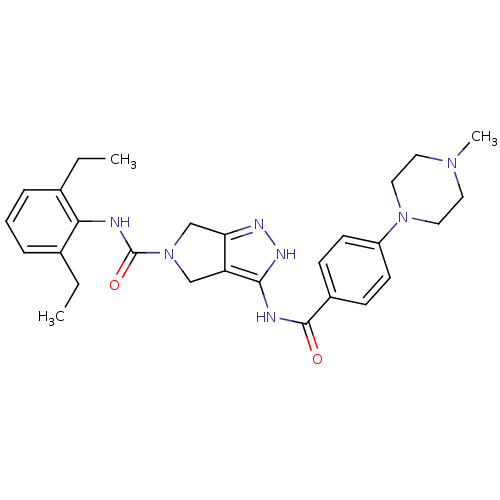
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 18 | 5-N-...)Show SMILES CCc1cccc(CC)c1NC(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1 Show InChI InChI=1S/C28H35N7O2/c1-4-19-7-6-8-20(5-2)25(19)29-28(37)35-17-23-24(18-35)31-32-26(23)30-27(36)21-9-11-22(12-10-21)34-15-13-33(3)14-16-34/h6-12H,4-5,13-18H2,1-3H3,(H,29,37)(H2,30,31,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50405982

(CHEMBL56602)Show InChI InChI=1S/C13H16N4/c1-8-3-9(2)5-10(4-8)6-11-7-16-13(15)17-12(11)14/h3-5,7H,6H2,1-2H3,(H4,14,15,16,17) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60612
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Escherichia coli Dihydrofolate reductase. |
J Med Chem 39: 4825-32 (1996)
Article DOI: 10.1021/jm960491r
BindingDB Entry DOI: 10.7270/Q2668GXG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425842

(CHEMBL2312292)Show SMILES COc1cc(CCNc2ncc(C(=O)NCCCN3CCCC3=O)c(NC3CCCC3)n2)ccc1O Show InChI InChI=1S/C26H36N6O4/c1-36-22-16-18(9-10-21(22)33)11-13-28-26-29-17-20(24(31-26)30-19-6-2-3-7-19)25(35)27-12-5-15-32-14-4-8-23(32)34/h9-10,16-17,19,33H,2-8,11-15H2,1H3,(H,27,35)(H2,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425844
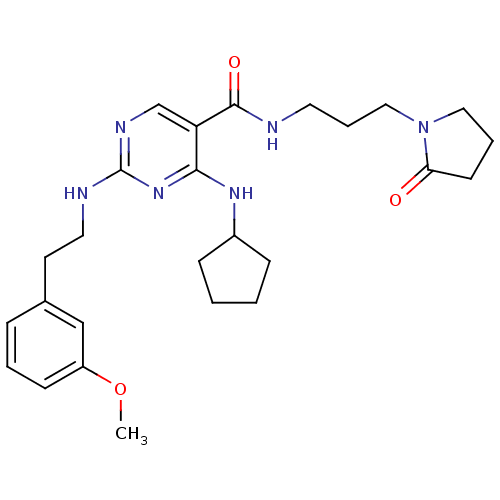
(CHEMBL2312318)Show SMILES COc1cccc(CCNc2ncc(C(=O)NCCCN3CCCC3=O)c(NC3CCCC3)n2)c1 Show InChI InChI=1S/C26H36N6O3/c1-35-21-10-4-7-19(17-21)12-14-28-26-29-18-22(24(31-26)30-20-8-2-3-9-20)25(34)27-13-6-16-32-15-5-11-23(32)33/h4,7,10,17-18,20H,2-3,5-6,8-9,11-16H2,1H3,(H,27,34)(H2,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12104
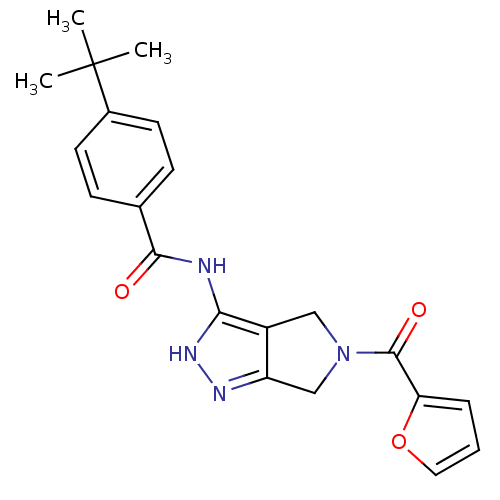
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 12 | 4-te...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)c1ccco1 Show InChI InChI=1S/C21H22N4O3/c1-21(2,3)14-8-6-13(7-9-14)19(26)22-18-15-11-25(12-16(15)23-24-18)20(27)17-5-4-10-28-17/h4-10H,11-12H2,1-3H3,(H2,22,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425873
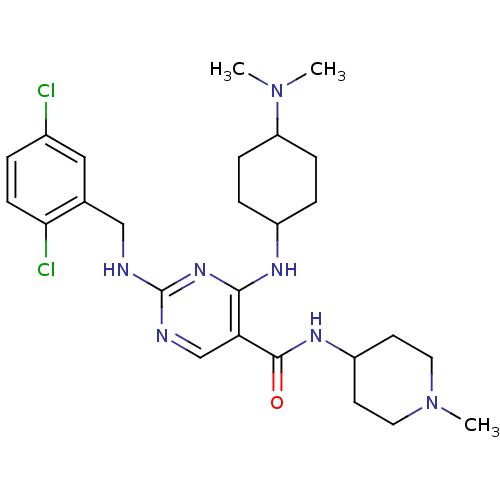
(CHEMBL2312648)Show SMILES CN(C)C1CCC(CC1)Nc1nc(NCc2cc(Cl)ccc2Cl)ncc1C(=O)NC1CCN(C)CC1 |(25.27,-50.31,;26.81,-50.32,;27.57,-51.65,;27.59,-48.99,;26.83,-47.65,;27.6,-46.32,;29.13,-46.34,;29.91,-47.66,;29.13,-49,;29.91,-45.01,;29.14,-43.67,;27.6,-43.67,;26.84,-42.33,;25.3,-42.33,;24.53,-43.66,;22.99,-43.66,;22.22,-44.99,;20.69,-44.99,;19.92,-46.32,;19.91,-43.65,;20.69,-42.32,;22.22,-42.32,;23,-40.99,;27.6,-41,;29.14,-41,;29.91,-42.34,;31.45,-42.34,;32.22,-43.67,;32.23,-41.01,;33.77,-41.01,;34.53,-42.34,;36.06,-42.35,;36.84,-41.02,;38.38,-41.03,;36.07,-39.68,;34.53,-39.67,)| Show InChI InChI=1S/C26H37Cl2N7O/c1-34(2)21-7-5-19(6-8-21)31-24-22(25(36)32-20-10-12-35(3)13-11-20)16-30-26(33-24)29-15-17-14-18(27)4-9-23(17)28/h4,9,14,16,19-21H,5-8,10-13,15H2,1-3H3,(H,32,36)(H2,29,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425868

(CHEMBL2312655)Show SMILES O[C@@H]1COC[C@H]1Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1cccnc1 |r| Show InChI InChI=1S/C19H17Cl2N5O2/c20-12-4-13(21)6-14(5-12)24-19-23-8-15(11-2-1-3-22-7-11)18(26-19)25-16-9-28-10-17(16)27/h1-8,16-17,27H,9-10H2,(H2,23,24,25,26)/t16-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50425832
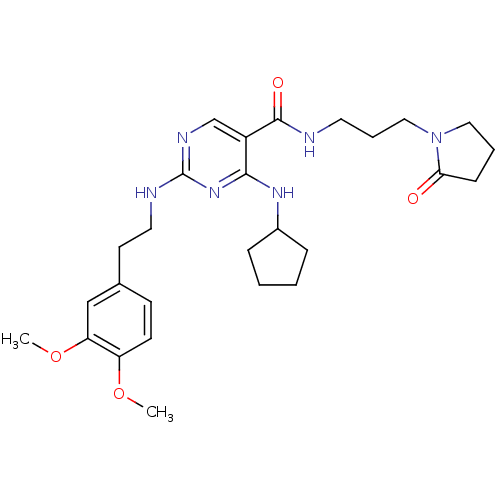
(CHEMBL2312290)Show SMILES COc1ccc(CCNc2ncc(C(=O)NCCCN3CCCC3=O)c(NC3CCCC3)n2)cc1OC Show InChI InChI=1S/C27H38N6O4/c1-36-22-11-10-19(17-23(22)37-2)12-14-29-27-30-18-21(25(32-27)31-20-7-3-4-8-20)26(35)28-13-6-16-33-15-5-9-24(33)34/h10-11,17-18,20H,3-9,12-16H2,1-2H3,(H,28,35)(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Axl (unknown origin) |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12107

(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 15 | 4-(4...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)Cc1cccs1 Show InChI InChI=1S/C23H26N6O2S/c1-27-8-10-28(11-9-27)17-6-4-16(5-7-17)23(31)24-22-19-14-29(15-20(19)25-26-22)21(30)13-18-3-2-12-32-18/h2-7,12H,8-11,13-15H2,1H3,(H2,24,25,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425843
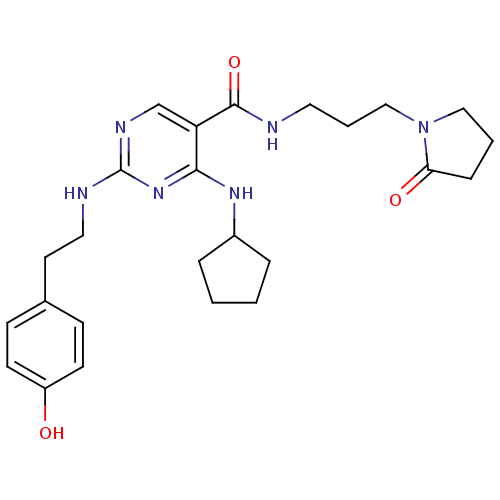
(CHEMBL2312291)Show SMILES Oc1ccc(CCNc2ncc(C(=O)NCCCN3CCCC3=O)c(NC3CCCC3)n2)cc1 Show InChI InChI=1S/C25H34N6O3/c32-20-10-8-18(9-11-20)12-14-27-25-28-17-21(23(30-25)29-19-5-1-2-6-19)24(34)26-13-4-16-31-15-3-7-22(31)33/h8-11,17,19,32H,1-7,12-16H2,(H,26,34)(H2,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425829

(CHEMBL2312304)Show SMILES Clc1ccc(Cl)c(CNc2ncc(C(=O)NCCCN3CCOC3=O)c(NC3CCCC3)n2)c1 Show InChI InChI=1S/C23H28Cl2N6O3/c24-16-6-7-19(25)15(12-16)13-27-22-28-14-18(20(30-22)29-17-4-1-2-5-17)21(32)26-8-3-9-31-10-11-34-23(31)33/h6-7,12,14,17H,1-5,8-11,13H2,(H,26,32)(H2,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425829

(CHEMBL2312304)Show SMILES Clc1ccc(Cl)c(CNc2ncc(C(=O)NCCCN3CCOC3=O)c(NC3CCCC3)n2)c1 Show InChI InChI=1S/C23H28Cl2N6O3/c24-16-6-7-19(25)15(12-16)13-27-22-28-14-18(20(30-22)29-17-4-1-2-5-17)21(32)26-8-3-9-31-10-11-34-23(31)33/h6-7,12,14,17H,1-5,8-11,13H2,(H,26,32)(H2,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425869

(CHEMBL2312653)Show SMILES Cc1cc(on1)-c1cnc(Nc2cc(Cl)cc(Cl)c2)nc1NC1CCNCC1 Show InChI InChI=1S/C19H20Cl2N6O/c1-11-6-17(28-27-11)16-10-23-19(25-15-8-12(20)7-13(21)9-15)26-18(16)24-14-2-4-22-5-3-14/h6-10,14,22H,2-5H2,1H3,(H2,23,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50138701

(6-Methyl-5-(3,4,5-trimethoxy-benzyl)-pyrimidine-2,...)Show InChI InChI=1S/C15H20N4O3/c1-8-10(14(16)19-15(17)18-8)5-9-6-11(20-2)13(22-4)12(7-9)21-3/h6-7H,5H2,1-4H3,(H4,16,17,18,19) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60612
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Escherichia coli Dihydrofolate reductase. |
J Med Chem 39: 4825-32 (1996)
Article DOI: 10.1021/jm960491r
BindingDB Entry DOI: 10.7270/Q2668GXG |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12106
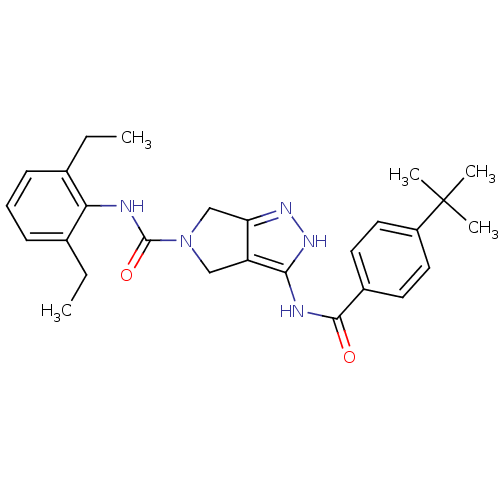
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 14 | 3-(4...)Show SMILES CCc1cccc(CC)c1NC(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)C(C)(C)C)c2C1 Show InChI InChI=1S/C27H33N5O2/c1-6-17-9-8-10-18(7-2)23(17)28-26(34)32-15-21-22(16-32)30-31-24(21)29-25(33)19-11-13-20(14-12-19)27(3,4)5/h8-14H,6-7,15-16H2,1-5H3,(H,28,34)(H2,29,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50425862

(CHEMBL2312654)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1cc(C)no1 |(9.04,-43.08,;10.39,-42.35,;11.7,-43.16,;10.44,-40.81,;9.13,-40,;9.18,-38.46,;10.53,-37.74,;11.85,-38.53,;11.8,-40.08,;10.57,-36.2,;9.81,-34.87,;8.27,-34.86,;7.51,-33.53,;5.96,-33.52,;5.2,-32.19,;5.97,-30.85,;5.21,-29.52,;5.98,-28.19,;3.67,-29.52,;2.89,-30.86,;1.35,-30.86,;3.66,-32.19,;8.27,-32.2,;9.8,-32.19,;10.58,-33.53,;12.11,-33.53,;12.6,-34.99,;14.14,-34.99,;15.06,-36.23,;14.61,-33.52,;13.35,-32.62,)| Show InChI InChI=1S/C22H26Cl2N6O/c1-13-8-20(31-29-13)19-12-25-22(27-17-10-14(23)9-15(24)11-17)28-21(19)26-16-4-6-18(7-5-16)30(2)3/h8-12,16,18H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CK1-delta (unknown origin) in presence of ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425849
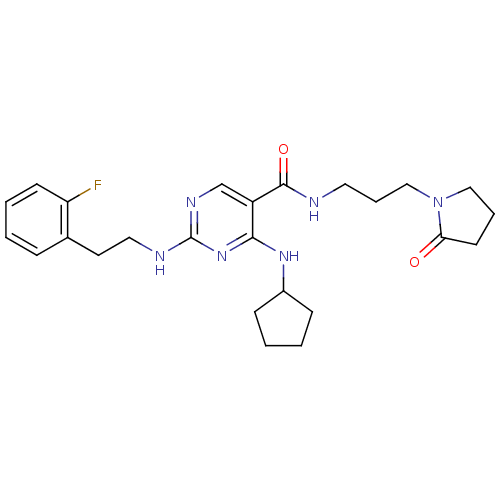
(CHEMBL2312313)Show SMILES Fc1ccccc1CCNc1ncc(C(=O)NCCCN2CCCC2=O)c(NC2CCCC2)n1 Show InChI InChI=1S/C25H33FN6O2/c26-21-10-4-1-7-18(21)12-14-28-25-29-17-20(23(31-25)30-19-8-2-3-9-19)24(34)27-13-6-16-32-15-5-11-22(32)33/h1,4,7,10,17,19H,2-3,5-6,8-9,11-16H2,(H,27,34)(H2,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50425864

(CHEMBL2312649)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |(47.36,-51.36,;48.9,-51.37,;49.67,-52.7,;49.68,-50.04,;48.92,-48.7,;49.7,-47.37,;51.23,-47.39,;52.01,-48.71,;51.22,-50.05,;52,-46.06,;51.24,-44.72,;49.7,-44.72,;48.94,-43.38,;47.4,-43.38,;46.63,-42.04,;47.41,-40.71,;46.64,-39.37,;47.41,-38.04,;45.1,-39.37,;44.33,-40.71,;42.79,-40.71,;45.1,-42.04,;49.7,-42.05,;51.24,-42.05,;52.01,-43.39,;53.55,-43.39,;54.32,-44.72,;54.32,-42.06,;55.86,-42.06,;56.62,-43.39,;58.16,-43.4,;58.94,-42.07,;60.48,-42.08,;58.17,-40.73,;56.62,-40.72,)| Show InChI InChI=1S/C25H35Cl2N7O/c1-33(2)21-6-4-18(5-7-21)29-23-22(24(35)30-19-8-10-34(3)11-9-19)15-28-25(32-23)31-20-13-16(26)12-17(27)14-20/h12-15,18-19,21H,4-11H2,1-3H3,(H,30,35)(H2,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAP4K4 (unknown origin) in presence of ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50425832

(CHEMBL2312290)Show SMILES COc1ccc(CCNc2ncc(C(=O)NCCCN3CCCC3=O)c(NC3CCCC3)n2)cc1OC Show InChI InChI=1S/C27H38N6O4/c1-36-22-11-10-19(17-23(22)37-2)12-14-29-27-30-18-21(25(32-27)31-20-7-3-4-8-20)26(35)28-13-6-16-33-15-5-9-24(33)34/h10-11,17-18,20H,3-9,12-16H2,1-2H3,(H,28,35)(H2,29,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mer (unknown origin) |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12105
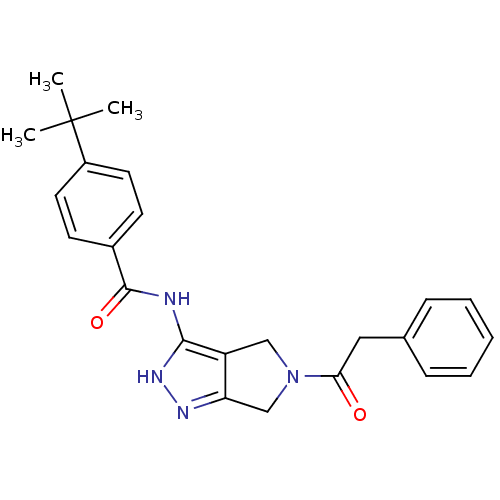
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 13 | 4-te...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)Cc1ccccc1 Show InChI InChI=1S/C24H26N4O2/c1-24(2,3)18-11-9-17(10-12-18)23(30)25-22-19-14-28(15-20(19)26-27-22)21(29)13-16-7-5-4-6-8-16/h4-12H,13-15H2,1-3H3,(H2,25,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12109

(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 17 | 4-(4...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)Cc1ccccc1 Show InChI InChI=1S/C25H28N6O2/c1-29-11-13-30(14-12-29)20-9-7-19(8-10-20)25(33)26-24-21-16-31(17-22(21)27-28-24)23(32)15-18-5-3-2-4-6-18/h2-10H,11-17H2,1H3,(H2,26,27,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425867

(CHEMBL2312656)Show SMILES O[C@@H]1COC[C@H]1Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H17Cl2FN4O2/c21-12-5-13(22)7-15(6-12)25-20-24-8-16(11-1-3-14(23)4-2-11)19(27-20)26-17-9-29-10-18(17)28/h1-8,17-18,28H,9-10H2,(H2,24,25,26,27)/t17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425877

(CHEMBL2312658)Show InChI InChI=1S/C21H29N5O3/c1-29-18-10-5-2-7-15(18)13-23-21-24-14-17(20(28)22-11-6-12-27)19(26-21)25-16-8-3-4-9-16/h2,5,7,10,14,16,27H,3-4,6,8-9,11-13H2,1H3,(H,22,28)(H2,23,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12108
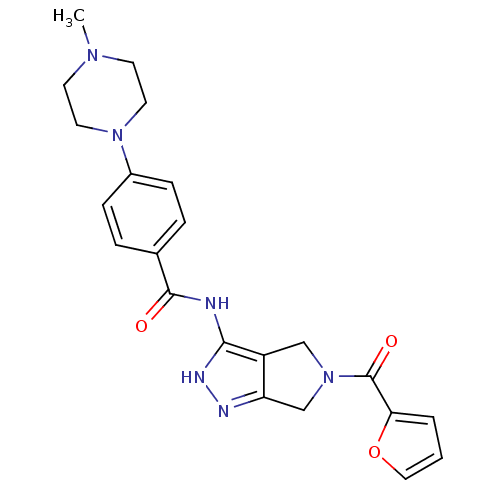
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 16 | CHEM...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)c1ccco1 Show InChI InChI=1S/C22H24N6O3/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)21(29)23-20-17-13-28(14-18(17)24-25-20)22(30)19-3-2-12-31-19/h2-7,12H,8-11,13-14H2,1H3,(H2,23,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50425862

(CHEMBL2312654)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1cc(C)no1 |(9.04,-43.08,;10.39,-42.35,;11.7,-43.16,;10.44,-40.81,;9.13,-40,;9.18,-38.46,;10.53,-37.74,;11.85,-38.53,;11.8,-40.08,;10.57,-36.2,;9.81,-34.87,;8.27,-34.86,;7.51,-33.53,;5.96,-33.52,;5.2,-32.19,;5.97,-30.85,;5.21,-29.52,;5.98,-28.19,;3.67,-29.52,;2.89,-30.86,;1.35,-30.86,;3.66,-32.19,;8.27,-32.2,;9.8,-32.19,;10.58,-33.53,;12.11,-33.53,;12.6,-34.99,;14.14,-34.99,;15.06,-36.23,;14.61,-33.52,;13.35,-32.62,)| Show InChI InChI=1S/C22H26Cl2N6O/c1-13-8-20(31-29-13)19-12-25-22(27-17-10-14(23)9-15(24)11-17)28-21(19)26-16-4-6-18(7-5-16)30(2)3/h8-12,16,18H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAP3K9 (unknown origin) in presence of ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425847
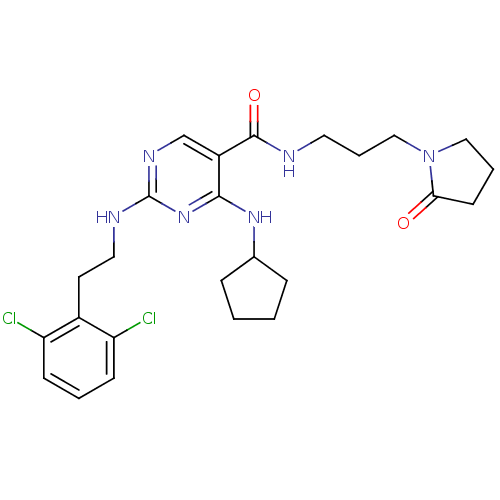
(CHEMBL2312315)Show SMILES Clc1cccc(Cl)c1CCNc1ncc(C(=O)NCCCN2CCCC2=O)c(NC2CCCC2)n1 Show InChI InChI=1S/C25H32Cl2N6O2/c26-20-8-3-9-21(27)18(20)11-13-29-25-30-16-19(23(32-25)31-17-6-1-2-7-17)24(35)28-12-5-15-33-14-4-10-22(33)34/h3,8-9,16-17H,1-2,4-7,10-15H2,(H,28,35)(H2,29,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425848
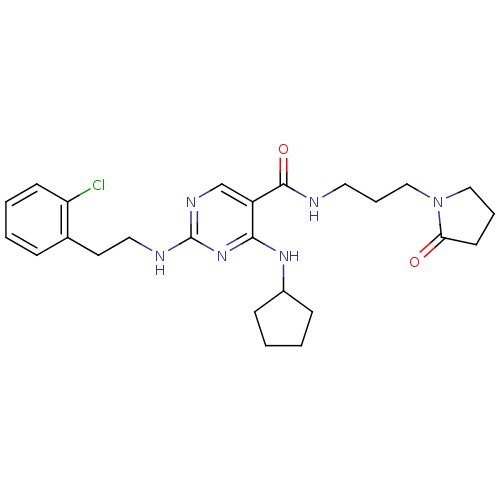
(CHEMBL2312314)Show SMILES Clc1ccccc1CCNc1ncc(C(=O)NCCCN2CCCC2=O)c(NC2CCCC2)n1 Show InChI InChI=1S/C25H33ClN6O2/c26-21-10-4-1-7-18(21)12-14-28-25-29-17-20(23(31-25)30-19-8-2-3-9-19)24(34)27-13-6-16-32-15-5-11-22(32)33/h1,4,7,10,17,19H,2-3,5-6,8-9,11-16H2,(H,27,34)(H2,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase
(Mus musculus) | BDBM50360610
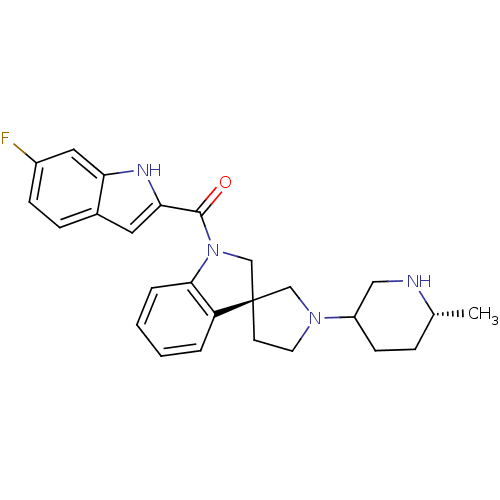
(CHEMBL1933552)Show SMILES C[C@@H]1CCC(CN1)N1CC[C@@]2(C1)CN(C(=O)c1cc3ccc(F)cc3[nH]1)c1ccccc21 |r| Show InChI InChI=1S/C26H29FN4O/c1-17-6-9-20(14-28-17)30-11-10-26(15-30)16-31(24-5-3-2-4-21(24)26)25(32)23-12-18-7-8-19(27)13-22(18)29-23/h2-5,7-8,12-13,17,20,28-29H,6,9-11,14-16H2,1H3/t17-,20?,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse Sky kinase assessed as inhibition of src substrate phosphorylation by ELISA |
Bioorg Med Chem Lett 22: 190-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.036
BindingDB Entry DOI: 10.7270/Q2CF9QJS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425874
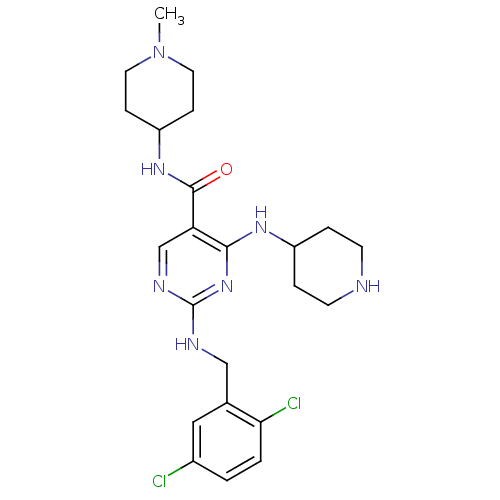
(CHEMBL2312647)Show SMILES CN1CCC(CC1)NC(=O)c1cnc(NCc2cc(Cl)ccc2Cl)nc1NC1CCNCC1 Show InChI InChI=1S/C23H31Cl2N7O/c1-32-10-6-18(7-11-32)30-22(33)19-14-28-23(27-13-15-12-16(24)2-3-20(15)25)31-21(19)29-17-4-8-26-9-5-17/h2-3,12,14,17-18,26H,4-11,13H2,1H3,(H,30,33)(H2,27,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425845
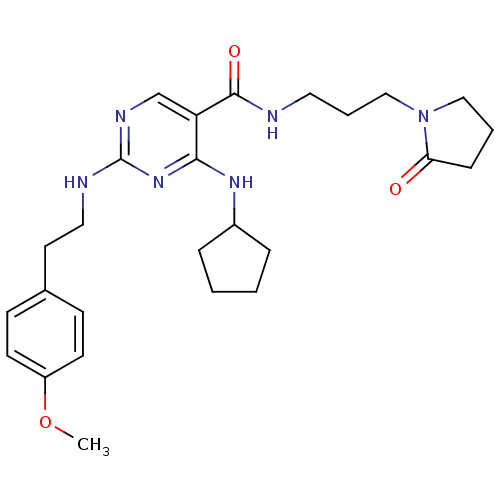
(CHEMBL2312317)Show SMILES COc1ccc(CCNc2ncc(C(=O)NCCCN3CCCC3=O)c(NC3CCCC3)n2)cc1 Show InChI InChI=1S/C26H36N6O3/c1-35-21-11-9-19(10-12-21)13-15-28-26-29-18-22(24(31-26)30-20-6-2-3-7-20)25(34)27-14-5-17-32-16-4-8-23(32)33/h9-12,18,20H,2-8,13-17H2,1H3,(H,27,34)(H2,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425833

(CHEMBL2312302)Show SMILES CCOc1ccccc1CNc1ncc(C(=O)NCCCN2CCCC2=O)c(NC2CCCC2)n1 Show InChI InChI=1S/C26H36N6O3/c1-2-35-22-12-6-3-9-19(22)17-28-26-29-18-21(24(31-26)30-20-10-4-5-11-20)25(34)27-14-8-16-32-15-7-13-23(32)33/h3,6,9,12,18,20H,2,4-5,7-8,10-11,13-17H2,1H3,(H,27,34)(H2,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50425862

(CHEMBL2312654)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1cc(C)no1 |(9.04,-43.08,;10.39,-42.35,;11.7,-43.16,;10.44,-40.81,;9.13,-40,;9.18,-38.46,;10.53,-37.74,;11.85,-38.53,;11.8,-40.08,;10.57,-36.2,;9.81,-34.87,;8.27,-34.86,;7.51,-33.53,;5.96,-33.52,;5.2,-32.19,;5.97,-30.85,;5.21,-29.52,;5.98,-28.19,;3.67,-29.52,;2.89,-30.86,;1.35,-30.86,;3.66,-32.19,;8.27,-32.2,;9.8,-32.19,;10.58,-33.53,;12.11,-33.53,;12.6,-34.99,;14.14,-34.99,;15.06,-36.23,;14.61,-33.52,;13.35,-32.62,)| Show InChI InChI=1S/C22H26Cl2N6O/c1-13-8-20(31-29-13)19-12-25-22(27-17-10-14(23)9-15(24)11-17)28-21(19)26-16-4-6-18(7-5-16)30(2)3/h8-12,16,18H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) in presence of ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase
(Mus musculus) | BDBM50360609
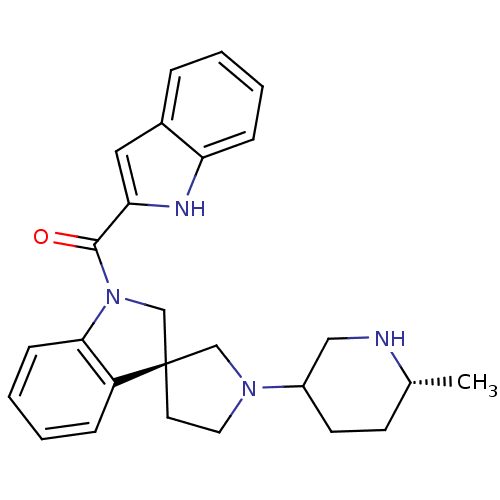
(CHEMBL1933551)Show SMILES C[C@@H]1CCC(CN1)N1CC[C@@]2(C1)CN(C(=O)c1cc3ccccc3[nH]1)c1ccccc21 |r| Show InChI InChI=1S/C26H30N4O/c1-18-10-11-20(15-27-18)29-13-12-26(16-29)17-30(24-9-5-3-7-21(24)26)25(31)23-14-19-6-2-4-8-22(19)28-23/h2-9,14,18,20,27-28H,10-13,15-17H2,1H3/t18-,20?,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse Sky kinase assessed as inhibition of src substrate phosphorylation by ELISA |
Bioorg Med Chem Lett 22: 190-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.036
BindingDB Entry DOI: 10.7270/Q2CF9QJS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
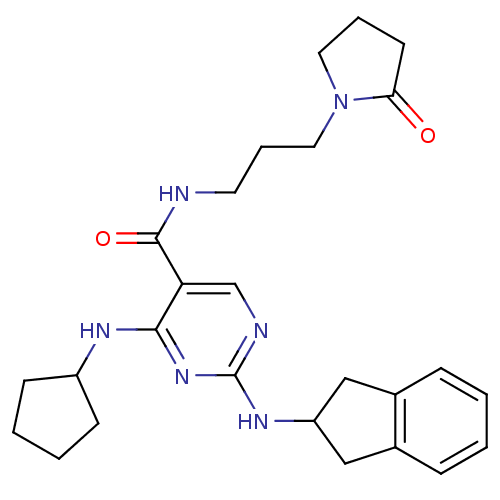
(Homo sapiens (Human)) | BDBM50425850
(CHEMBL2312312)Show SMILES O=C(NCCCN1CCCC1=O)c1cnc(NC2Cc3ccccc3C2)nc1NC1CCCC1 Show InChI InChI=1S/C26H34N6O2/c33-23-11-5-13-32(23)14-6-12-27-25(34)22-17-28-26(31-24(22)29-20-9-3-4-10-20)30-21-15-18-7-1-2-8-19(18)16-21/h1-2,7-8,17,20-21H,3-6,9-16H2,(H,27,34)(H2,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425832

(CHEMBL2312290)Show SMILES COc1ccc(CCNc2ncc(C(=O)NCCCN3CCCC3=O)c(NC3CCCC3)n2)cc1OC Show InChI InChI=1S/C27H38N6O4/c1-36-22-11-10-19(17-23(22)37-2)12-14-29-27-30-18-21(25(32-27)31-20-7-3-4-8-20)26(35)28-13-6-16-33-15-5-9-24(33)34/h10-11,17-18,20H,3-9,12-16H2,1-2H3,(H,28,35)(H2,29,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) in presence of 60 uM ATP by ELISA |
Bioorg Med Chem Lett 23: 1046-50 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.013
BindingDB Entry DOI: 10.7270/Q26D5V9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
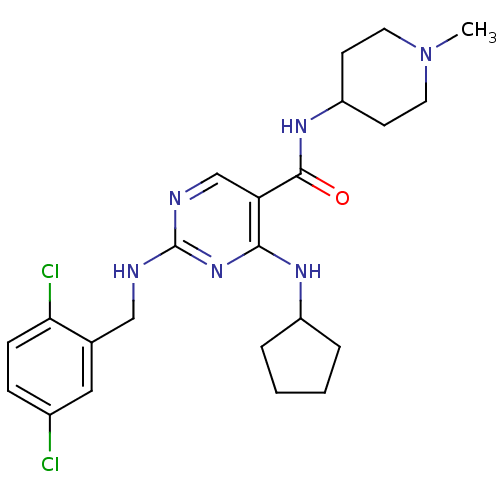
(Homo sapiens (Human)) | BDBM50425865
(CHEMBL2312646)Show SMILES CN1CCC(CC1)NC(=O)c1cnc(NCc2cc(Cl)ccc2Cl)nc1NC1CCCC1 Show InChI InChI=1S/C23H30Cl2N6O/c1-31-10-8-18(9-11-31)29-22(32)19-14-27-23(30-21(19)28-17-4-2-3-5-17)26-13-15-12-16(24)6-7-20(15)25/h6-7,12,14,17-18H,2-5,8-11,13H2,1H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data