 Found 830 hits with Last Name = 'christopoulos' and Initial = 'a'
Found 830 hits with Last Name = 'christopoulos' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50021819

(CHEMBL3298828)Show SMILES COc1nc2sc(C(=O)NCCCCCCN3CCC(CC3)c3ccc(Cl)cc3)c(N)c2c(C)c1Cl Show InChI InChI=1S/C27H34Cl2N4O2S/c1-17-21-23(30)24(36-27(21)32-26(35-2)22(17)29)25(34)31-13-5-3-4-6-14-33-15-11-19(12-16-33)18-7-9-20(28)10-8-18/h7-10,19H,3-6,11-16,30H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis |
J Med Chem 57: 4924-39 (2014)
Article DOI: 10.1021/jm500457x
BindingDB Entry DOI: 10.7270/Q29Z96FS |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from alpha4beta2 nAChR expressed in human recombinant SH-SY5Y cell membranes after 120 mins |
J Med Chem 60: 9239-9250 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01113
BindingDB Entry DOI: 10.7270/Q2445PW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50246607

(CHEMBL4083241)Show InChI InChI=1S/C11H16N2O/c1-3-10(7-12-5-1)9-14-11-4-2-6-13-8-11/h1,3,5,7,11,13H,2,4,6,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from alpha4beta2 nAChR expressed in human recombinant SH-SY5Y cell membranes after 120 mins |
J Med Chem 60: 9239-9250 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01113
BindingDB Entry DOI: 10.7270/Q2445PW2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50233623

(CHEMBL4104013)Show SMILES O=C1COc2ccccc2N1CCCN1CCN(CC1)c1cccc2[nH]c(=O)oc12 Show InChI InChI=1S/C22H24N4O4/c27-20-15-29-19-8-2-1-6-17(19)26(20)10-4-9-24-11-13-25(14-12-24)18-7-3-5-16-21(18)30-22(28)23-16/h1-3,5-8H,4,9-15H2,(H,23,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis |
J Med Chem 58: 1550-5 (2015)
Article DOI: 10.1021/jm5013243
BindingDB Entry DOI: 10.7270/Q2F76FTG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50233623

(CHEMBL4104013)Show SMILES O=C1COc2ccccc2N1CCCN1CCN(CC1)c1cccc2[nH]c(=O)oc12 Show InChI InChI=1S/C22H24N4O4/c27-20-15-29-19-8-2-1-6-17(19)26(20)10-4-9-24-11-13-25(14-12-24)18-7-3-5-16-21(18)30-22(28)23-16/h1-3,5-8H,4,9-15H2,(H,23,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis |
J Med Chem 58: 1550-5 (2015)
Article DOI: 10.1021/jm5013243
BindingDB Entry DOI: 10.7270/Q2F76FTG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50233626
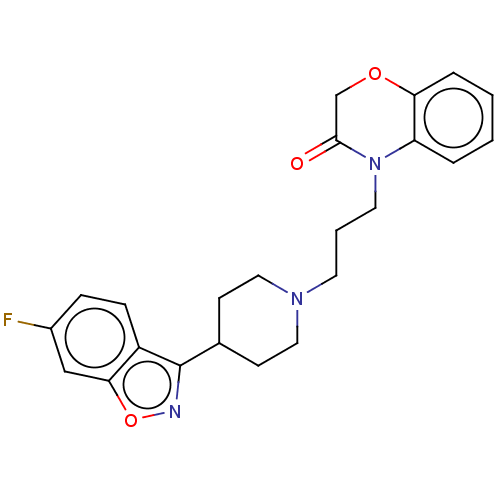
(CHEMBL4062602)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCN2C(=O)COc3ccccc23)CC1 Show InChI InChI=1S/C23H24FN3O3/c24-17-6-7-18-21(14-17)30-25-23(18)16-8-12-26(13-9-16)10-3-11-27-19-4-1-2-5-20(19)29-15-22(27)28/h1-2,4-7,14,16H,3,8-13,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method |
J Med Chem 58: 1550-5 (2015)
Article DOI: 10.1021/jm5013243
BindingDB Entry DOI: 10.7270/Q2F76FTG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50233626

(CHEMBL4062602)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCN2C(=O)COc3ccccc23)CC1 Show InChI InChI=1S/C23H24FN3O3/c24-17-6-7-18-21(14-17)30-25-23(18)16-8-12-26(13-9-16)10-3-11-27-19-4-1-2-5-20(19)29-15-22(27)28/h1-2,4-7,14,16H,3,8-13,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method |
J Med Chem 58: 1550-5 (2015)
Article DOI: 10.1021/jm5013243
BindingDB Entry DOI: 10.7270/Q2F76FTG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50087826
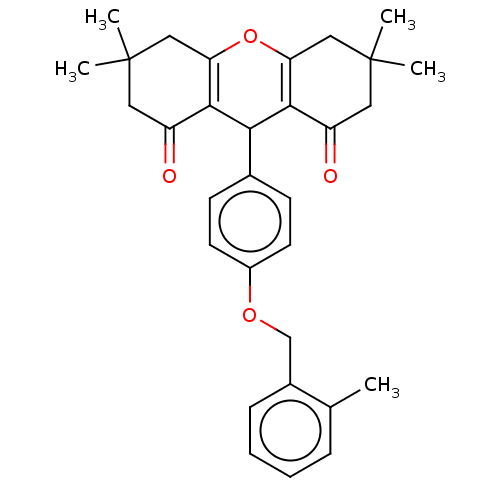
(CHEMBL3426789)Show SMILES Cc1ccccc1COc1ccc(cc1)C1C2=C(CC(C)(C)CC2=O)OC2=C1C(=O)CC(C)(C)C2 |c:29,t:18| Show InChI InChI=1S/C31H34O4/c1-19-8-6-7-9-21(19)18-34-22-12-10-20(11-13-22)27-28-23(32)14-30(2,3)16-25(28)35-26-17-31(4,5)15-24(33)29(26)27/h6-13,27H,14-18H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human delta opioid receptor expressed in CHO cell membranes assessed as TAN67 Ki at 10 uM after 90 mins by [3H]-dip... |
J Med Chem 58: 4220-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00007
BindingDB Entry DOI: 10.7270/Q27P9149 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50233626

(CHEMBL4062602)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCN2C(=O)COc3ccccc23)CC1 Show InChI InChI=1S/C23H24FN3O3/c24-17-6-7-18-21(14-17)30-25-23(18)16-8-12-26(13-9-16)10-3-11-27-19-4-1-2-5-20(19)29-15-22(27)28/h1-2,4-7,14,16H,3,8-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis |
J Med Chem 58: 1550-5 (2015)
Article DOI: 10.1021/jm5013243
BindingDB Entry DOI: 10.7270/Q2F76FTG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50233626

(CHEMBL4062602)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCN2C(=O)COc3ccccc23)CC1 Show InChI InChI=1S/C23H24FN3O3/c24-17-6-7-18-21(14-17)30-25-23(18)16-8-12-26(13-9-16)10-3-11-27-19-4-1-2-5-20(19)29-15-22(27)28/h1-2,4-7,14,16H,3,8-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis |
J Med Chem 58: 1550-5 (2015)
Article DOI: 10.1021/jm5013243
BindingDB Entry DOI: 10.7270/Q2F76FTG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50021818

(CHEMBL3298827)Show SMILES COc1ccccc1N1CCN(CCCCCNC(=O)c2sc3nc(OC)c(Cl)c(C)c3c2N)CC1 Show InChI InChI=1S/C26H34ClN5O3S/c1-17-20-22(28)23(36-26(20)30-25(35-3)21(17)27)24(33)29-11-7-4-8-12-31-13-15-32(16-14-31)18-9-5-6-10-19(18)34-2/h5-6,9-10H,4,7-8,11-16,28H2,1-3H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis |
J Med Chem 57: 4924-39 (2014)
Article DOI: 10.1021/jm500457x
BindingDB Entry DOI: 10.7270/Q29Z96FS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 4.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50448377

(CHEMBL3121473)Show InChI InChI=1S/C10H17N2O2/c1-12(2,3)7-4-5-8-13-10-6-9-14-11-10/h6-9H2,1-3H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopolamine bromide from human recombinant muscarinic M2 receptor expressed in HEK293T cell membranes after 1 hr by liqu... |
J Med Chem 60: 9239-9250 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01113
BindingDB Entry DOI: 10.7270/Q2445PW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50087826

(CHEMBL3426789)Show SMILES Cc1ccccc1COc1ccc(cc1)C1C2=C(CC(C)(C)CC2=O)OC2=C1C(=O)CC(C)(C)C2 |c:29,t:18| Show InChI InChI=1S/C31H34O4/c1-19-8-6-7-9-21(19)18-34-22-12-10-20(11-13-22)27-28-23(32)14-30(2,3)16-25(28)35-26-17-31(4,5)15-24(33)29(26)27/h6-13,27H,14-18H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human delta opioid receptor expressed in CHO cell membranes assessed as SNC80 Ki at 10 uM after 90 mins by [3H]-dip... |
J Med Chem 58: 4220-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00007
BindingDB Entry DOI: 10.7270/Q27P9149 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 5.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 5.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50233625
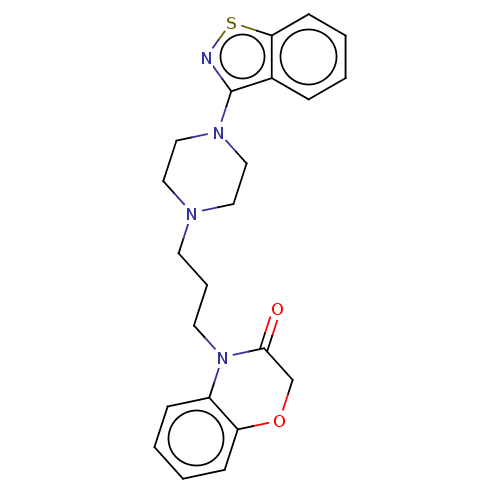
(CHEMBL4090568)Show InChI InChI=1S/C22H24N4O2S/c27-21-16-28-19-8-3-2-7-18(19)26(21)11-5-10-24-12-14-25(15-13-24)22-17-6-1-4-9-20(17)29-23-22/h1-4,6-9H,5,10-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method |
J Med Chem 58: 1550-5 (2015)
Article DOI: 10.1021/jm5013243
BindingDB Entry DOI: 10.7270/Q2F76FTG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50233625

(CHEMBL4090568)Show InChI InChI=1S/C22H24N4O2S/c27-21-16-28-19-8-3-2-7-18(19)26(21)11-5-10-24-12-14-25(15-13-24)22-17-6-1-4-9-20(17)29-23-22/h1-4,6-9H,5,10-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method |
J Med Chem 58: 1550-5 (2015)
Article DOI: 10.1021/jm5013243
BindingDB Entry DOI: 10.7270/Q2F76FTG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 6.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50021817
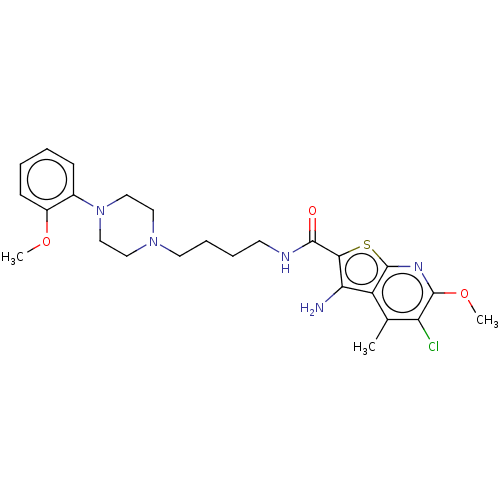
(CHEMBL3298826)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2sc3nc(OC)c(Cl)c(C)c3c2N)CC1 Show InChI InChI=1S/C25H32ClN5O3S/c1-16-19-21(27)22(35-25(19)29-24(34-3)20(16)26)23(32)28-10-6-7-11-30-12-14-31(15-13-30)17-8-4-5-9-18(17)33-2/h4-5,8-9H,6-7,10-15,27H2,1-3H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis |
J Med Chem 57: 4924-39 (2014)
Article DOI: 10.1021/jm500457x
BindingDB Entry DOI: 10.7270/Q29Z96FS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50087826

(CHEMBL3426789)Show SMILES Cc1ccccc1COc1ccc(cc1)C1C2=C(CC(C)(C)CC2=O)OC2=C1C(=O)CC(C)(C)C2 |c:29,t:18| Show InChI InChI=1S/C31H34O4/c1-19-8-6-7-9-21(19)18-34-22-12-10-20(11-13-22)27-28-23(32)14-30(2,3)16-25(28)35-26-17-31(4,5)15-24(33)29(26)27/h6-13,27H,14-18H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human delta opioid receptor expressed in CHO cell membranes assessed as leu-enkephalin Ki at 10 uM after 90 mins by... |
J Med Chem 58: 4220-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00007
BindingDB Entry DOI: 10.7270/Q27P9149 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NPA from dopamine D2L receptor (unknown origin) expressed in CHO cell membranes |
J Med Chem 56: 9199-221 (2013)
Article DOI: 10.1021/jm401318w
BindingDB Entry DOI: 10.7270/Q2RF5WGV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 8.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 8.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50240606
((2-methyl-3-(2-morpholinoethyl)-1H-indol-1-yl)(nap...)Show SMILES Cc1c(CCN2CCOCC2)c2ccccc2n1C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C26H26N2O2/c1-19-21(13-14-27-15-17-30-18-16-27)23-10-4-5-12-25(23)28(19)26(29)24-11-6-8-20-7-2-3-9-22(20)24/h2-12H,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Activity at CB2 receptor (unknown origin) |
Eur J Med Chem 43: 513-39 (2008)
Article DOI: 10.1016/j.ejmech.2007.04.007
BindingDB Entry DOI: 10.7270/Q2G73DGR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50021771
(CHEMBL3298896)Show SMILES COc1ccccc1N1CCN(CCCCCNC(=O)c2sc3nc(C)cc(C)c3c2N)CC1 Show InChI InChI=1S/C26H35N5O2S/c1-18-17-19(2)29-26-22(18)23(27)24(34-26)25(32)28-11-7-4-8-12-30-13-15-31(16-14-30)20-9-5-6-10-21(20)33-3/h5-6,9-10,17H,4,7-8,11-16,27H2,1-3H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis |
J Med Chem 57: 4924-39 (2014)
Article DOI: 10.1021/jm500457x
BindingDB Entry DOI: 10.7270/Q29Z96FS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 9.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50443100
(CHEMBL3085820)Show SMILES CC(C)(C)OC(=O)N[C@H]1CC[C@H](CCN2CCCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:8.7,wD:11.11,(21.57,-16.54,;20.24,-15.77,;20.24,-14.23,;21.57,-14.99,;18.91,-16.54,;17.57,-15.77,;17.58,-14.23,;16.24,-16.54,;14.91,-15.77,;13.57,-16.53,;12.24,-15.77,;12.24,-14.22,;10.91,-13.45,;9.58,-14.23,;8.24,-13.46,;7.06,-14.44,;5.55,-14.13,;4.84,-12.77,;5.48,-11.37,;6.98,-10.99,;8.2,-11.92,;4.73,-10.03,;5.52,-8.71,;4.77,-7.37,;3.23,-7.34,;2.44,-8.68,;.9,-8.66,;3.19,-10.01,;2.41,-11.34,;13.57,-13.45,;14.91,-14.23,)| Show InChI InChI=1S/C24H37Cl2N3O2/c1-24(2,3)31-23(30)27-19-10-8-18(9-11-19)12-15-28-13-5-14-29(17-16-28)21-7-4-6-20(25)22(21)26/h4,6-7,18-19H,5,8-17H2,1-3H3,(H,27,30)/t18-,19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis |
J Med Chem 56: 9199-221 (2013)
Article DOI: 10.1021/jm401318w
BindingDB Entry DOI: 10.7270/Q2RF5WGV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50021770
(CHEMBL3298895)Show SMILES Cc1cc(C)c2c(N)c(sc2n1)C(=O)NCCCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C25H31Cl2N5OS/c1-16-15-17(2)30-25-20(16)22(28)23(34-25)24(33)29-9-4-3-5-10-31-11-13-32(14-12-31)19-8-6-7-18(26)21(19)27/h6-8,15H,3-5,9-14,28H2,1-2H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis |
J Med Chem 57: 4924-39 (2014)
Article DOI: 10.1021/jm500457x
BindingDB Entry DOI: 10.7270/Q29Z96FS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50234418
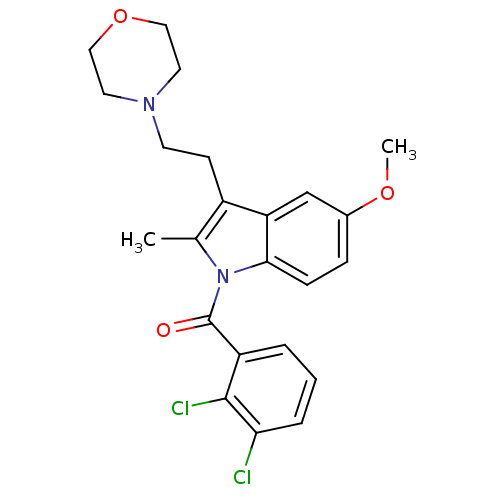
((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...)Show SMILES COc1ccc2n(C(=O)c3cccc(Cl)c3Cl)c(C)c(CCN3CCOCC3)c2c1 Show InChI InChI=1S/C23H24Cl2N2O3/c1-15-17(8-9-26-10-12-30-13-11-26)19-14-16(29-2)6-7-21(19)27(15)23(28)18-4-3-5-20(24)22(18)25/h3-7,14H,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Activity at CB2 receptor (unknown origin) |
Eur J Med Chem 43: 513-39 (2008)
Article DOI: 10.1016/j.ejmech.2007.04.007
BindingDB Entry DOI: 10.7270/Q2G73DGR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50021802

(CHEMBL3299096)Show InChI InChI=1S/C21H35N3O3/c1-21(2,3)27-20(25)22-12-8-5-9-13-23-14-16-24(17-15-23)18-10-6-7-11-19(18)26-4/h6-7,10-11H,5,8-9,12-17H2,1-4H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis |
J Med Chem 57: 4924-39 (2014)
Article DOI: 10.1021/jm500457x
BindingDB Entry DOI: 10.7270/Q29Z96FS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50072228
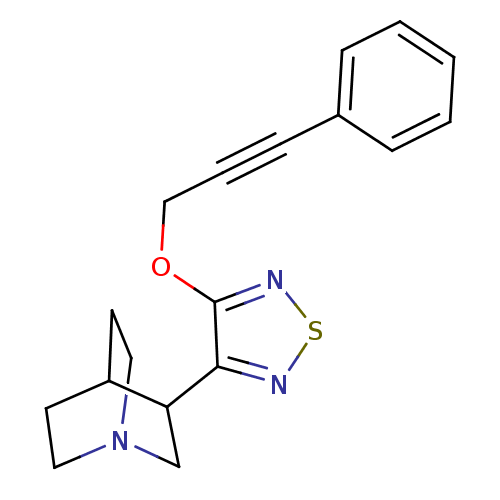
(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1 |(14.79,-5.45,;13.26,-5.32,;12.37,-6.6,;12.89,-8.07,;11.66,-9,;10.38,-8.12,;10.82,-6.64,;9.88,-5.4,;8.44,-5.96,;7.22,-5.01,;8.49,-3.99,;8.96,-4.86,;10.1,-3.86,;8.91,-2.91,;7.44,-3.72,;15.47,-6.86,;16.24,-8.18,;16.99,-9.52,;18.54,-9.52,;19.29,-10.85,;18.51,-12.2,;16.98,-12.2,;16.22,-10.85,)| Show InChI InChI=1S/C18H19N3OS/c1-2-5-14(6-3-1)7-4-12-22-18-17(19-23-20-18)16-13-21-10-8-15(16)9-11-21/h1-3,5-6,15-16H,8-13H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50021769
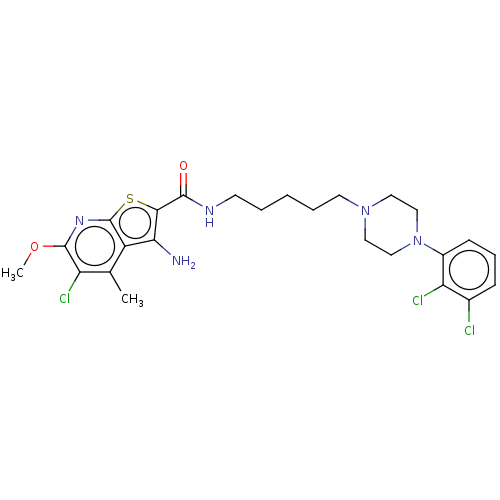
(CHEMBL3298830)Show SMILES COc1nc2sc(C(=O)NCCCCCN3CCN(CC3)c3cccc(Cl)c3Cl)c(N)c2c(C)c1Cl Show InChI InChI=1S/C25H30Cl3N5O2S/c1-15-18-21(29)22(36-25(18)31-24(35-2)19(15)27)23(34)30-9-4-3-5-10-32-11-13-33(14-12-32)17-8-6-7-16(26)20(17)28/h6-8H,3-5,9-14,29H2,1-2H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis |
J Med Chem 57: 4924-39 (2014)
Article DOI: 10.1021/jm500457x
BindingDB Entry DOI: 10.7270/Q29Z96FS |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50021796
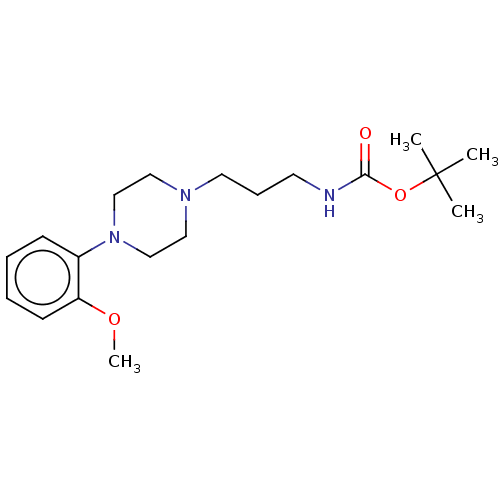
(CHEMBL3299094)Show InChI InChI=1S/C19H31N3O3/c1-19(2,3)25-18(23)20-10-7-11-21-12-14-22(15-13-21)16-8-5-6-9-17(16)24-4/h5-6,8-9H,7,10-15H2,1-4H3,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis |
J Med Chem 57: 4924-39 (2014)
Article DOI: 10.1021/jm500457x
BindingDB Entry DOI: 10.7270/Q29Z96FS |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50233625

(CHEMBL4090568)Show InChI InChI=1S/C22H24N4O2S/c27-21-16-28-19-8-3-2-7-18(19)26(21)11-5-10-24-12-14-25(15-13-24)22-17-6-1-4-9-20(17)29-23-22/h1-4,6-9H,5,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis |
J Med Chem 58: 1550-5 (2015)
Article DOI: 10.1021/jm5013243
BindingDB Entry DOI: 10.7270/Q2F76FTG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50233625

(CHEMBL4090568)Show InChI InChI=1S/C22H24N4O2S/c27-21-16-28-19-8-3-2-7-18(19)26(21)11-5-10-24-12-14-25(15-13-24)22-17-6-1-4-9-20(17)29-23-22/h1-4,6-9H,5,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis |
J Med Chem 58: 1550-5 (2015)
Article DOI: 10.1021/jm5013243
BindingDB Entry DOI: 10.7270/Q2F76FTG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis |
J Med Chem 56: 9199-221 (2013)
Article DOI: 10.1021/jm401318w
BindingDB Entry DOI: 10.7270/Q2RF5WGV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50021800
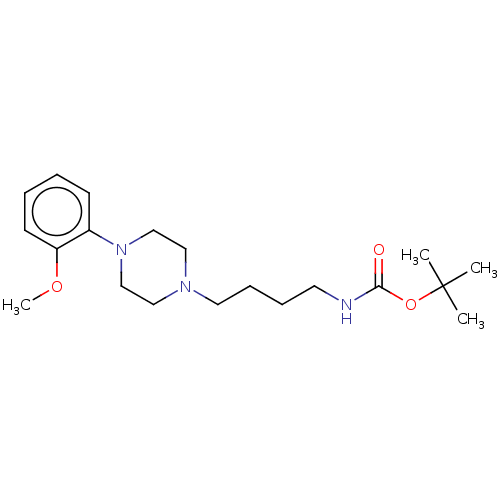
(CHEMBL3299095)Show InChI InChI=1S/C20H33N3O3/c1-20(2,3)26-19(24)21-11-7-8-12-22-13-15-23(16-14-22)17-9-5-6-10-18(17)25-4/h5-6,9-10H,7-8,11-16H2,1-4H3,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis |
J Med Chem 57: 4924-39 (2014)
Article DOI: 10.1021/jm500457x
BindingDB Entry DOI: 10.7270/Q29Z96FS |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50021816

(CHEMBL3298759)Show SMILES COc1ccccc1N1CCN(CCCNC(=O)c2sc3nc(OC)c(Cl)c(C)c3c2N)CC1 Show InChI InChI=1S/C24H30ClN5O3S/c1-15-18-20(26)21(34-24(18)28-23(33-3)19(15)25)22(31)27-9-6-10-29-11-13-30(14-12-29)16-7-4-5-8-17(16)32-2/h4-5,7-8H,6,9-14,26H2,1-3H3,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis |
J Med Chem 57: 4924-39 (2014)
Article DOI: 10.1021/jm500457x
BindingDB Entry DOI: 10.7270/Q29Z96FS |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50443102

(CHEMBL3085819)Show SMILES CC(=O)N[C@H]1CC[C@H](CCN2CCc3ccc(cc3C2)C#N)CC1 |r,wU:4.3,wD:7.7,(83.78,-17.14,;82.45,-16.37,;82.45,-14.83,;81.11,-17.14,;79.78,-16.37,;78.44,-17.13,;77.12,-16.37,;77.12,-14.82,;75.78,-14.05,;74.45,-14.83,;73.12,-14.06,;73.11,-12.51,;71.76,-11.73,;70.43,-12.52,;69.09,-11.76,;67.76,-12.53,;67.76,-14.07,;69.1,-14.84,;70.43,-14.07,;71.77,-14.84,;66.43,-14.84,;65.1,-15.61,;78.44,-14.05,;79.78,-14.83,)| Show InChI InChI=1S/C20H27N3O/c1-15(24)22-20-6-3-16(4-7-20)8-10-23-11-9-18-5-2-17(13-21)12-19(18)14-23/h2,5,12,16,20H,3-4,6-11,14H2,1H3,(H,22,24)/t16-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis |
J Med Chem 56: 9199-221 (2013)
Article DOI: 10.1021/jm401318w
BindingDB Entry DOI: 10.7270/Q2RF5WGV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50246733

(CHEMBL4100938)Show InChI InChI=1S/C13H18NO/c1-14(2,3)8-6-11-4-5-12-7-9-15-13(12)10-11/h4-5,7,9-10H,6,8H2,1-3H3/q+1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopolamine bromide from human recombinant muscarinic M1 receptor expressed in HEK293T cell membranes after 1 hr by liqu... |
J Med Chem 60: 9239-9250 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01113
BindingDB Entry DOI: 10.7270/Q2445PW2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-2] from wild-type human CCK2R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
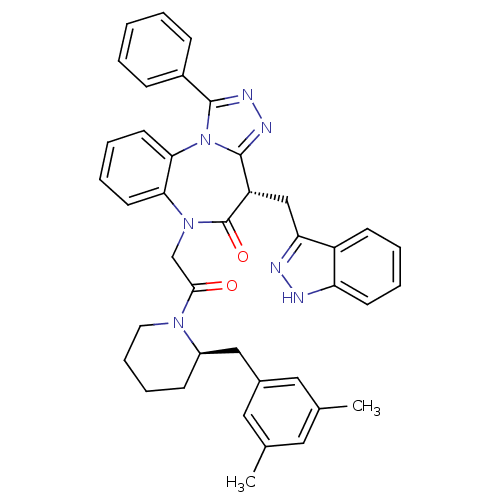
(Homo sapiens (Human)) | BDBM50380728
(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
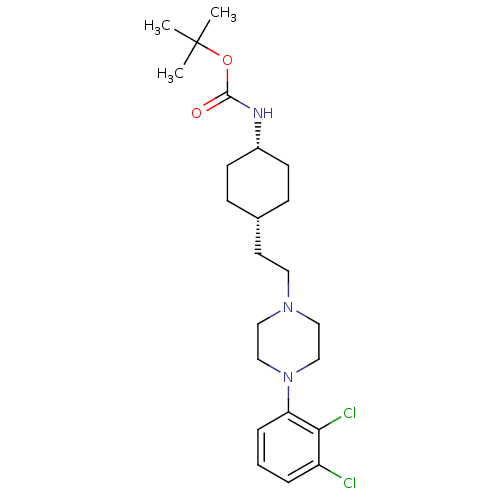
(Homo sapiens (Human)) | BDBM50443096
(CHEMBL3085824)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:11.11,8.7,(46.33,-48.72,;44.99,-47.94,;45,-46.4,;46.32,-47.16,;43.66,-48.71,;42.33,-47.94,;42.33,-46.4,;40.99,-48.71,;39.66,-47.94,;39.66,-46.4,;38.32,-45.62,;37,-46.39,;35.66,-45.63,;34.33,-46.4,;32.99,-45.63,;31.65,-46.41,;30.31,-45.64,;30.3,-44.09,;31.64,-43.3,;32.99,-44.08,;28.96,-43.33,;27.64,-44.11,;26.31,-43.35,;26.29,-41.81,;27.62,-41.03,;27.61,-39.49,;28.96,-41.79,;30.29,-41.01,;37,-47.94,;38.32,-48.71,)| Show InChI InChI=1S/C23H35Cl2N3O2/c1-23(2,3)30-22(29)26-18-9-7-17(8-10-18)11-12-27-13-15-28(16-14-27)20-6-4-5-19(24)21(20)25/h4-6,17-18H,7-16H2,1-3H3,(H,26,29)/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis |
J Med Chem 56: 9199-221 (2013)
Article DOI: 10.1021/jm401318w
BindingDB Entry DOI: 10.7270/Q2RF5WGV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50443099

(CHEMBL3085821)Show SMILES CC(C)(C)OC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:8.7,wD:11.11,(43.73,-15.34,;42.4,-14.57,;42.4,-13.03,;43.72,-13.78,;41.06,-15.33,;39.73,-14.56,;39.73,-13.02,;38.4,-15.33,;37.06,-14.56,;35.73,-15.33,;34.4,-14.56,;34.4,-13.01,;33.06,-12.25,;31.73,-13.02,;30.4,-12.25,;29.05,-13.03,;27.71,-12.27,;27.7,-10.71,;29.04,-9.92,;30.39,-10.7,;26.37,-9.95,;26.36,-8.41,;25.02,-7.65,;23.69,-8.43,;23.71,-9.97,;22.38,-10.76,;25.05,-10.73,;25.06,-12.27,;35.73,-12.24,;37.06,-13.02,)| Show InChI InChI=1S/C23H35Cl2N3O2/c1-23(2,3)30-22(29)26-18-9-7-17(8-10-18)11-12-27-13-15-28(16-14-27)20-6-4-5-19(24)21(20)25/h4-6,17-18H,7-16H2,1-3H3,(H,26,29)/t17-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis |
J Med Chem 56: 9199-221 (2013)
Article DOI: 10.1021/jm401318w
BindingDB Entry DOI: 10.7270/Q2RF5WGV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data