 Found 3536 hits with Last Name = 'chung' and Initial = 'cw'
Found 3536 hits with Last Name = 'chung' and Initial = 'cw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228930
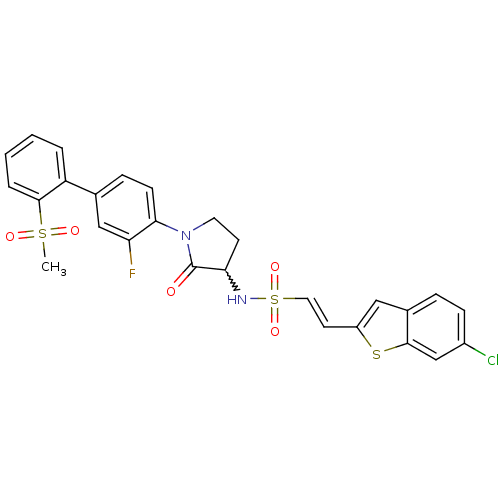
((E)-2-(6-chloro-benzo[b]thiophen-2-yl)-ethenesulfo...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3cc4ccc(Cl)cc4s3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C27H22ClFN2O5S3/c1-38(33,34)26-5-3-2-4-21(26)17-7-9-24(22(29)15-17)31-12-10-23(27(31)32)30-39(35,36)13-11-20-14-18-6-8-19(28)16-25(18)37-20/h2-9,11,13-16,23,30H,10,12H2,1H3/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572134
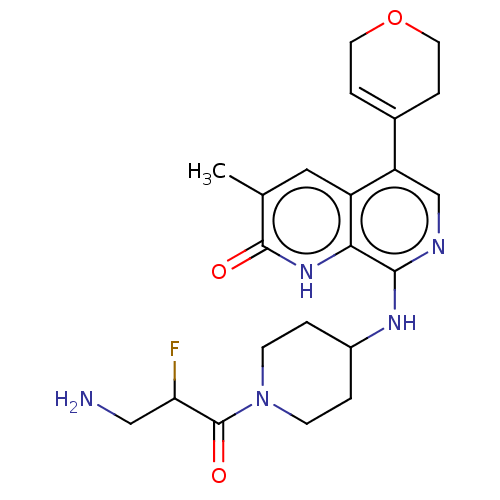
(CHEMBL4868363)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:28| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228950
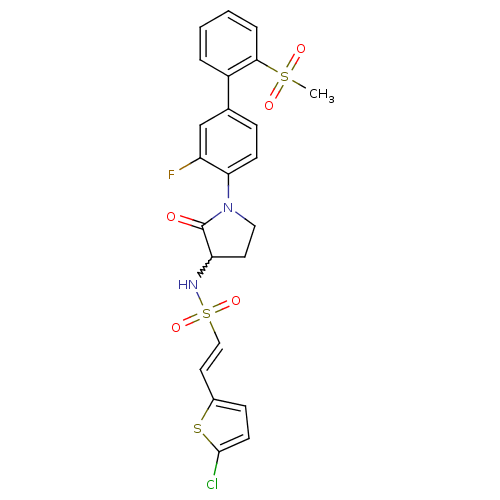
((E)-2-(5-chloro-thiophen-2-yl)-ethenesulfonic acid...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C23H20ClFN2O5S3/c1-34(29,30)21-5-3-2-4-17(21)15-6-8-20(18(25)14-15)27-12-10-19(23(27)28)26-35(31,32)13-11-16-7-9-22(24)33-16/h2-9,11,13-14,19,26H,10,12H2,1H3/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572127
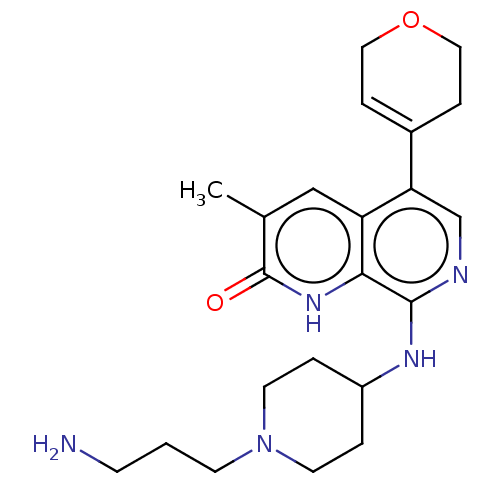
(CHEMBL4850335)Show SMILES Cc1cc2c(cnc(NC3CCN(CCCN)CC3)c2[nH]c1=O)C1=CCOCC1 |t:26| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228954
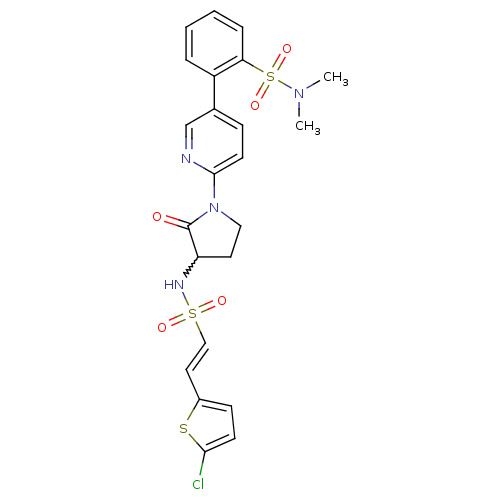
((E)-2-(6-(3-(2-(5-chlorothiophen-2-yl)vinylsulfona...)Show SMILES CN(C)S(=O)(=O)c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |w:21.23| Show InChI InChI=1S/C23H23ClN4O5S3/c1-27(2)36(32,33)20-6-4-3-5-18(20)16-7-10-22(25-15-16)28-13-11-19(23(28)29)26-35(30,31)14-12-17-8-9-21(24)34-17/h3-10,12,14-15,19,26H,11,13H2,1-2H3/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263211
(2-Cyclobutylamino-N-[4'-(cyclopropylmethyl-carbamo...)Show SMILES Cc1ccc(NC(=O)c2ccnc(NC3CCC3)c2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H30N4O2/c1-18-5-12-24(32-28(34)22-13-14-29-26(15-22)31-23-3-2-4-23)16-25(18)20-8-10-21(11-9-20)27(33)30-17-19-6-7-19/h5,8-16,19,23H,2-4,6-7,17H2,1H3,(H,29,31)(H,30,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228940
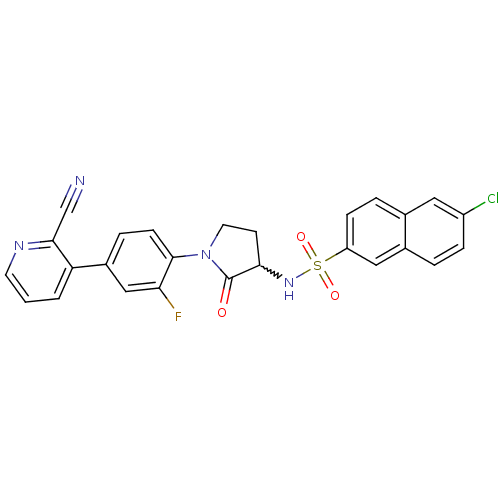
(6-chloro-N-(1-(4-(2-cyanopyridin-3-yl)-2-fluorophe...)Show SMILES Fc1cc(ccc1N1CCC(NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)-c1cccnc1C#N |w:10.11| Show InChI InChI=1S/C26H18ClFN4O3S/c27-19-6-3-17-13-20(7-4-16(17)12-19)36(34,35)31-23-9-11-32(26(23)33)25-8-5-18(14-22(25)28)21-2-1-10-30-24(21)15-29/h1-8,10,12-14,23,31H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572130
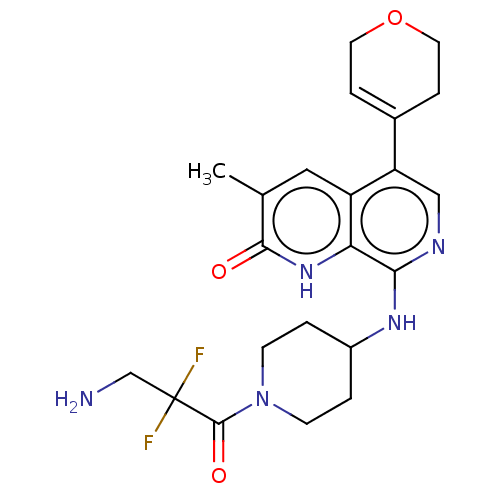
(CHEMBL4864027)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:29| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572131
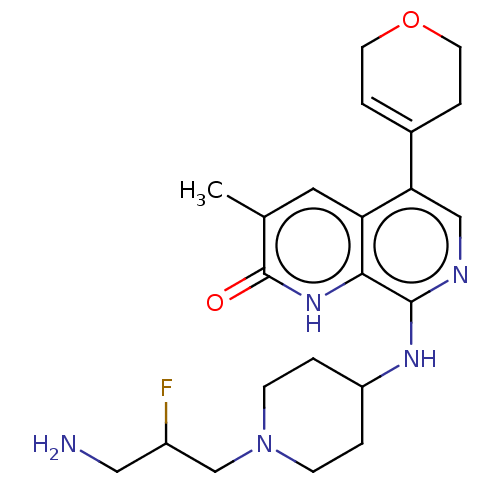
(CHEMBL4873236)Show SMILES Cc1cc2c(cnc(NC3CCN(CC(F)CN)CC3)c2[nH]c1=O)C1=CCOCC1 |t:27| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228932
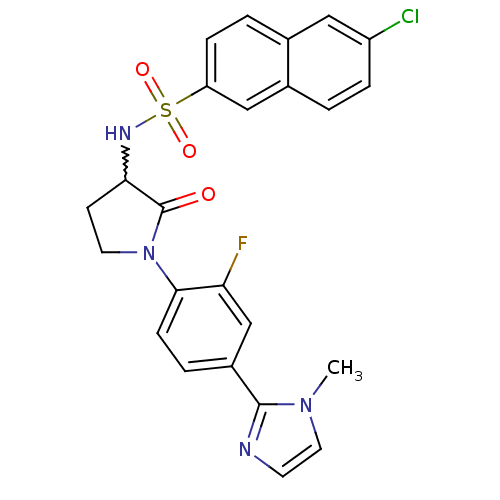
(6-chloro-N-(1-(2-fluoro-4-(1-methyl-1H-imidazol-2-...)Show SMILES Cn1ccnc1-c1ccc(N2CCC(NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |w:13.14| Show InChI InChI=1S/C24H20ClFN4O3S/c1-29-11-9-27-23(29)17-4-7-22(20(26)14-17)30-10-8-21(24(30)31)28-34(32,33)19-6-3-15-12-18(25)5-2-16(15)13-19/h2-7,9,11-14,21,28H,8,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228947
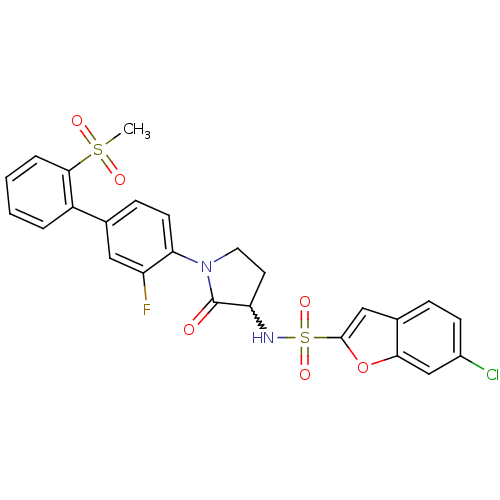
(6-chloro-benzofuran-2-sulfonic acid [1-(3-fluoro-2...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CCC(NS(=O)(=O)c3cc4ccc(Cl)cc4o3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C25H20ClFN2O6S2/c1-36(31,32)23-5-3-2-4-18(23)15-7-9-21(19(27)12-15)29-11-10-20(25(29)30)28-37(33,34)24-13-16-6-8-17(26)14-22(16)35-24/h2-9,12-14,20,28H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2A
(Homo sapiens (Human)) | BDBM50234923
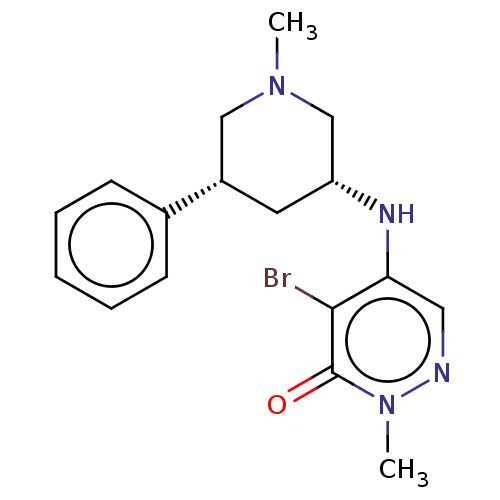
(CHEMBL4069412)Show SMILES CN1C[C@@H](C[C@@H](C1)c1ccccc1)Nc1cnn(C)c(=O)c1Br |r| Show InChI InChI=1S/C17H21BrN4O/c1-21-10-13(12-6-4-3-5-7-12)8-14(11-21)20-15-9-19-22(2)17(23)16(15)18/h3-7,9,13-14,20H,8,10-11H2,1-2H3/t13-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length GCN5 expressed in bacterial expression system by BROMOscan method |
J Med Chem 60: 695-709 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01566
BindingDB Entry DOI: 10.7270/Q2W09863 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50234923

(CHEMBL4069412)Show SMILES CN1C[C@@H](C[C@@H](C1)c1ccccc1)Nc1cnn(C)c(=O)c1Br |r| Show InChI InChI=1S/C17H21BrN4O/c1-21-10-13(12-6-4-3-5-7-12)8-14(11-21)20-15-9-19-22(2)17(23)16(15)18/h3-7,9,13-14,20H,8,10-11H2,1-2H3/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length PCAF bromodomain expressed in mammalian expression system by BROMOscan method |
J Med Chem 60: 695-709 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01566
BindingDB Entry DOI: 10.7270/Q2W09863 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2A
(Homo sapiens (Human)) | BDBM50234923

(CHEMBL4069412)Show SMILES CN1C[C@@H](C[C@@H](C1)c1ccccc1)Nc1cnn(C)c(=O)c1Br |r| Show InChI InChI=1S/C17H21BrN4O/c1-21-10-13(12-6-4-3-5-7-12)8-14(11-21)20-15-9-19-22(2)17(23)16(15)18/h3-7,9,13-14,20H,8,10-11H2,1-2H3/t13-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length GCN5 expressed in bacterial expression system by BROMOscan method |
J Med Chem 60: 695-709 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01566
BindingDB Entry DOI: 10.7270/Q2W09863 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572133
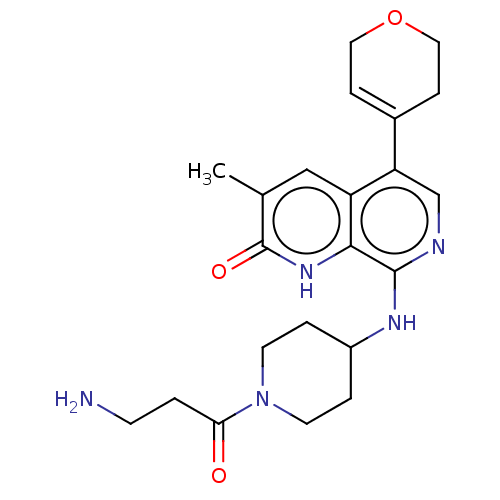
(CHEMBL4854161)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)CCN)c2[nH]c1=O)C1=CCOCC1 |t:27| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50234923

(CHEMBL4069412)Show SMILES CN1C[C@@H](C[C@@H](C1)c1ccccc1)Nc1cnn(C)c(=O)c1Br |r| Show InChI InChI=1S/C17H21BrN4O/c1-21-10-13(12-6-4-3-5-7-12)8-14(11-21)20-15-9-19-22(2)17(23)16(15)18/h3-7,9,13-14,20H,8,10-11H2,1-2H3/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length PCAF bromodomain expressed in mammalian expression system by BROMOscan method |
J Med Chem 60: 695-709 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01566
BindingDB Entry DOI: 10.7270/Q2W09863 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263261
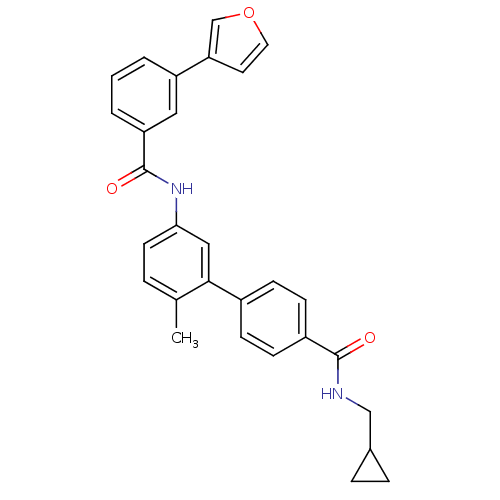
(5'-(3-Furan-3-yl-benzoylamino)-2'-methyl-biphenyl-...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)-c2ccoc2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C29H26N2O3/c1-19-5-12-26(31-29(33)24-4-2-3-23(15-24)25-13-14-34-18-25)16-27(19)21-8-10-22(11-9-21)28(32)30-17-20-6-7-20/h2-5,8-16,18,20H,6-7,17H2,1H3,(H,30,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228935
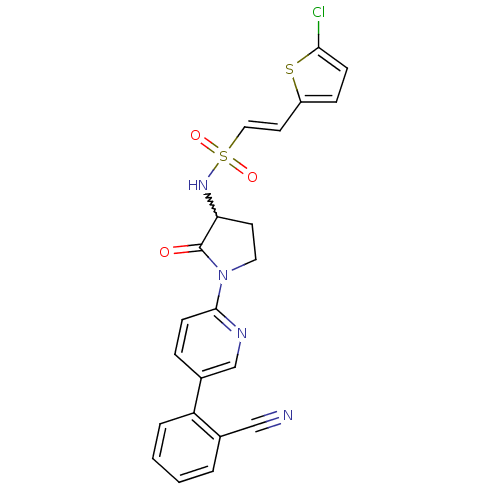
((E)-2-(5-chloro-thiophen-2-yl)-ethenesulfonic acid...)Show SMILES Clc1ccc(\C=C\S(=O)(=O)NC2CCN(C2=O)c2ccc(cn2)-c2ccccc2C#N)s1 |w:11.10| Show InChI InChI=1S/C22H17ClN4O3S2/c23-20-7-6-17(31-20)10-12-32(29,30)26-19-9-11-27(22(19)28)21-8-5-16(14-25-21)18-4-2-1-3-15(18)13-24/h1-8,10,12,14,19,26H,9,11H2/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263129
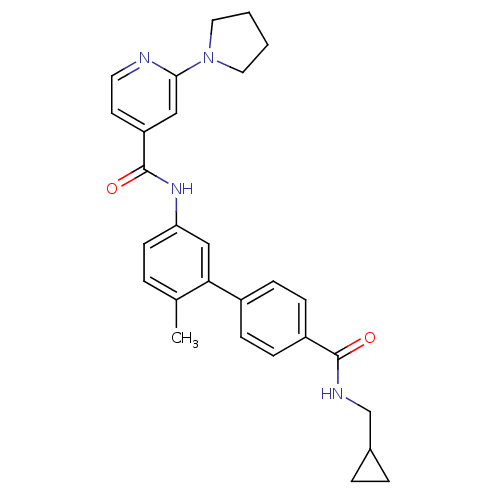
(CHEMBL477182 | N-[4'-(Cyclopropylmethyl-carbamoyl)...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCCC2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H30N4O2/c1-19-4-11-24(31-28(34)23-12-13-29-26(16-23)32-14-2-3-15-32)17-25(19)21-7-9-22(10-8-21)27(33)30-18-20-5-6-20/h4,7-13,16-17,20H,2-3,5-6,14-15,18H2,1H3,(H,30,33)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263295
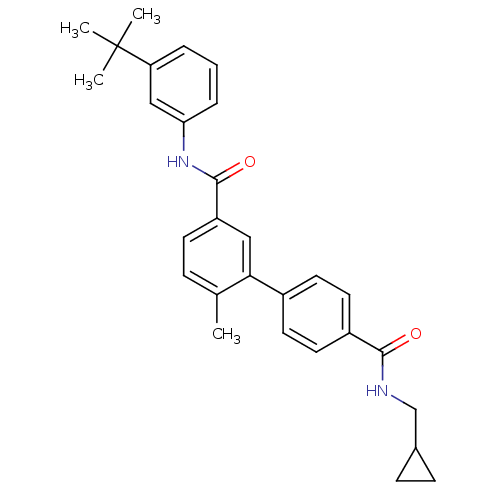
(6-Methyl-biphenyl-3,4'-dicarboxylic acid 3-[(3-ter...)Show SMILES Cc1ccc(cc1-c1ccc(cc1)C(=O)NCC1CC1)C(=O)Nc1cccc(c1)C(C)(C)C Show InChI InChI=1S/C29H32N2O2/c1-19-8-11-23(28(33)31-25-7-5-6-24(17-25)29(2,3)4)16-26(19)21-12-14-22(15-13-21)27(32)30-18-20-9-10-20/h5-8,11-17,20H,9-10,18H2,1-4H3,(H,30,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17654

(5-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(morph...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ncc(s2)-c2ccc(Cl)s2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C18H21ClN4O5S3/c1-11(16(24)22-6-8-28-9-7-22)23-5-4-12(17(23)25)21-31(26,27)18-20-10-14(30-18)13-2-3-15(19)29-13/h2-3,10-12,21H,4-9H2,1H3/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK
| Assay Description
The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... |
J Med Chem 50: 1546-57 (2007)
Article DOI: 10.1021/jm060870c
BindingDB Entry DOI: 10.7270/Q2F18X06 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9
(Homo sapiens (Human)) | BDBM50147620
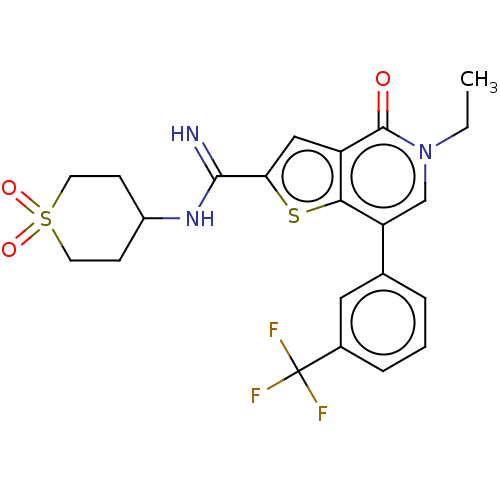
(CHEMBL3769507)Show SMILES CCn1cc(-c2cccc(c2)C(F)(F)F)c2sc(cc2c1=O)C(=N)NC1CCS(=O)(=O)CC1 Show InChI InChI=1S/C22H22F3N3O3S2/c1-2-28-12-17(13-4-3-5-14(10-13)22(23,24)25)19-16(21(28)29)11-18(32-19)20(26)27-15-6-8-33(30,31)9-7-15/h3-5,10-12,15H,2,6-9H2,1H3,(H2,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length BRD9 (R130 to V259 residues) expressed in bacterial expression system by BROMOscan assay |
J Med Chem 63: 5816-5840 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00075
BindingDB Entry DOI: 10.7270/Q2MG7T12 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228941
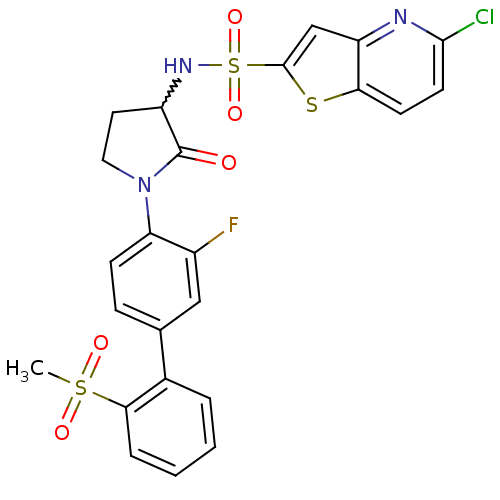
(5-chloro-thieno[3,2-b]pyridine-2-sulfonic acid [1-...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CCC(NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C24H19ClFN3O5S3/c1-36(31,32)21-5-3-2-4-15(21)14-6-7-19(16(26)12-14)29-11-10-17(24(29)30)28-37(33,34)23-13-18-20(35-23)8-9-22(25)27-18/h2-9,12-13,17,28H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50359016
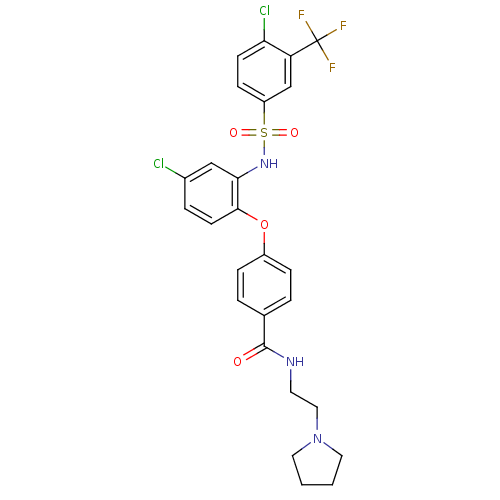
(CHEMBL1924017)Show SMILES FC(F)(F)c1cc(ccc1Cl)S(=O)(=O)Nc1cc(Cl)ccc1Oc1ccc(cc1)C(=O)NCCN1CCCC1 Show InChI InChI=1S/C26H24Cl2F3N3O4S/c27-18-5-10-24(23(15-18)33-39(36,37)20-8-9-22(28)21(16-20)26(29,30)31)38-19-6-3-17(4-7-19)25(35)32-11-14-34-12-1-2-13-34/h3-10,15-16,33H,1-2,11-14H2,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs |
Bioorg Med Chem Lett 21: 7291-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.038
BindingDB Entry DOI: 10.7270/Q2KK9C6D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263260
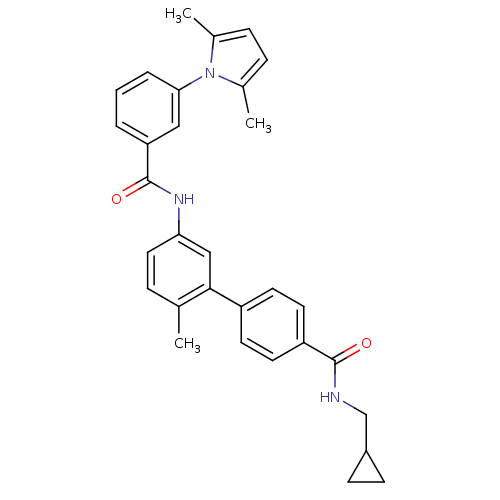
(5'-[3-(2,5-Dimethyl-pyrrol-1-yl)-benzoylamino]-2'-...)Show SMILES Cc1ccc(C)n1-c1cccc(c1)C(=O)Nc1ccc(C)c(c1)-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C31H31N3O2/c1-20-7-16-27(18-29(20)24-12-14-25(15-13-24)30(35)32-19-23-10-11-23)33-31(36)26-5-4-6-28(17-26)34-21(2)8-9-22(34)3/h4-9,12-18,23H,10-11,19H2,1-3H3,(H,32,35)(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263167
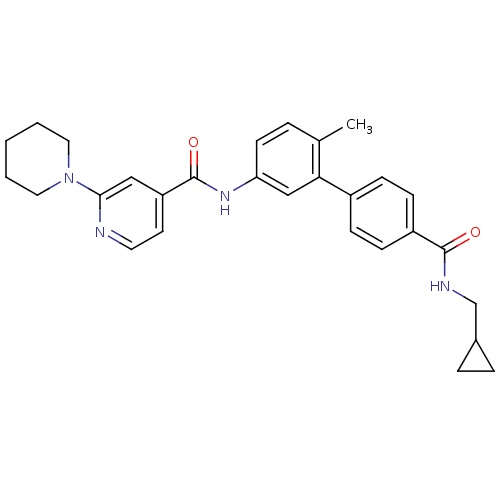
(3,4,5,6-Tetrahydro-2H-[1,2']bipyridinyl-4'-carboxy...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCCCC2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C29H32N4O2/c1-20-5-12-25(32-29(35)24-13-14-30-27(17-24)33-15-3-2-4-16-33)18-26(20)22-8-10-23(11-9-22)28(34)31-19-21-6-7-21/h5,8-14,17-18,21H,2-4,6-7,15-16,19H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228936
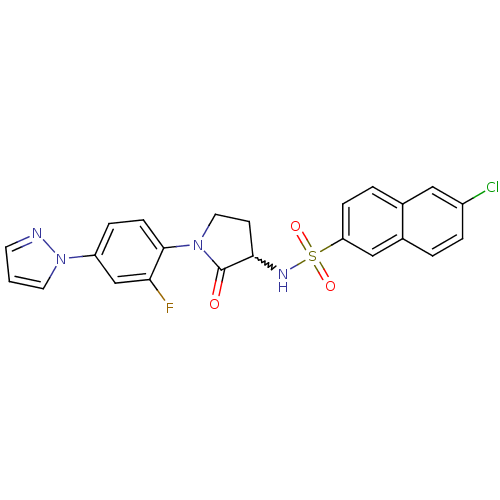
(6-chloro-N-(1-(2-fluoro-4-(1H-pyrazol-1-yl)phenyl)...)Show SMILES Fc1cc(ccc1N1CCC(NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)-n1cccn1 |w:10.11| Show InChI InChI=1S/C23H18ClFN4O3S/c24-17-4-2-16-13-19(6-3-15(16)12-17)33(31,32)27-21-8-11-28(23(21)30)22-7-5-18(14-20(22)25)29-10-1-9-26-29/h1-7,9-10,12-14,21,27H,8,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263212
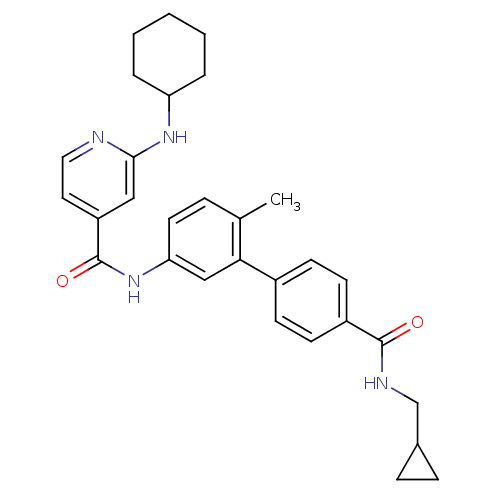
(2-Cyclohexylamino-N-[4'-(cyclopropylmethyl-carbamo...)Show SMILES Cc1ccc(NC(=O)c2ccnc(NC3CCCCC3)c2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C30H34N4O2/c1-20-7-14-26(18-27(20)22-10-12-23(13-11-22)29(35)32-19-21-8-9-21)34-30(36)24-15-16-31-28(17-24)33-25-5-3-2-4-6-25/h7,10-18,21,25H,2-6,8-9,19H2,1H3,(H,31,33)(H,32,35)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50359013
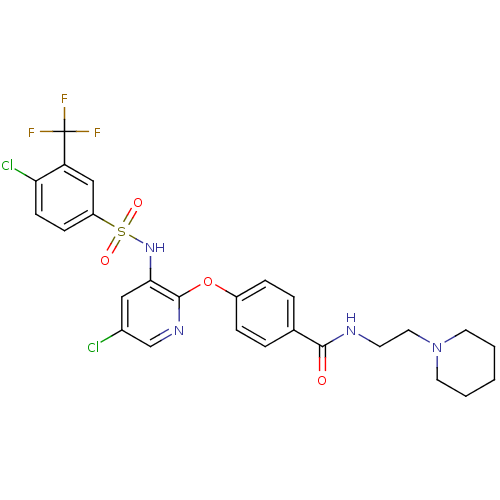
(CHEMBL1924014)Show SMILES FC(F)(F)c1cc(ccc1Cl)S(=O)(=O)Nc1cc(Cl)cnc1Oc1ccc(cc1)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C26H25Cl2F3N4O4S/c27-18-14-23(34-40(37,38)20-8-9-22(28)21(15-20)26(29,30)31)25(33-16-18)39-19-6-4-17(5-7-19)24(36)32-10-13-35-11-2-1-3-12-35/h4-9,14-16,34H,1-3,10-13H2,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs |
Bioorg Med Chem Lett 21: 7291-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.038
BindingDB Entry DOI: 10.7270/Q2KK9C6D |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17641
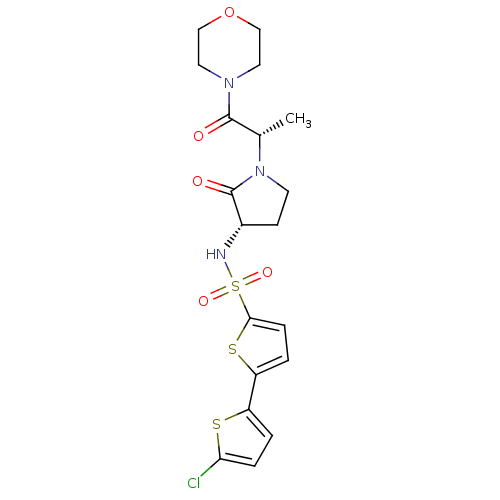
(5-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(morph...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ccc(s2)-c2ccc(Cl)s2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C19H22ClN3O5S3/c1-12(18(24)22-8-10-28-11-9-22)23-7-6-13(19(23)25)21-31(26,27)17-5-3-15(30-17)14-2-4-16(20)29-14/h2-5,12-13,21H,6-11H2,1H3/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK
| Assay Description
The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... |
J Med Chem 50: 1546-57 (2007)
Article DOI: 10.1021/jm060870c
BindingDB Entry DOI: 10.7270/Q2F18X06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17643
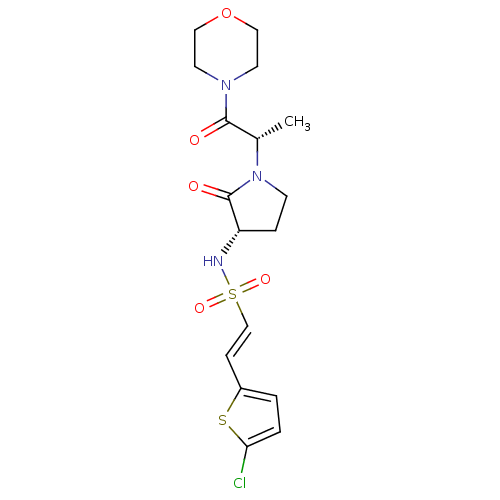
((E)-2-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(m...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C17H22ClN3O5S2/c1-12(16(22)20-7-9-26-10-8-20)21-6-4-14(17(21)23)19-28(24,25)11-5-13-2-3-15(18)27-13/h2-3,5,11-12,14,19H,4,6-10H2,1H3/b11-5+/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK
| Assay Description
The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... |
J Med Chem 50: 1546-57 (2007)
Article DOI: 10.1021/jm060870c
BindingDB Entry DOI: 10.7270/Q2F18X06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50359018

(CHEMBL1924019)Show SMILES COc1cc(Oc2ccc(cc2)C(=O)NCCN2CCCC2)c(NS(=O)(=O)c2ccc(Cl)c(c2)C(F)(F)F)cc1Cl Show InChI InChI=1S/C27H26Cl2F3N3O5S/c1-39-24-16-25(40-18-6-4-17(5-7-18)26(36)33-10-13-35-11-2-3-12-35)23(15-22(24)29)34-41(37,38)19-8-9-21(28)20(14-19)27(30,31)32/h4-9,14-16,34H,2-3,10-13H2,1H3,(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs |
Bioorg Med Chem Lett 21: 7291-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.038
BindingDB Entry DOI: 10.7270/Q2KK9C6D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263168

(CHEMBL476351 | N-[4'-(Cyclopropylmethyl-carbamoyl)...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCOCC2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H30N4O3/c1-19-2-9-24(31-28(34)23-10-11-29-26(16-23)32-12-14-35-15-13-32)17-25(19)21-5-7-22(8-6-21)27(33)30-18-20-3-4-20/h2,5-11,16-17,20H,3-4,12-15,18H2,1H3,(H,30,33)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228949
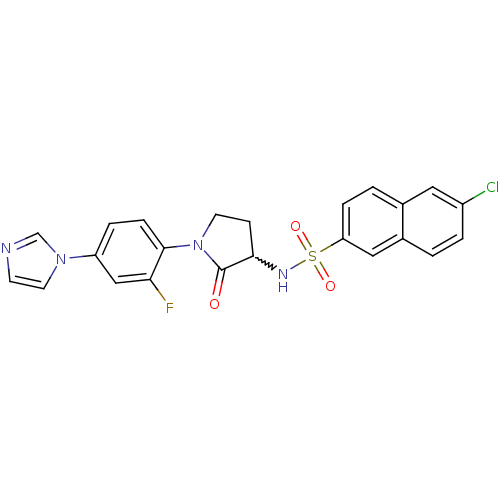
(6-chloro-N-(1-(2-fluoro-4-(1H-imidazol-1-yl)phenyl...)Show SMILES Fc1cc(ccc1N1CCC(NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)-n1ccnc1 |w:10.11| Show InChI InChI=1S/C23H18ClFN4O3S/c24-17-3-1-16-12-19(5-2-15(16)11-17)33(31,32)27-21-7-9-29(23(21)30)22-6-4-18(13-20(22)25)28-10-8-26-14-28/h1-6,8,10-14,21,27H,7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263210
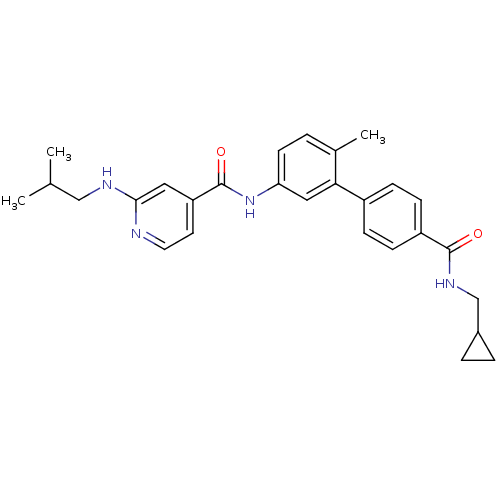
(CHEMBL478234 | N-[4'-(Cyclopropylmethyl-carbamoyl)...)Show SMILES CC(C)CNc1cc(ccn1)C(=O)Nc1ccc(C)c(c1)-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H32N4O2/c1-18(2)16-30-26-14-23(12-13-29-26)28(34)32-24-11-4-19(3)25(15-24)21-7-9-22(10-8-21)27(33)31-17-20-5-6-20/h4,7-15,18,20H,5-6,16-17H2,1-3H3,(H,29,30)(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
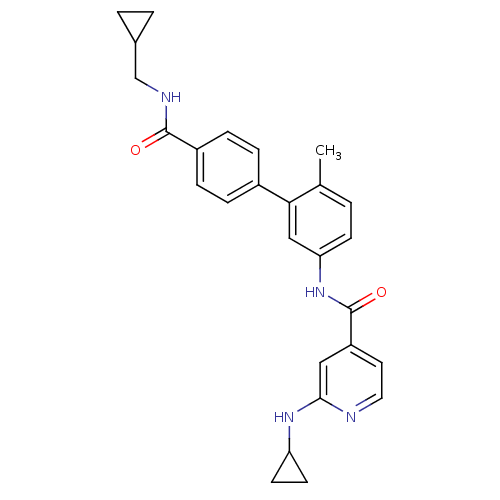
Coagulation factor X
(Homo sapiens (Human)) | BDBM17653
(2-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(morph...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2cnc(s2)-c2ccc(Cl)s2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C18H21ClN4O5S3/c1-11(17(24)22-6-8-28-9-7-22)23-5-4-12(18(23)25)21-31(26,27)15-10-20-16(30-15)13-2-3-14(19)29-13/h2-3,10-12,21H,4-9H2,1H3/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK
| Assay Description
The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... |
J Med Chem 50: 1546-57 (2007)
Article DOI: 10.1021/jm060870c
BindingDB Entry DOI: 10.7270/Q2F18X06 |
More data for this
Ligand-Target Pair | |
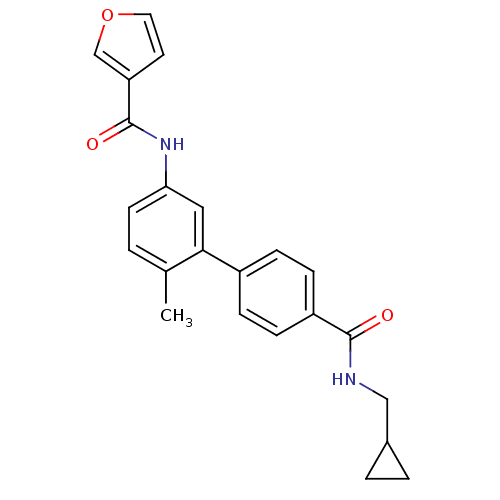
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263170
(2-Cyclopropylamino-N-[4'-(cyclopropylmethyl-carbam...)Show SMILES Cc1ccc(NC(=O)c2ccnc(NC3CC3)c2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C27H28N4O2/c1-17-2-9-23(31-27(33)21-12-13-28-25(14-21)30-22-10-11-22)15-24(17)19-5-7-20(8-6-19)26(32)29-16-18-3-4-18/h2,5-9,12-15,18,22H,3-4,10-11,16H2,1H3,(H,28,30)(H,29,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50262865
(CHEMBL477583 | Furan-3-carboxylic acid [4'-(cyclop...)Show SMILES Cc1ccc(NC(=O)c2ccoc2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C23H22N2O3/c1-15-2-9-20(25-23(27)19-10-11-28-14-19)12-21(15)17-5-7-18(8-6-17)22(26)24-13-16-3-4-16/h2,5-12,14,16H,3-4,13H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha-mediated ATF2 phosphorylation by TR-FRET assay |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263168

(CHEMBL476351 | N-[4'-(Cyclopropylmethyl-carbamoyl)...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCOCC2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H30N4O3/c1-19-2-9-24(31-28(34)23-10-11-29-26(16-23)32-12-14-35-15-13-32)17-25(19)21-5-7-22(8-6-21)27(33)30-18-20-3-4-20/h2,5-11,16-17,20H,3-4,12-15,18H2,1H3,(H,30,33)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha-mediated ATF2 phosphorylation by TR-FRET assay |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50359032
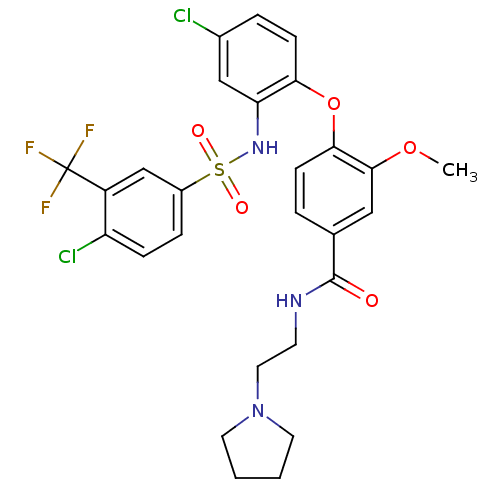
(CHEMBL1924024)Show SMILES COc1cc(ccc1Oc1ccc(Cl)cc1NS(=O)(=O)c1ccc(Cl)c(c1)C(F)(F)F)C(=O)NCCN1CCCC1 Show InChI InChI=1S/C27H26Cl2F3N3O5S/c1-39-25-14-17(26(36)33-10-13-35-11-2-3-12-35)4-8-24(25)40-23-9-5-18(28)15-22(23)34-41(37,38)19-6-7-21(29)20(16-19)27(30,31)32/h4-9,14-16,34H,2-3,10-13H2,1H3,(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs |
Bioorg Med Chem Lett 21: 7291-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.038
BindingDB Entry DOI: 10.7270/Q2KK9C6D |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12538
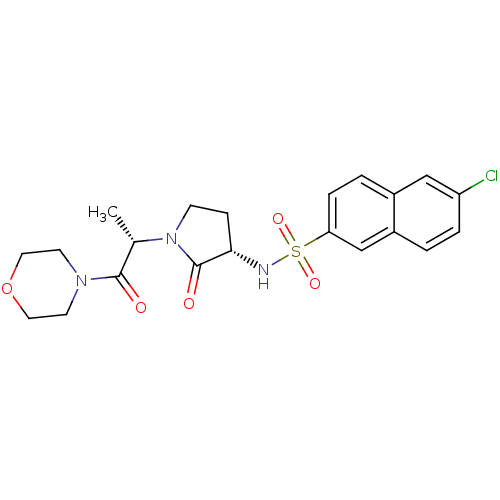
(6-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C21H24ClN3O5S/c1-14(20(26)24-8-10-30-11-9-24)25-7-6-19(21(25)27)23-31(28,29)18-5-3-15-12-17(22)4-2-16(15)13-18/h2-5,12-14,19,23H,6-11H2,1H3/t14-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK
| Assay Description
The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... |
J Med Chem 50: 1546-57 (2007)
Article DOI: 10.1021/jm060870c
BindingDB Entry DOI: 10.7270/Q2F18X06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228946
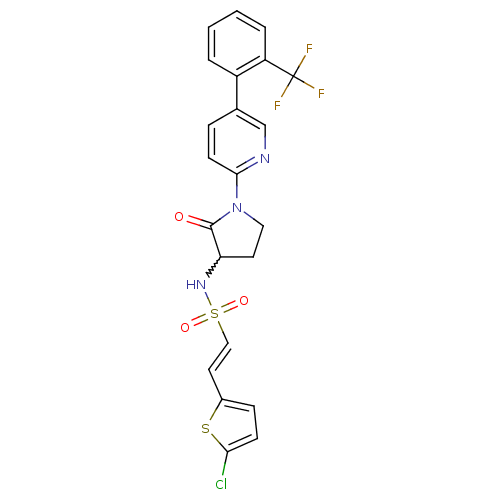
((E)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(5-(2-(tr...)Show SMILES FC(F)(F)c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |w:19.21| Show InChI InChI=1S/C22H17ClF3N3O3S2/c23-19-7-6-15(33-19)10-12-34(31,32)28-18-9-11-29(21(18)30)20-8-5-14(13-27-20)16-3-1-2-4-17(16)22(24,25)26/h1-8,10,12-13,18,28H,9,11H2/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228953
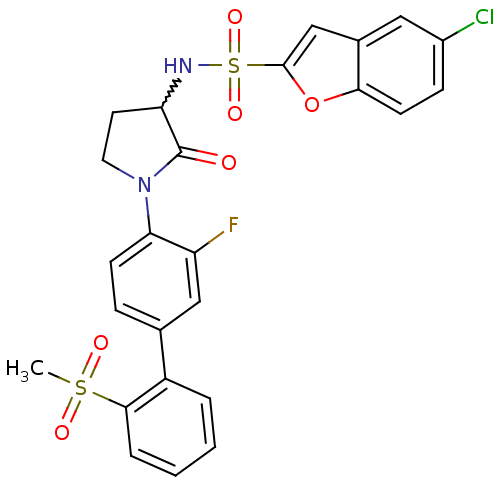
(5-chloro-benzofuran-2-sulfonic acid [1-(3-fluoro-2...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CCC(NS(=O)(=O)c3cc4cc(Cl)ccc4o3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C25H20ClFN2O6S2/c1-36(31,32)23-5-3-2-4-18(23)15-6-8-21(19(27)13-15)29-11-10-20(25(29)30)28-37(33,34)24-14-16-12-17(26)7-9-22(16)35-24/h2-9,12-14,20,28H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263209
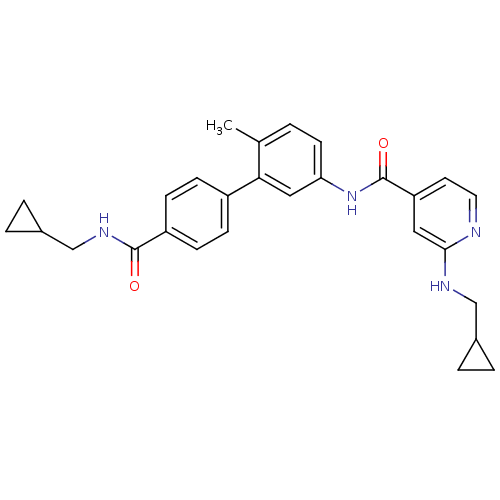
(2-(Cyclopropylmethyl-amino)-N-[4'-(cyclopropylmeth...)Show SMILES Cc1ccc(NC(=O)c2ccnc(NCC3CC3)c2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H30N4O2/c1-18-2-11-24(32-28(34)23-12-13-29-26(14-23)30-16-19-3-4-19)15-25(18)21-7-9-22(10-8-21)27(33)31-17-20-5-6-20/h2,7-15,19-20H,3-6,16-17H2,1H3,(H,29,30)(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228951
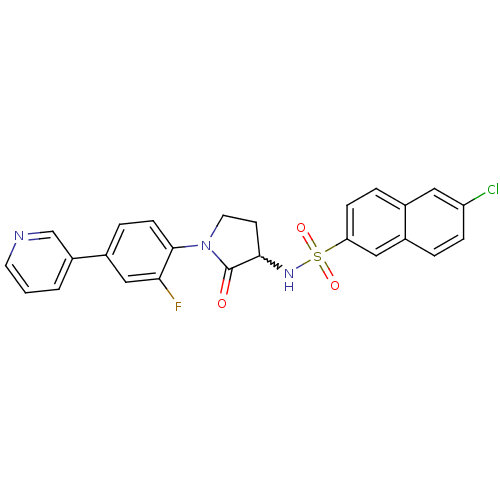
(6-chloro-N-(1-(2-fluoro-4-(pyridin-3-yl)phenyl)-2-...)Show SMILES Fc1cc(ccc1N1CCC(NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)-c1cccnc1 |w:10.11| Show InChI InChI=1S/C25H19ClFN3O3S/c26-20-6-3-17-13-21(7-4-16(17)12-20)34(32,33)29-23-9-11-30(25(23)31)24-8-5-18(14-22(24)27)19-2-1-10-28-15-19/h1-8,10,12-15,23,29H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50359022
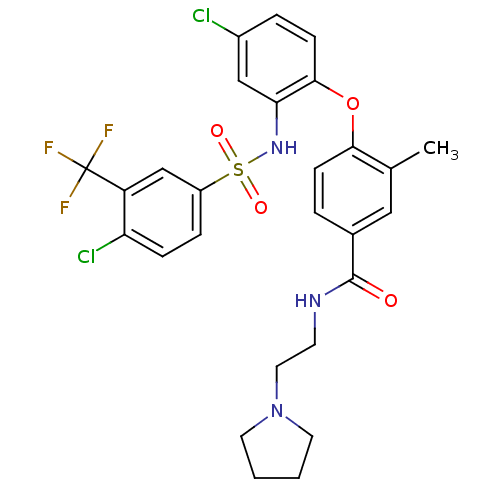
(CHEMBL1924023)Show SMILES Cc1cc(ccc1Oc1ccc(Cl)cc1NS(=O)(=O)c1ccc(Cl)c(c1)C(F)(F)F)C(=O)NCCN1CCCC1 Show InChI InChI=1S/C27H26Cl2F3N3O4S/c1-17-14-18(26(36)33-10-13-35-11-2-3-12-35)4-8-24(17)39-25-9-5-19(28)15-23(25)34-40(37,38)20-6-7-22(29)21(16-20)27(30,31)32/h4-9,14-16,34H,2-3,10-13H2,1H3,(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs |
Bioorg Med Chem Lett 21: 7291-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.038
BindingDB Entry DOI: 10.7270/Q2KK9C6D |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50359017
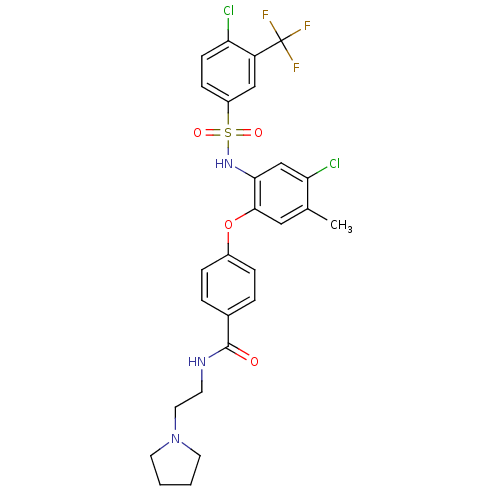
(CHEMBL1924018)Show SMILES Cc1cc(Oc2ccc(cc2)C(=O)NCCN2CCCC2)c(NS(=O)(=O)c2ccc(Cl)c(c2)C(F)(F)F)cc1Cl Show InChI InChI=1S/C27H26Cl2F3N3O4S/c1-17-14-25(39-19-6-4-18(5-7-19)26(36)33-10-13-35-11-2-3-12-35)24(16-23(17)29)34-40(37,38)20-8-9-22(28)21(15-20)27(30,31)32/h4-9,14-16,34H,2-3,10-13H2,1H3,(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs |
Bioorg Med Chem Lett 21: 7291-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.038
BindingDB Entry DOI: 10.7270/Q2KK9C6D |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228945
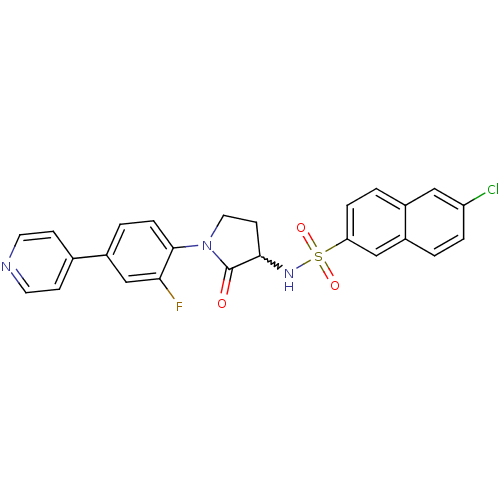
(6-chloro-N-(1-(2-fluoro-4-(pyridin-4-yl)phenyl)-2-...)Show SMILES Fc1cc(ccc1N1CCC(NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)-c1ccncc1 |w:10.11| Show InChI InChI=1S/C25H19ClFN3O3S/c26-20-4-1-18-14-21(5-2-17(18)13-20)34(32,33)29-23-9-12-30(25(23)31)24-6-3-19(15-22(24)27)16-7-10-28-11-8-16/h1-8,10-11,13-15,23,29H,9,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263167

(3,4,5,6-Tetrahydro-2H-[1,2']bipyridinyl-4'-carboxy...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCCCC2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C29H32N4O2/c1-20-5-12-25(32-29(35)24-13-14-30-27(17-24)33-15-3-2-4-16-33)18-26(20)22-8-10-23(11-9-22)28(34)31-19-21-6-7-21/h5,8-14,17-18,21H,2-4,6-7,15-16,19H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha-mediated ATF2 phosphorylation by TR-FRET assay |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data