 Found 201 hits with Last Name = 'dominguez' and Initial = 'j'
Found 201 hits with Last Name = 'dominguez' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389083
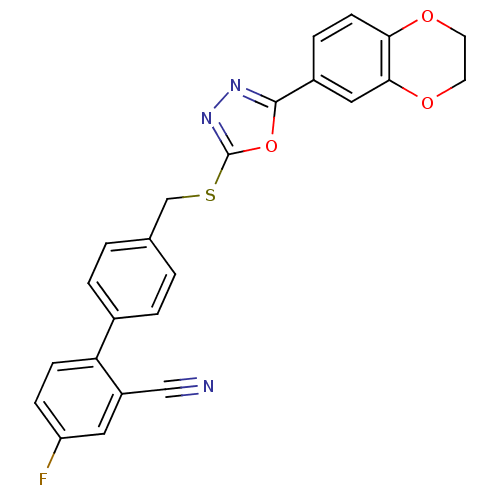
(CHEMBL2064535)Show SMILES Fc1ccc(-c2ccc(CSc3nnc(o3)-c3ccc4OCCOc4c3)cc2)c(c1)C#N Show InChI InChI=1S/C24H16FN3O3S/c25-19-6-7-20(18(11-19)13-26)16-3-1-15(2-4-16)14-32-24-28-27-23(31-24)17-5-8-21-22(12-17)30-10-9-29-21/h1-8,11-12H,9-10,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
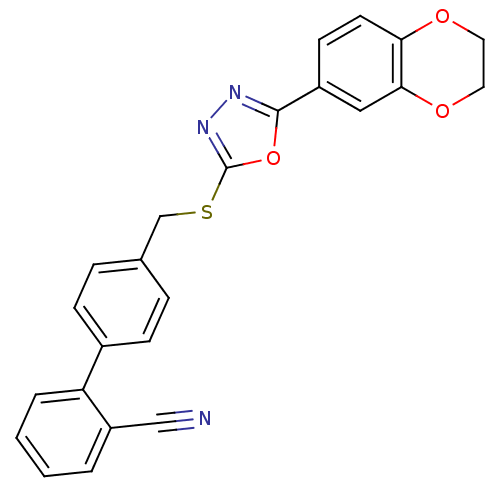
(Homo sapiens (Human)) | BDBM50389079
(CHEMBL2064532)Show SMILES Fc1ccc(-c2ccc(CSc3nnc(o3)-c3ccc4OCOc4c3)cc2)c(c1)C#N Show InChI InChI=1S/C23H14FN3O3S/c24-18-6-7-19(17(9-18)11-25)15-3-1-14(2-4-15)12-31-23-27-26-22(30-23)16-5-8-20-21(10-16)29-13-28-20/h1-10H,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
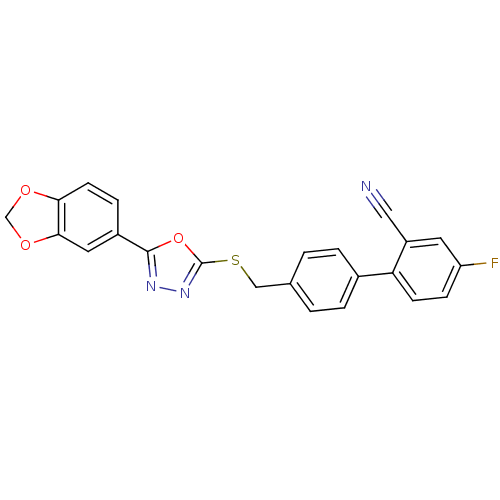
(Homo sapiens (Human)) | BDBM50389087
(CHEMBL2064533)Show SMILES N#Cc1ccccc1-c1ccc(CSc2nnc(o2)-c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C23H15N3O3S/c24-12-18-3-1-2-4-19(18)16-7-5-15(6-8-16)13-30-23-26-25-22(29-23)17-9-10-20-21(11-17)28-14-27-20/h1-11H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389081
(CHEMBL2064534)Show SMILES N#Cc1ccccc1-c1ccc(CSc2nnc(o2)-c2ccc3OCCOc3c2)cc1 Show InChI InChI=1S/C24H17N3O3S/c25-14-19-3-1-2-4-20(19)17-7-5-16(6-8-17)15-31-24-27-26-23(30-24)18-9-10-21-22(13-18)29-12-11-28-21/h1-10,13H,11-12,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389081

(CHEMBL2064534)Show SMILES N#Cc1ccccc1-c1ccc(CSc2nnc(o2)-c2ccc3OCCOc3c2)cc1 Show InChI InChI=1S/C24H17N3O3S/c25-14-19-3-1-2-4-20(19)17-7-5-16(6-8-17)15-31-24-27-26-23(30-24)18-9-10-21-22(13-18)29-12-11-28-21/h1-10,13H,11-12,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Falcipain-2
(Plasmodium falciparum) | BDBM12041
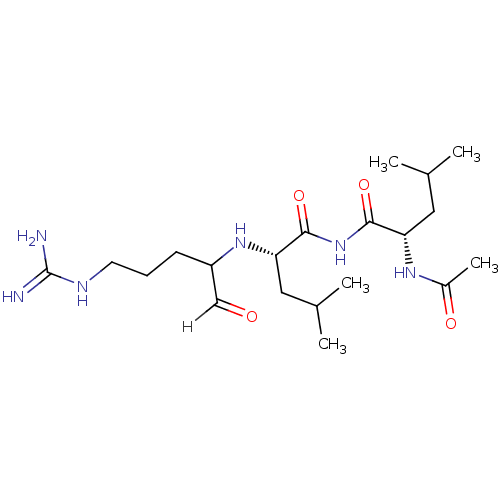
((2S)-2-({5-[(diaminomethylidene)amino]-1-oxopentan...)Show SMILES CC(C)C[C@H](NC(CCCNC(N)=N)C=O)C(=O)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C20H38N6O4/c1-12(2)9-16(24-14(5)28)18(29)26-19(30)17(10-13(3)4)25-15(11-27)7-6-8-23-20(21)22/h11-13,15-17,25H,6-10H2,1-5H3,(H,24,28)(H4,21,22,23)(H,26,29,30)/t15?,16-,17-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Universidad Central de Venezuela
| Assay Description
The substrate peptide terminating in AMC is processed by falcipain-2 with or without inhibitors, and the accumulation of AMC was monitored in a Labsy... |
J Med Chem 48: 3654-8 (2005)
Article DOI: 10.1021/jm058208o
BindingDB Entry DOI: 10.7270/Q2GF0RRD |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
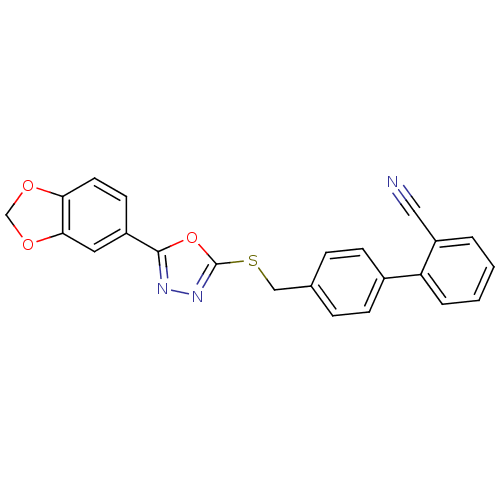
(Homo sapiens (Human)) | BDBM50389080
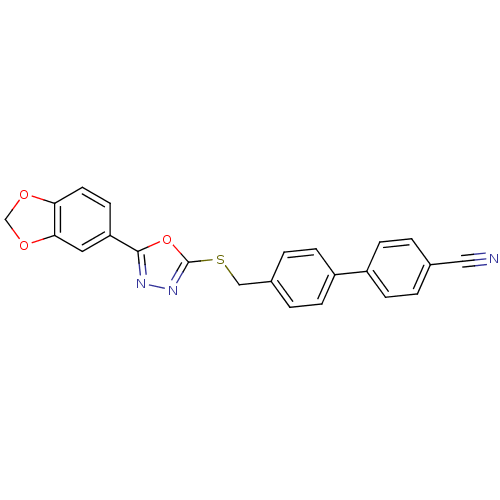
(CHEMBL2064515)Show SMILES N#Cc1ccc(cc1)-c1ccc(CSc2nnc(o2)-c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C23H15N3O3S/c24-12-15-1-5-17(6-2-15)18-7-3-16(4-8-18)13-30-23-26-25-22(29-23)19-9-10-20-21(11-19)28-14-27-20/h1-11H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389086
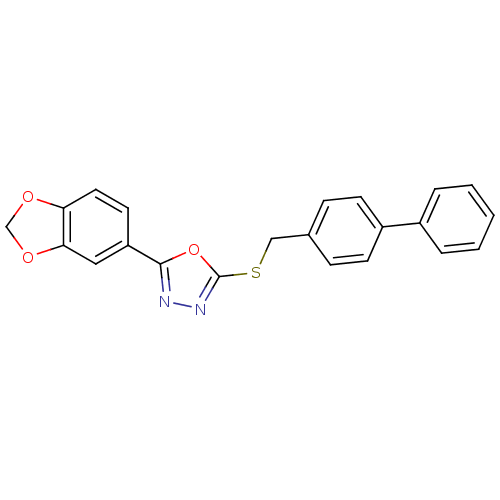
(CHEMBL2064531)Show SMILES C(Sc1nnc(o1)-c1ccc2OCOc2c1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H16N2O3S/c1-2-4-16(5-3-1)17-8-6-15(7-9-17)13-28-22-24-23-21(27-22)18-10-11-19-20(12-18)26-14-25-19/h1-12H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389085
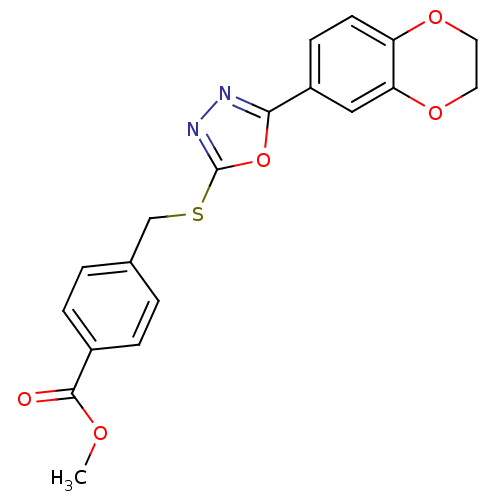
(CHEMBL2064520)Show SMILES COC(=O)c1ccc(CSc2nnc(o2)-c2ccc3OCCOc3c2)cc1 Show InChI InChI=1S/C19H16N2O5S/c1-23-18(22)13-4-2-12(3-5-13)11-27-19-21-20-17(26-19)14-6-7-15-16(10-14)25-9-8-24-15/h2-7,10H,8-9,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389078
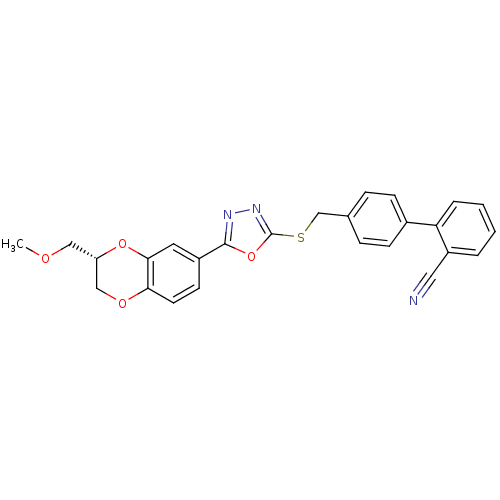
(CHEMBL2064539)Show SMILES COC[C@H]1COc2ccc(cc2O1)-c1nnc(SCc2ccc(cc2)-c2ccccc2C#N)o1 |r| Show InChI InChI=1S/C26H21N3O4S/c1-30-14-21-15-31-23-11-10-19(12-24(23)32-21)25-28-29-26(33-25)34-16-17-6-8-18(9-7-17)22-5-3-2-4-20(22)13-27/h2-12,21H,14-16H2,1H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389088

(CHEMBL2064516)Show InChI InChI=1S/C17H11N3O3S/c18-8-11-2-1-3-12(6-11)9-24-17-20-19-16(23-17)13-4-5-14-15(7-13)22-10-21-14/h1-7H,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389089
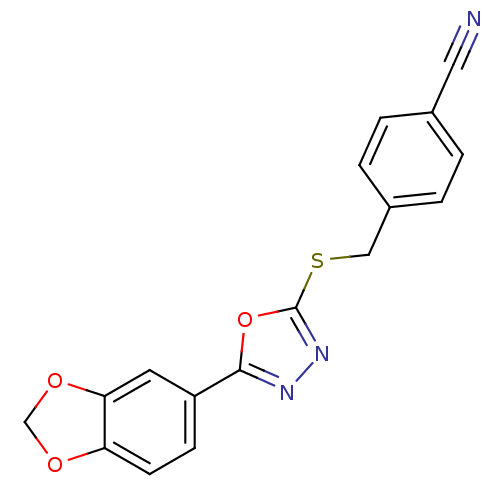
(CHEMBL2064517)Show InChI InChI=1S/C17H11N3O3S/c18-8-11-1-3-12(4-2-11)9-24-17-20-19-16(23-17)13-5-6-14-15(7-13)22-10-21-14/h1-7H,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389082
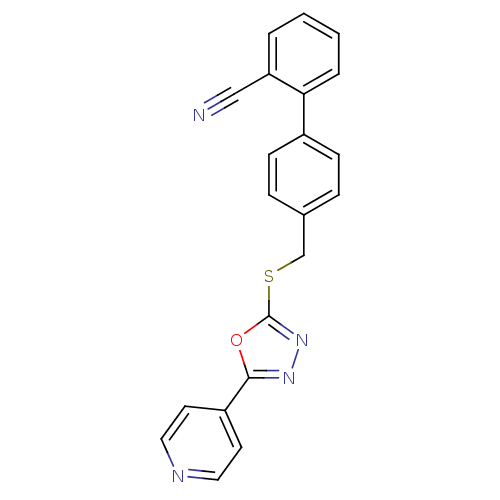
(CHEMBL2064536)Show InChI InChI=1S/C21H14N4OS/c22-13-18-3-1-2-4-19(18)16-7-5-15(6-8-16)14-27-21-25-24-20(26-21)17-9-11-23-12-10-17/h1-12H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389079

(CHEMBL2064532)Show SMILES Fc1ccc(-c2ccc(CSc3nnc(o3)-c3ccc4OCOc4c3)cc2)c(c1)C#N Show InChI InChI=1S/C23H14FN3O3S/c24-18-6-7-19(17(9-18)11-25)15-3-1-14(2-4-15)12-31-23-27-26-22(30-23)16-5-8-20-21(10-16)29-13-28-20/h1-10H,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50115777

((E)-1-(4'-Fluorophenyl)-3-(3'',4'',5''-trimethoxyp...)Show InChI InChI=1S/C18H17FO4/c1-21-16-10-12(11-17(22-2)18(16)23-3)4-9-15(20)13-5-7-14(19)8-6-13/h4-11H,1-3H3/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required to produce 50% inhibition of the response was determined |
Bioorg Med Chem Lett 12: 1951-4 (2002)
BindingDB Entry DOI: 10.7270/Q2M61JKW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389085

(CHEMBL2064520)Show SMILES COC(=O)c1ccc(CSc2nnc(o2)-c2ccc3OCCOc3c2)cc1 Show InChI InChI=1S/C19H16N2O5S/c1-23-18(22)13-4-2-12(3-5-13)11-27-19-21-20-17(26-19)14-6-7-15-16(10-14)25-9-8-24-15/h2-7,10H,8-9,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389087

(CHEMBL2064533)Show SMILES N#Cc1ccccc1-c1ccc(CSc2nnc(o2)-c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C23H15N3O3S/c24-12-18-3-1-2-4-19(18)16-7-5-15(6-8-16)13-30-23-26-25-22(29-23)17-9-10-20-21(11-17)28-14-27-20/h1-11H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389082

(CHEMBL2064536)Show InChI InChI=1S/C21H14N4OS/c22-13-18-3-1-2-4-19(18)16-7-5-15(6-8-16)14-27-21-25-24-20(26-21)17-9-11-23-12-10-17/h1-12H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389091
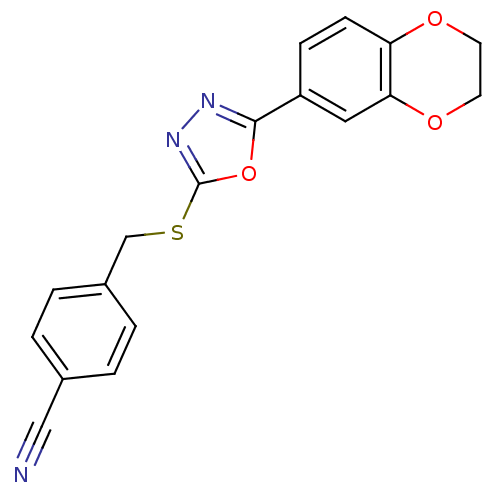
(CHEMBL2064519)Show InChI InChI=1S/C18H13N3O3S/c19-10-12-1-3-13(4-2-12)11-25-18-21-20-17(24-18)14-5-6-15-16(9-14)23-8-7-22-15/h1-6,9H,7-8,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389078

(CHEMBL2064539)Show SMILES COC[C@H]1COc2ccc(cc2O1)-c1nnc(SCc2ccc(cc2)-c2ccccc2C#N)o1 |r| Show InChI InChI=1S/C26H21N3O4S/c1-30-14-21-15-31-23-11-10-19(12-24(23)32-21)25-28-29-26(33-25)34-16-17-6-8-18(9-7-17)22-5-3-2-4-20(22)13-27/h2-12,21H,14-16H2,1H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Falcipain-2
(Plasmodium falciparum) | BDBM12042

((2R,3R)-3-{[(1S)-1-[(4-carbamimidamidobutyl)carbam...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#8]-[#6@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10+,11+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Universidad Central de Venezuela
| Assay Description
The substrate peptide terminating in AMC is processed by falcipain-2 with or without inhibitors, and the accumulation of AMC was monitored in a Labsy... |
J Med Chem 48: 3654-8 (2005)
Article DOI: 10.1021/jm058208o
BindingDB Entry DOI: 10.7270/Q2GF0RRD |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389088

(CHEMBL2064516)Show InChI InChI=1S/C17H11N3O3S/c18-8-11-2-1-3-12(6-11)9-24-17-20-19-16(23-17)13-4-5-14-15(7-13)22-10-21-14/h1-7H,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389090
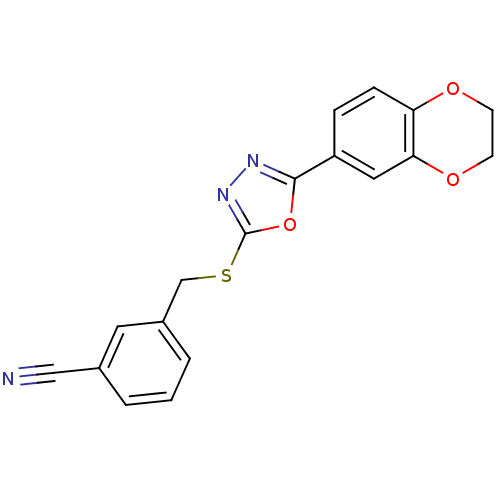
(CHEMBL2064518)Show InChI InChI=1S/C18H13N3O3S/c19-10-12-2-1-3-13(8-12)11-25-18-21-20-17(24-18)14-4-5-15-16(9-14)23-7-6-22-15/h1-5,8-9H,6-7,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389090

(CHEMBL2064518)Show InChI InChI=1S/C18H13N3O3S/c19-10-12-2-1-3-13(8-12)11-25-18-21-20-17(24-18)14-4-5-15-16(9-14)23-7-6-22-15/h1-5,8-9H,6-7,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50352490
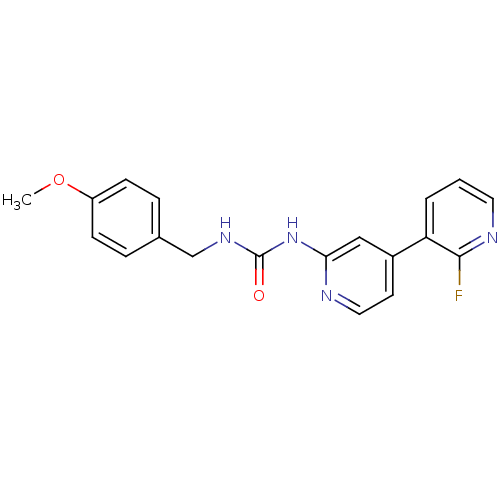
(CHEMBL1824331)Show InChI InChI=1S/C19H17FN4O2/c1-26-15-6-4-13(5-7-15)12-23-19(25)24-17-11-14(8-10-21-17)16-3-2-9-22-18(16)20/h2-11H,12H2,1H3,(H2,21,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta activity using Ser/Thr 9 peptide as substrate by FRET assay |
Bioorg Med Chem Lett 21: 5610-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.131
BindingDB Entry DOI: 10.7270/Q2765FP3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389093

(CHEMBL2064522)Show SMILES C(Sc1nnc(o1)-c1ccc2OCOc2c1)c1ccc(cc1)-c1nnn[nH]1 Show InChI InChI=1S/C17H12N6O3S/c1-3-11(15-18-22-23-19-15)4-2-10(1)8-27-17-21-20-16(26-17)12-5-6-13-14(7-12)25-9-24-13/h1-7H,8-9H2,(H,18,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389078

(CHEMBL2064539)Show SMILES COC[C@H]1COc2ccc(cc2O1)-c1nnc(SCc2ccc(cc2)-c2ccccc2C#N)o1 |r| Show InChI InChI=1S/C26H21N3O4S/c1-30-14-21-15-31-23-11-10-19(12-24(23)32-21)25-28-29-26(33-25)34-16-17-6-8-18(9-7-17)22-5-3-2-4-20(22)13-27/h2-12,21H,14-16H2,1H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389089

(CHEMBL2064517)Show InChI InChI=1S/C17H11N3O3S/c18-8-11-1-3-12(4-2-11)9-24-17-20-19-16(23-17)13-5-6-14-15(7-13)22-10-21-14/h1-7H,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389091

(CHEMBL2064519)Show InChI InChI=1S/C18H13N3O3S/c19-10-12-1-3-13(4-2-12)11-25-18-21-20-17(24-18)14-5-6-15-16(9-14)23-8-7-22-15/h1-6,9H,7-8,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389093

(CHEMBL2064522)Show SMILES C(Sc1nnc(o1)-c1ccc2OCOc2c1)c1ccc(cc1)-c1nnn[nH]1 Show InChI InChI=1S/C17H12N6O3S/c1-3-11(15-18-22-23-19-15)4-2-10(1)8-27-17-21-20-16(26-17)12-5-6-13-14(7-12)25-9-24-13/h1-7H,8-9H2,(H,18,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389086

(CHEMBL2064531)Show SMILES C(Sc1nnc(o1)-c1ccc2OCOc2c1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H16N2O3S/c1-2-4-16(5-3-1)17-8-6-15(7-9-17)13-28-22-24-23-21(27-22)18-10-11-19-20(12-18)26-14-25-19/h1-12H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389083

(CHEMBL2064535)Show SMILES Fc1ccc(-c2ccc(CSc3nnc(o3)-c3ccc4OCCOc4c3)cc2)c(c1)C#N Show InChI InChI=1S/C24H16FN3O3S/c25-19-6-7-20(18(11-19)13-26)16-3-1-15(2-4-16)14-32-24-28-27-23(31-24)17-5-8-21-22(12-17)30-10-9-29-21/h1-8,11-12H,9-10,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389084
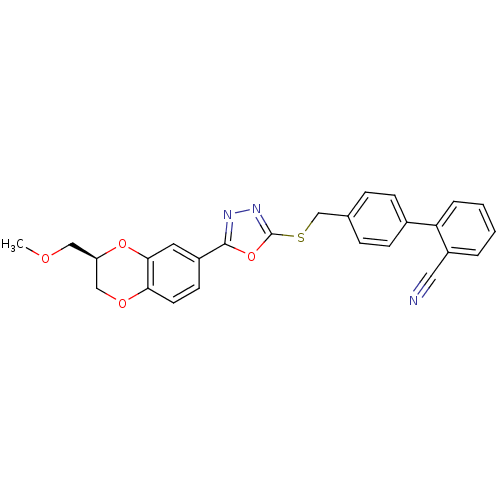
(CHEMBL2064538)Show SMILES COC[C@@H]1COc2ccc(cc2O1)-c1nnc(SCc2ccc(cc2)-c2ccccc2C#N)o1 |r| Show InChI InChI=1S/C26H21N3O4S/c1-30-14-21-15-31-23-11-10-19(12-24(23)32-21)25-28-29-26(33-25)34-16-17-6-8-18(9-7-17)22-5-3-2-4-20(22)13-27/h2-12,21H,14-16H2,1H3/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389078

(CHEMBL2064539)Show SMILES COC[C@H]1COc2ccc(cc2O1)-c1nnc(SCc2ccc(cc2)-c2ccccc2C#N)o1 |r| Show InChI InChI=1S/C26H21N3O4S/c1-30-14-21-15-31-23-11-10-19(12-24(23)32-21)25-28-29-26(33-25)34-16-17-6-8-18(9-7-17)22-5-3-2-4-20(22)13-27/h2-12,21H,14-16H2,1H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50115783
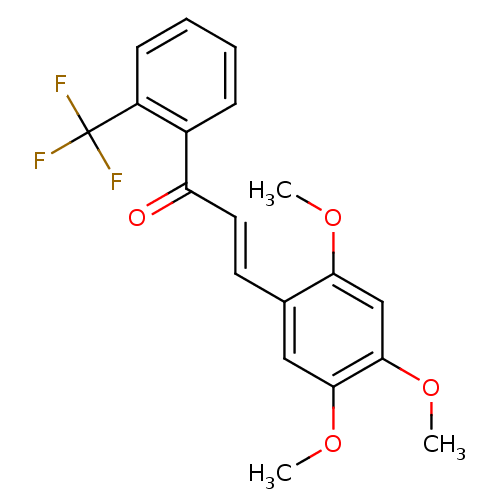
(3-(5-Ethoxy-2,4-dimethoxy-phenyl)-1-(2-trifluorome...)Show SMILES COc1cc(OC)c(\C=C\C(=O)c2ccccc2C(F)(F)F)cc1OC Show InChI InChI=1S/C19H17F3O4/c1-24-16-11-18(26-3)17(25-2)10-12(16)8-9-15(23)13-6-4-5-7-14(13)19(20,21)22/h4-11H,1-3H3/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required to produce 50% inhibition of the response was determined; range-0.150.47 uM |
Bioorg Med Chem Lett 12: 1951-4 (2002)
BindingDB Entry DOI: 10.7270/Q2M61JKW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389080

(CHEMBL2064515)Show SMILES N#Cc1ccc(cc1)-c1ccc(CSc2nnc(o2)-c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C23H15N3O3S/c24-12-15-1-5-17(6-2-15)18-7-3-16(4-8-18)13-30-23-26-25-22(29-23)19-9-10-20-21(11-19)28-14-27-20/h1-11H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8552
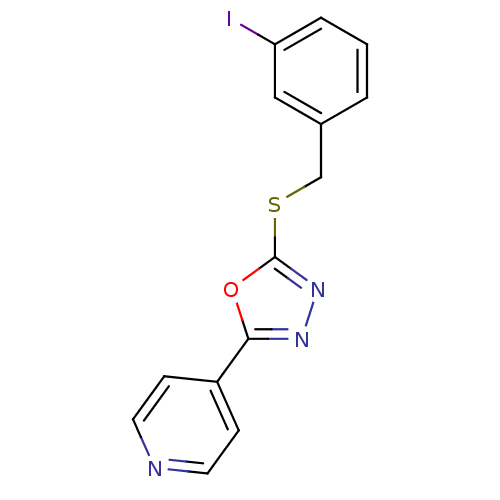
(2-{[(3-iodophenyl)methyl]sulfanyl}-5-(pyridin-4-yl...)Show InChI InChI=1S/C14H10IN3OS/c15-12-3-1-2-10(8-12)9-20-14-18-17-13(19-14)11-4-6-16-7-5-11/h1-8H,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50115779

(1-(2,4-Difluoro-phenyl)-3-(5-ethoxy-2,4-dimethoxy-...)Show InChI InChI=1S/C18H16F2O4/c1-22-16-10-18(24-3)17(23-2)8-11(16)4-7-15(21)13-6-5-12(19)9-14(13)20/h4-10H,1-3H3/b7-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required to produce 50% inhibition of the response was determined; range-0.431.12 uM |
Bioorg Med Chem Lett 12: 1951-4 (2002)
BindingDB Entry DOI: 10.7270/Q2M61JKW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50389084

(CHEMBL2064538)Show SMILES COC[C@@H]1COc2ccc(cc2O1)-c1nnc(SCc2ccc(cc2)-c2ccccc2C#N)o1 |r| Show InChI InChI=1S/C26H21N3O4S/c1-30-14-21-15-31-23-11-10-19(12-24(23)32-21)25-28-29-26(33-25)34-16-17-6-8-18(9-7-17)22-5-3-2-4-20(22)13-27/h2-12,21H,14-16H2,1H3/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 758 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8552

(2-{[(3-iodophenyl)methyl]sulfanyl}-5-(pyridin-4-yl...)Show InChI InChI=1S/C14H10IN3OS/c15-12-3-1-2-10(8-12)9-20-14-18-17-13(19-14)11-4-6-16-7-5-11/h1-8H,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50115780

(3-(2,4-Dimethoxy-phenyl)-1-(2-trifluoromethyl-phen...)Show InChI InChI=1S/C18H15F3O3/c1-23-13-9-7-12(17(11-13)24-2)8-10-16(22)14-5-3-4-6-15(14)18(19,20)21/h3-11H,1-2H3/b10-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required to produce 50% inhibition of the response was determined; range-0.461.81 uM |
Bioorg Med Chem Lett 12: 1951-4 (2002)
BindingDB Entry DOI: 10.7270/Q2M61JKW |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50115778

(3-(2,5-Dimethoxy-phenyl)-1-(2-trifluoromethyl-phen...)Show InChI InChI=1S/C18H15F3O3/c1-23-13-8-10-17(24-2)12(11-13)7-9-16(22)14-5-3-4-6-15(14)18(19,20)21/h3-11H,1-2H3/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required to produce 50% inhibition of the response was determined; range-0.431.85 uM |
Bioorg Med Chem Lett 12: 1951-4 (2002)
BindingDB Entry DOI: 10.7270/Q2M61JKW |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50115781
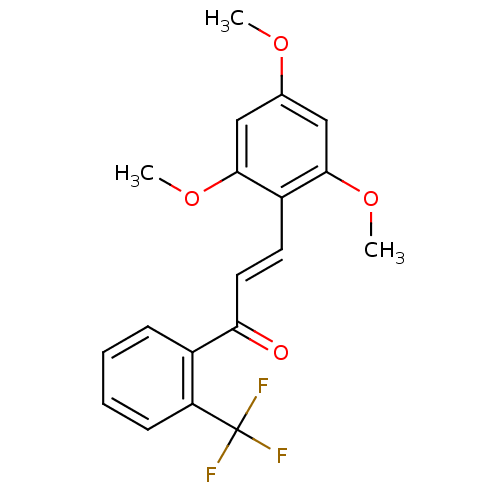
((E)-1-(2-(trifluoromethyl)phenyl)-3-(2,4,6-trimeth...)Show SMILES COc1cc(OC)c(\C=C\C(=O)c2ccccc2C(F)(F)F)c(OC)c1 Show InChI InChI=1S/C19H17F3O4/c1-24-12-10-17(25-2)14(18(11-12)26-3)8-9-16(23)13-6-4-5-7-15(13)19(20,21)22/h4-11H,1-3H3/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required to produce 50% inhibition of the response was determined; range-0.811.70 uM |
Bioorg Med Chem Lett 12: 1951-4 (2002)
BindingDB Entry DOI: 10.7270/Q2M61JKW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8548
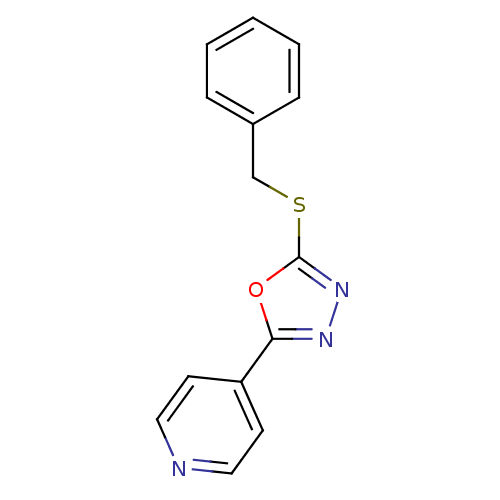
(2-(benzylsulfanyl)-5-(pyridin-4-yl)-1,3,4-oxadiazo...)Show InChI InChI=1S/C14H11N3OS/c1-2-4-11(5-3-1)10-19-14-17-16-13(18-14)12-6-8-15-9-7-12/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using pIRS-1 as substrate preincubated for 15 mins |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Falcipain-2
(Plasmodium falciparum) | BDBM12037
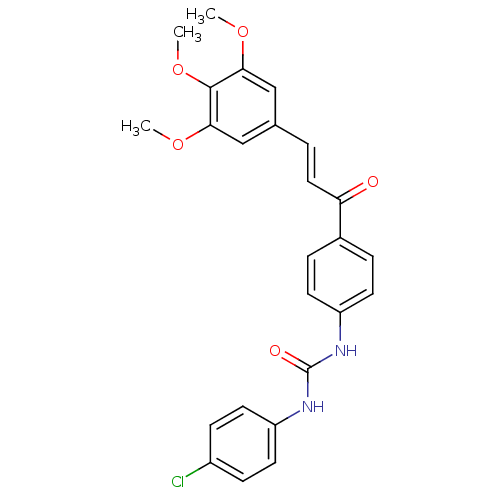
(3-(4-chlorophenyl)-1-{4-[(2E)-3-(3,4,5-trimethoxyp...)Show SMILES COc1cc(\C=C\C(=O)c2ccc(NC(=O)Nc3ccc(Cl)cc3)cc2)cc(OC)c1OC Show InChI InChI=1S/C25H23ClN2O5/c1-31-22-14-16(15-23(32-2)24(22)33-3)4-13-21(29)17-5-9-19(10-6-17)27-25(30)28-20-11-7-18(26)8-12-20/h4-15H,1-3H3,(H2,27,28,30)/b13-4+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Universidad Central de Venezuela
| Assay Description
The substrate peptide terminating in AMC is processed by falcipain-2 with or without inhibitors, and the accumulation of AMC was monitored in a Labsy... |
J Med Chem 48: 3654-8 (2005)
Article DOI: 10.1021/jm058208o
BindingDB Entry DOI: 10.7270/Q2GF0RRD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50115782

(1-(3-Trifluoromethyl-phenyl)-3-(2,4,6-trimethoxy-p...)Show SMILES COc1cc(OC)c(\C=C\C(=O)c2cccc(c2)C(F)(F)F)c(OC)c1 Show InChI InChI=1S/C19H17F3O4/c1-24-14-10-17(25-2)15(18(11-14)26-3)7-8-16(23)12-5-4-6-13(9-12)19(20,21)22/h4-11H,1-3H3/b8-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required to produce 50% inhibition of the response was determined; range-0.541.16 uM |
Bioorg Med Chem Lett 12: 1951-4 (2002)
BindingDB Entry DOI: 10.7270/Q2M61JKW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8548

(2-(benzylsulfanyl)-5-(pyridin-4-yl)-1,3,4-oxadiazo...)Show InChI InChI=1S/C14H11N3OS/c1-2-4-11(5-3-1)10-19-14-17-16-13(18-14)12-6-8-15-9-7-12/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3alpha |
J Med Chem 55: 4407-24 (2012)
Article DOI: 10.1021/jm300309a
BindingDB Entry DOI: 10.7270/Q2MG7QK0 |
More data for this
Ligand-Target Pair | |
Falcipain-2
(Plasmodium falciparum) | BDBM12032
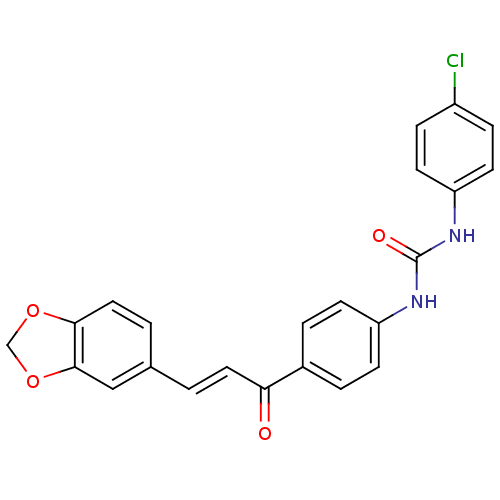
(1-{4-[(2E)-3-(2H-1,3-benzodioxol-5-yl)prop-2-enoyl...)Show SMILES Clc1ccc(NC(=O)Nc2ccc(cc2)C(=O)\C=C\c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C23H17ClN2O4/c24-17-5-9-19(10-6-17)26-23(28)25-18-7-3-16(4-8-18)20(27)11-1-15-2-12-21-22(13-15)30-14-29-21/h1-13H,14H2,(H2,25,26,28)/b11-1+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Universidad Central de Venezuela
| Assay Description
The substrate peptide terminating in AMC is processed by falcipain-2 with or without inhibitors, and the accumulation of AMC was monitored in a Labsy... |
J Med Chem 48: 3654-8 (2005)
Article DOI: 10.1021/jm058208o
BindingDB Entry DOI: 10.7270/Q2GF0RRD |
More data for this
Ligand-Target Pair | |
Falcipain-2
(Plasmodium falciparum) | BDBM12035
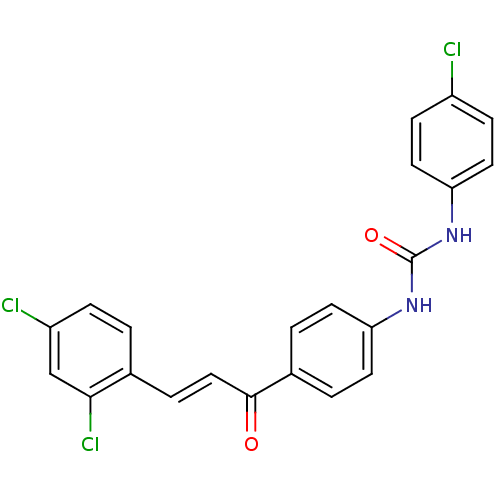
(3-(4-chlorophenyl)-1-{4-[(2E)-3-(2,4-dichloropheny...)Show SMILES Clc1ccc(NC(=O)Nc2ccc(cc2)C(=O)\C=C\c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C22H15Cl3N2O2/c23-16-6-10-19(11-7-16)27-22(29)26-18-8-2-15(3-9-18)21(28)12-4-14-1-5-17(24)13-20(14)25/h1-13H,(H2,26,27,29)/b12-4+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Universidad Central de Venezuela
| Assay Description
The substrate peptide terminating in AMC is processed by falcipain-2 with or without inhibitors, and the accumulation of AMC was monitored in a Labsy... |
J Med Chem 48: 3654-8 (2005)
Article DOI: 10.1021/jm058208o
BindingDB Entry DOI: 10.7270/Q2GF0RRD |
More data for this
Ligand-Target Pair | |
Falcipain-2
(Plasmodium falciparum) | BDBM12036
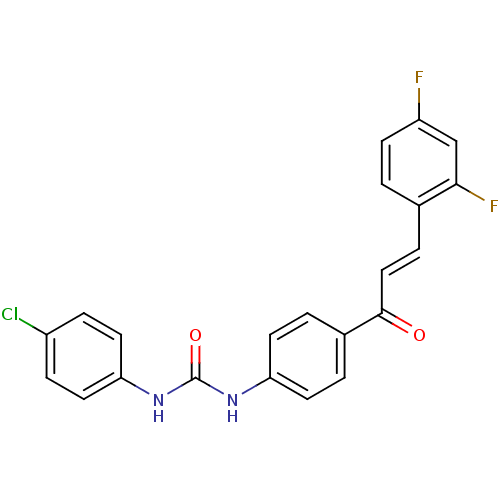
(3-(4-chlorophenyl)-1-{4-[(2E)-3-(2,4-difluoropheny...)Show SMILES Fc1ccc(\C=C\C(=O)c2ccc(NC(=O)Nc3ccc(Cl)cc3)cc2)c(F)c1 Show InChI InChI=1S/C22H15ClF2N2O2/c23-16-5-10-19(11-6-16)27-22(29)26-18-8-2-15(3-9-18)21(28)12-4-14-1-7-17(24)13-20(14)25/h1-13H,(H2,26,27,29)/b12-4+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Universidad Central de Venezuela
| Assay Description
The substrate peptide terminating in AMC is processed by falcipain-2 with or without inhibitors, and the accumulation of AMC was monitored in a Labsy... |
J Med Chem 48: 3654-8 (2005)
Article DOI: 10.1021/jm058208o
BindingDB Entry DOI: 10.7270/Q2GF0RRD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data