

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075813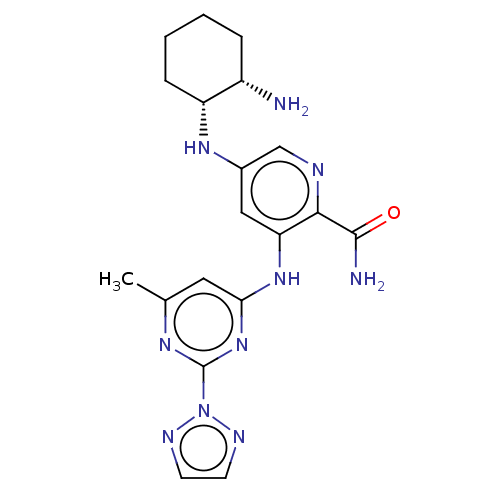 (CHEMBL3415598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075735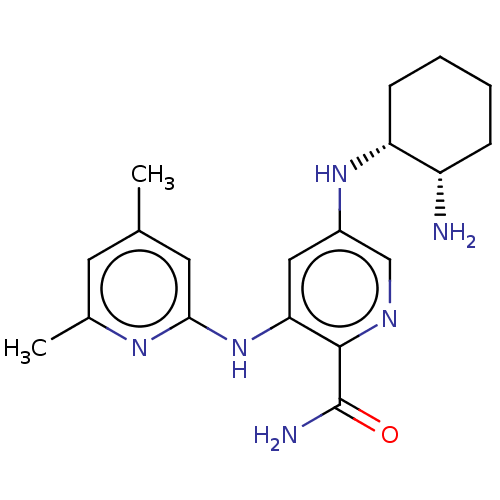 (CHEMBL3415583) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075744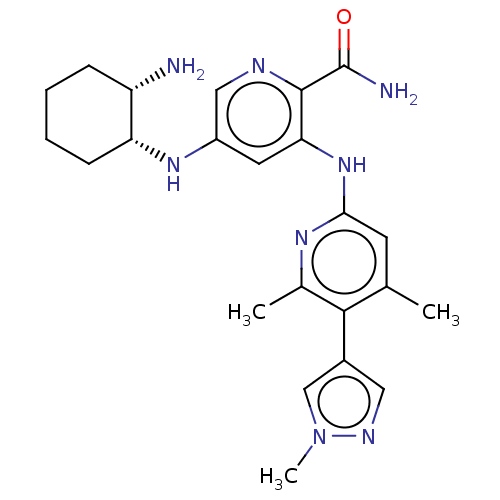 (CHEMBL3415594) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075740 (CHEMBL3415589) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075922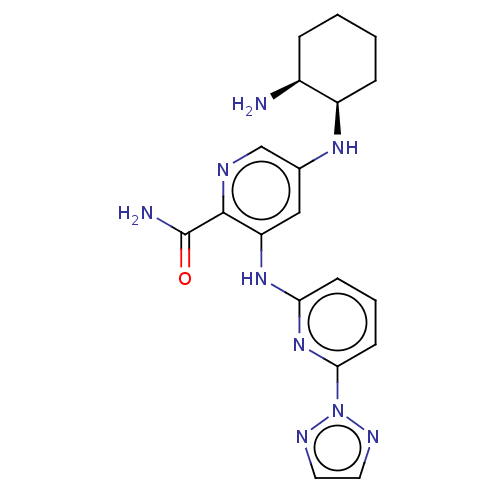 (CHEMBL3415606) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075747 (CHEMBL3415597) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075819 (CHEMBL3415604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182100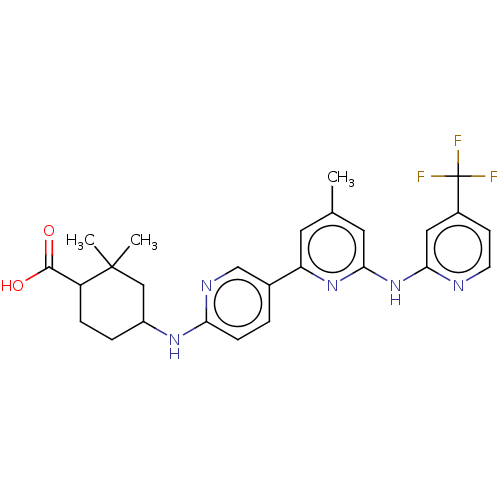 (US9145391, 3-4 | US9145391, 3-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182147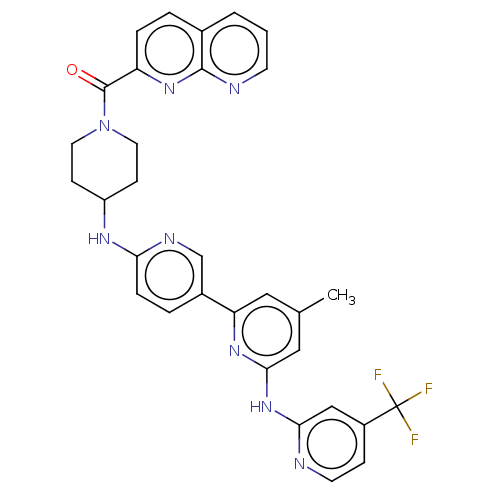 (US9145391, 4-44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182240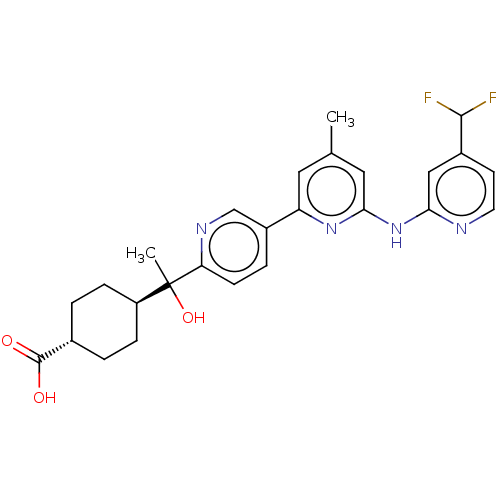 (US9145391, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182298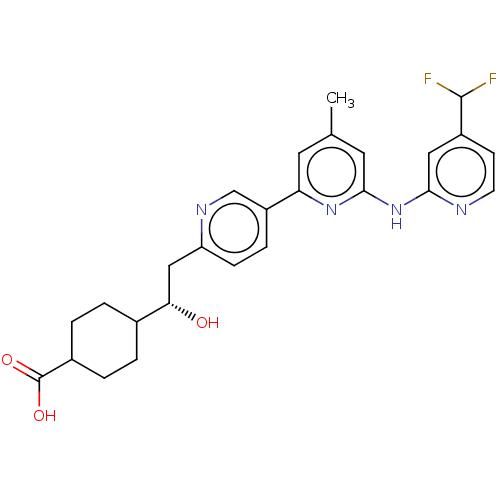 (US9145391, 12-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182241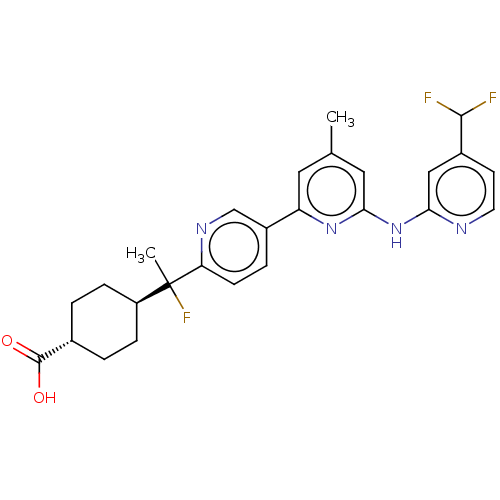 (US9145391, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182242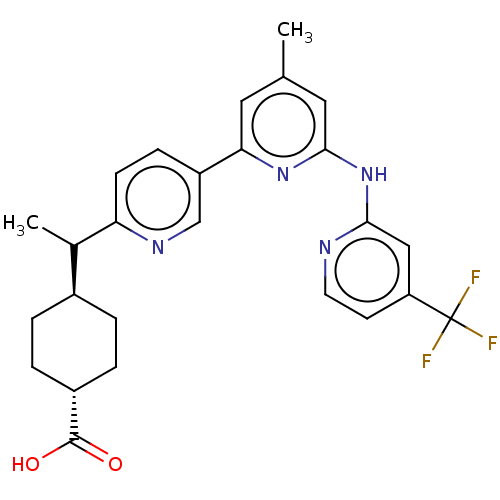 (US9145391, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182244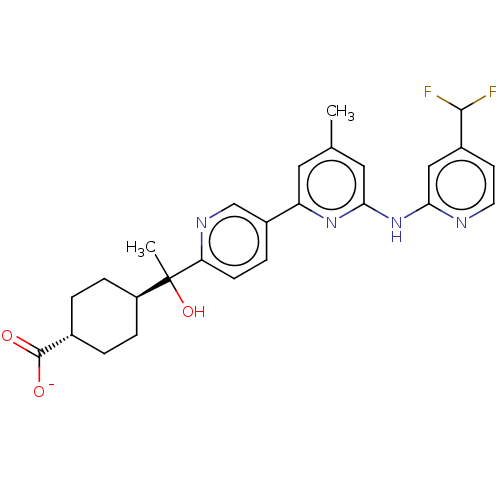 (US9145391, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075738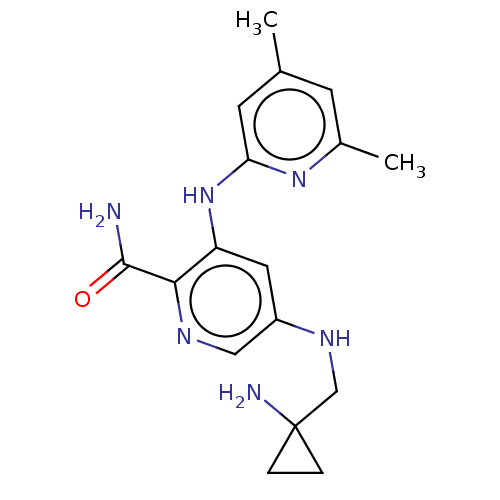 (CHEMBL3415587) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075742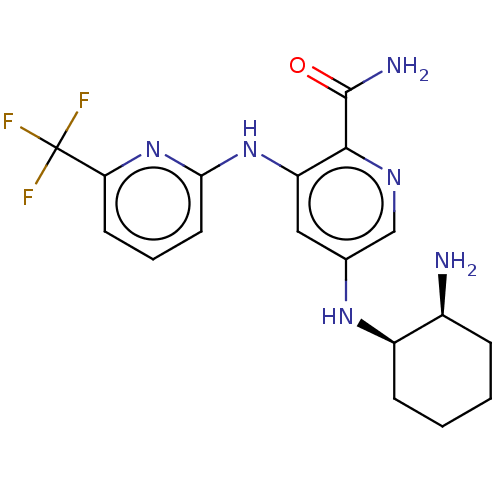 (CHEMBL3415592) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182098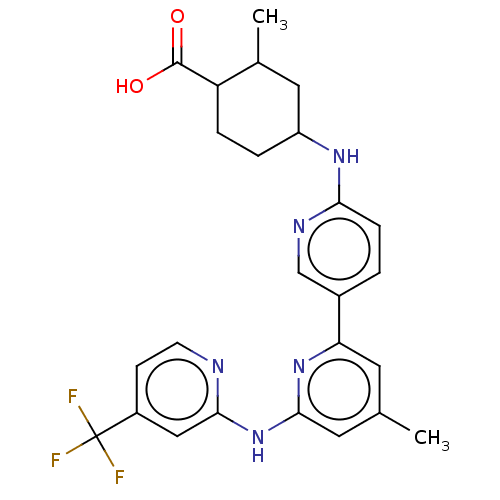 (US9145391, 3-2 | US9145391, 3-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182067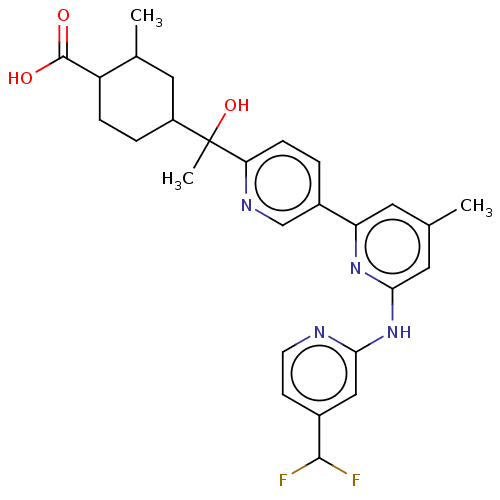 (US9145391, 1-35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182065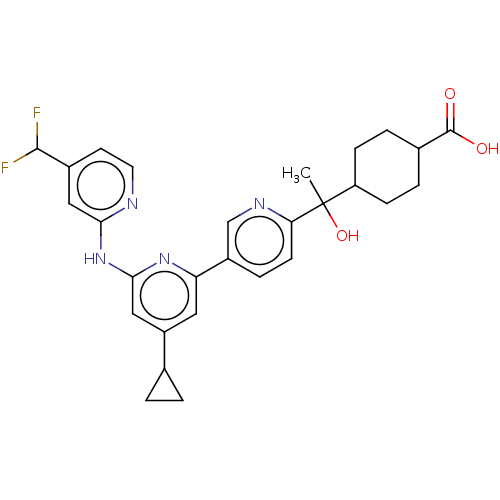 (US9145391, 1-33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182063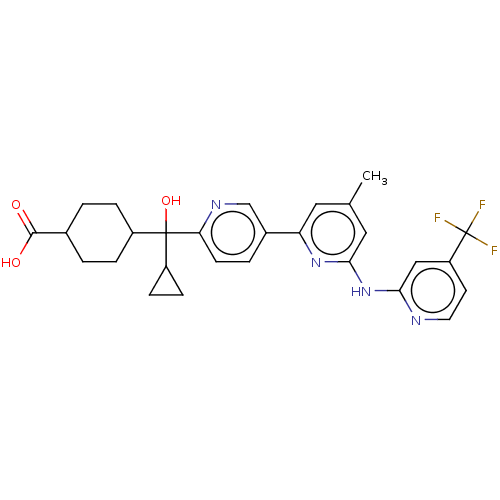 (US9145391, 1-31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182062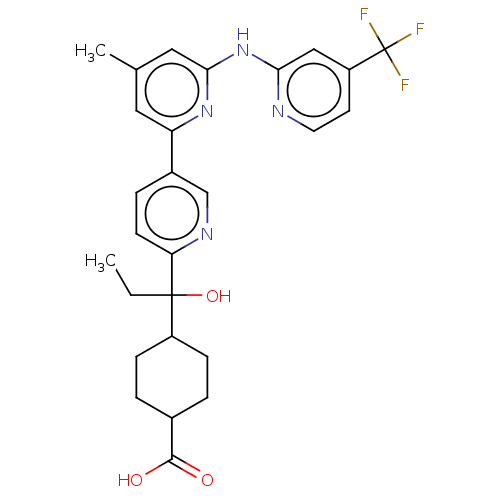 (US9145391, 1-30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394068 (5-(4-{[trans-4-(4- fluoropiperidin-1- yl)cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182038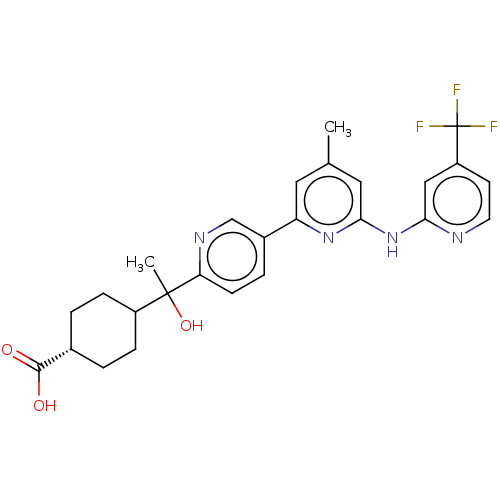 (US9145391, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182052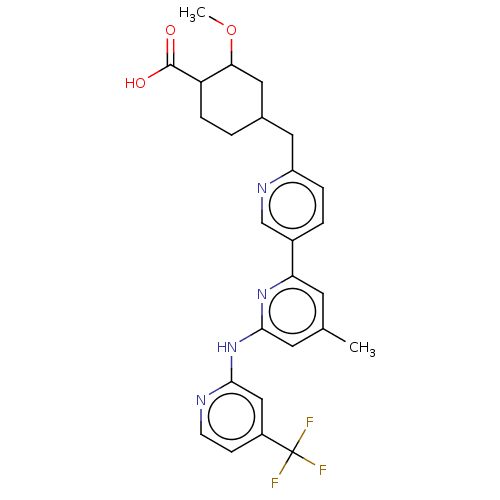 (US9145391, 1-20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394067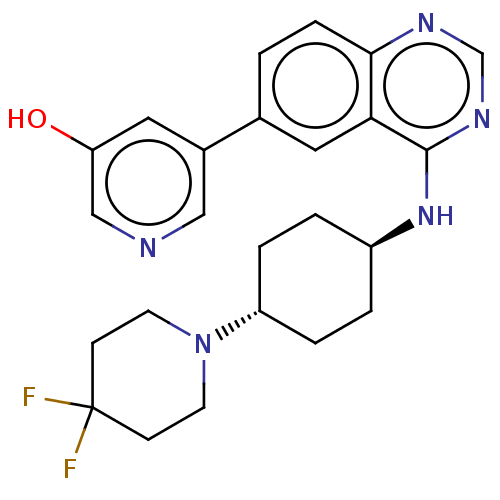 (5-(4-{[trans-4-(4,4- difluoropiperidin-1- yl)cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394063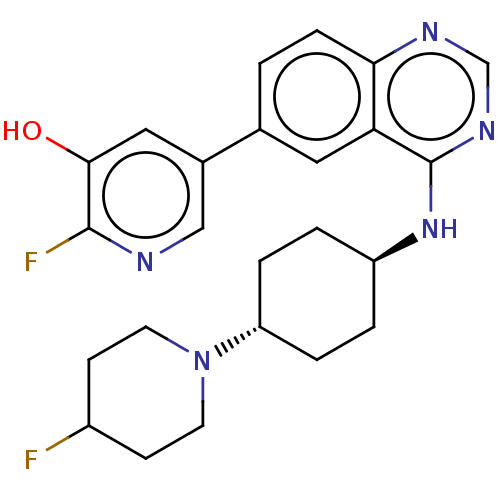 (2-fluoro-5-(4-{[trans-4- (4-fluoropiperidin-1- yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394056 (5-[4-({trans-4-[(2R,6S)- 2,6-dimethylmorpholin-4- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394054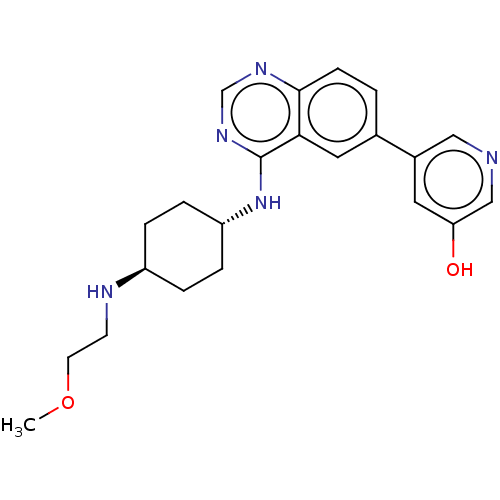 (5-[4-({trans-4-[(2- methoxyethyl)amino]cyclo- hexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075732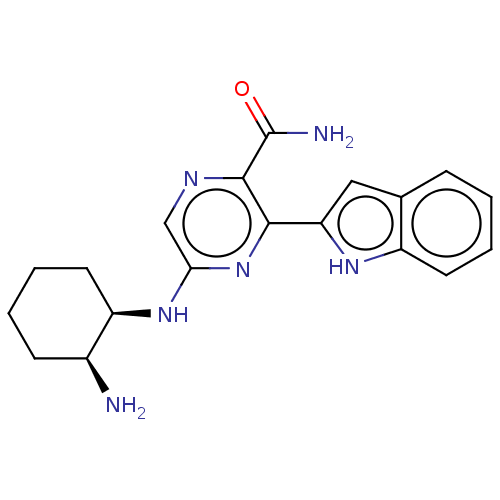 (CHEMBL3414584 | US9775839, 2.1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM393974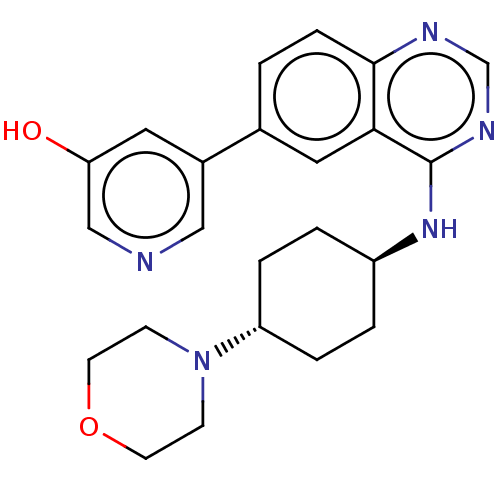 (5-(4-{[trans-4- (morpholin-4- yl)cyclohexyl]amino}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394042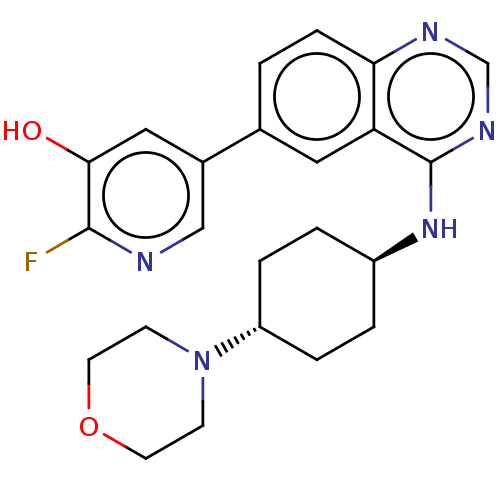 (2-fluoro-5-(4-{[trans-4- (morpholin-4- yl)cyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394051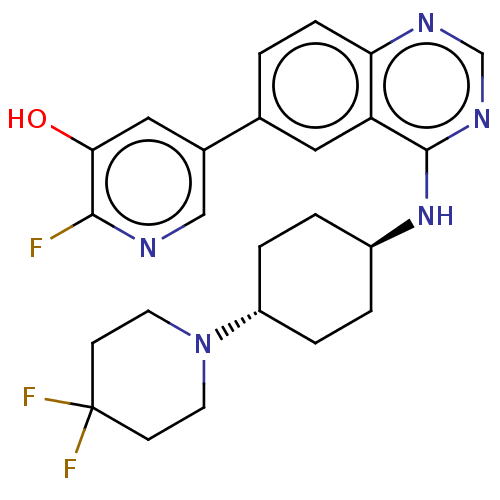 (US9969749, Example 5-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075730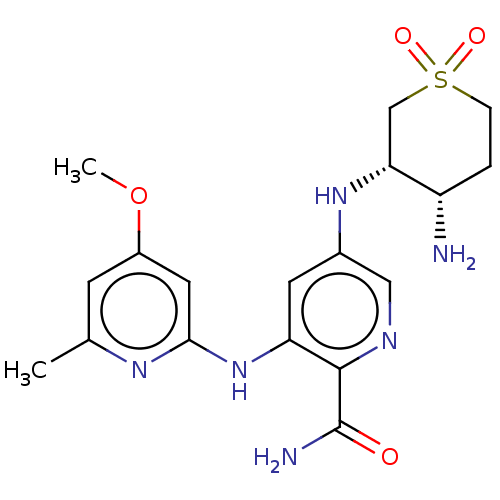 (CHEMBL3415599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075733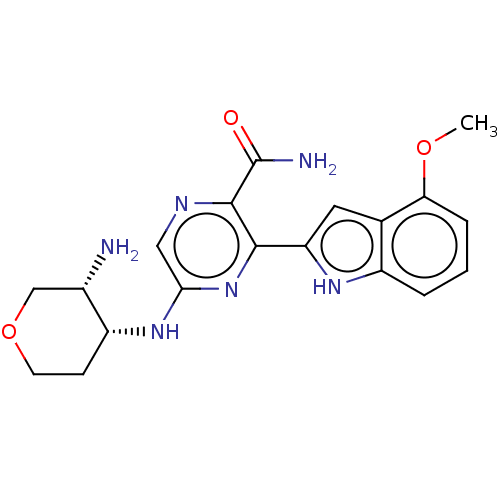 (CHEMBL3415610 | US9775839, 8.5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075739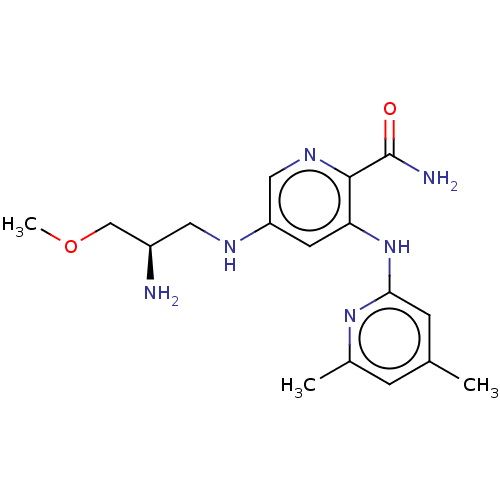 (CHEMBL3415588) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075743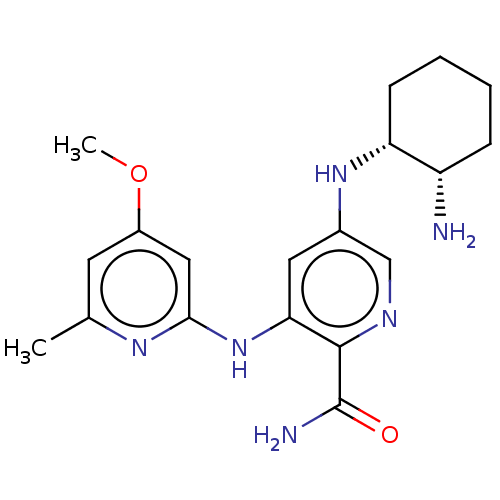 (CHEMBL3415593) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075745 (CHEMBL3415595) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394055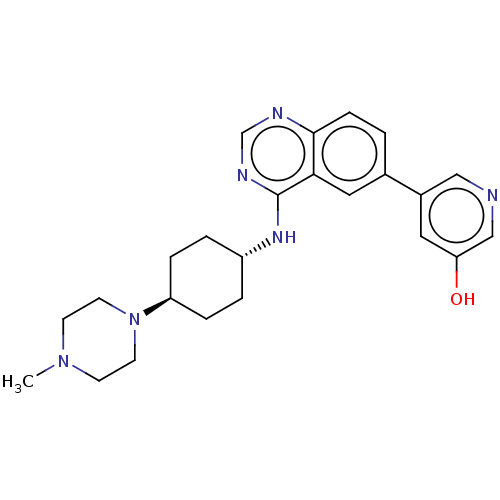 (5-(4-{[trans-4-(4- methylpiperazin-1- yl)cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394064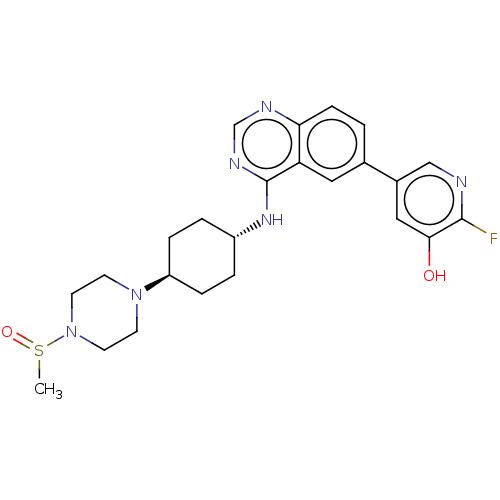 (2-fluoro-5-[4-({trans-4- [4- (methylsulfonyl)piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394066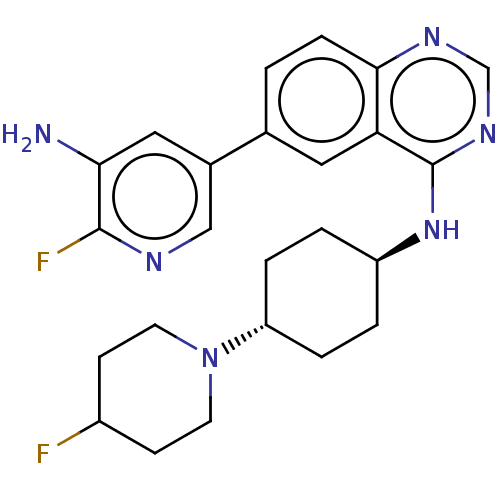 (6-(5-amino-6- fluoropyridin-3-yl)-N- [trans-4-(4- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394069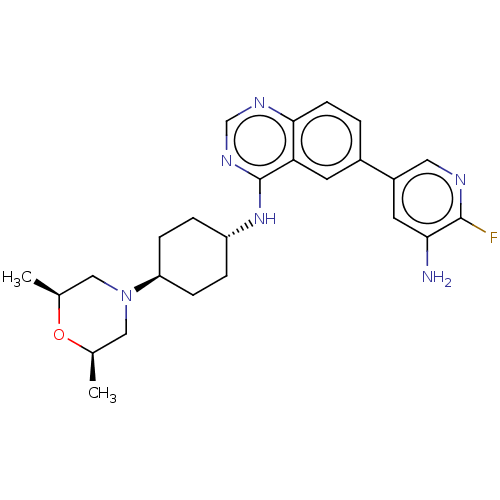 (6-(5-amino-6- fluoropyridin-3-yl)-N- {trans-4-[(2R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394074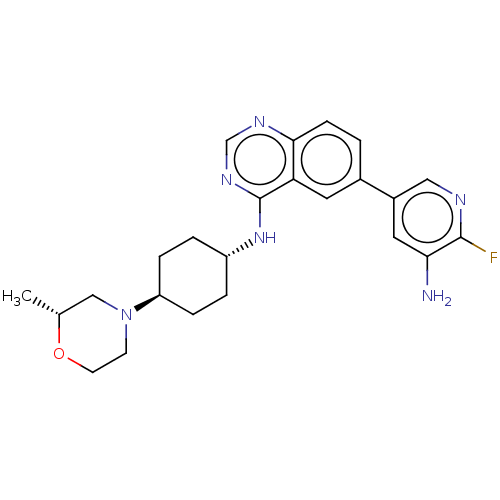 (6-(5-amino-6- fluoropyridin-3-yl)-N- {trans-4-[(2R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394075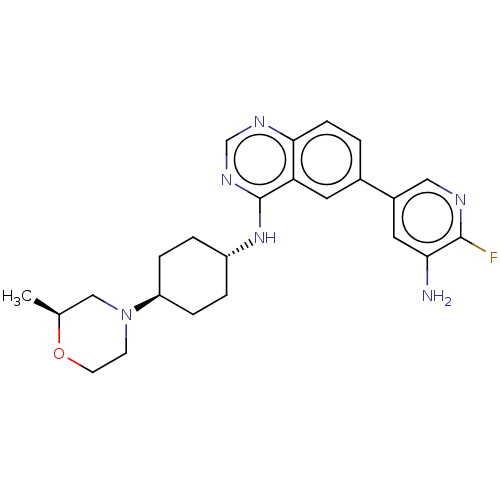 (6-(5-amino-6- fluoropyridin-3-yl)-N- {trans-4-[(2S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394076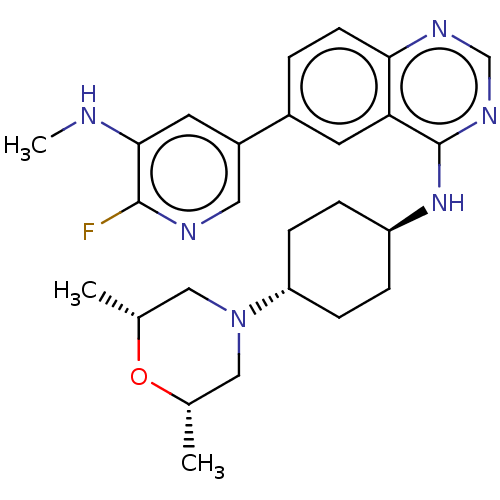 (N-{trans-4-[(2R,6S)-2,6- dimethylmorpholin-4- yl]c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394078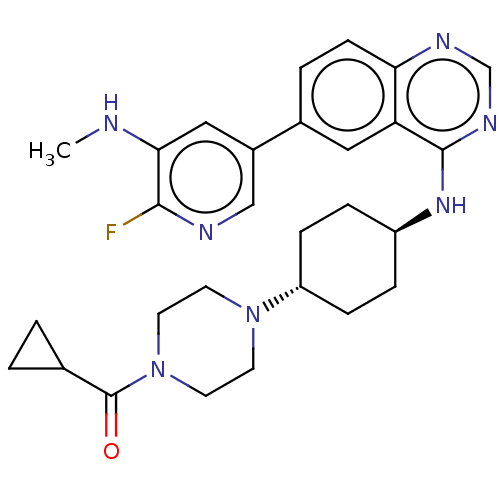 (US9969749, Example 5-28 | cyclopropyl{4-[trans-4- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394080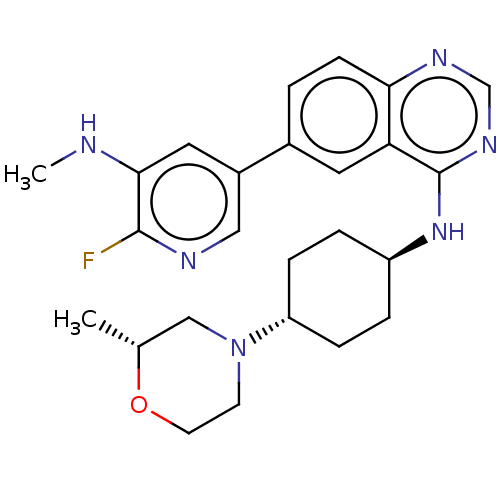 (6-[6-fluoro-5- (methylamino)pyridin-3- yl]-N-{tran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM394081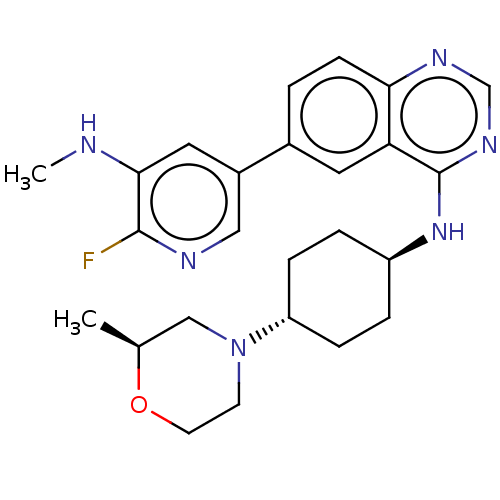 (6-[6-fluoro-5- (methylamino)pyridin-3- yl]-N-{tran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... | J Med Chem 52: 1873-84 (2009) BindingDB Entry DOI: 10.7270/Q2CN767V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50586268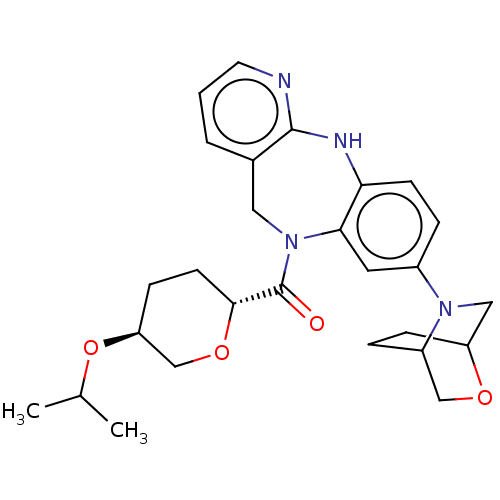 (CHEMBL5079148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00089 BindingDB Entry DOI: 10.7270/Q2RJ4PCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182227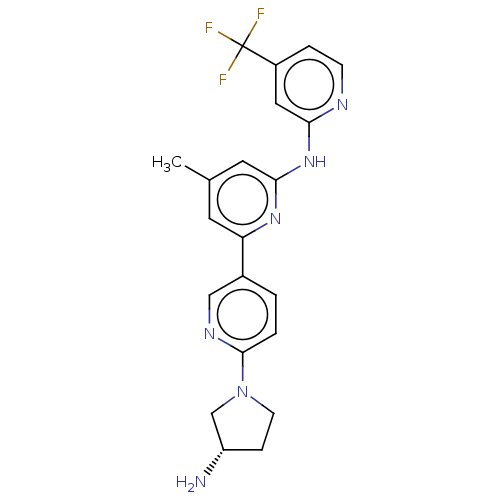 (US9145391, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182238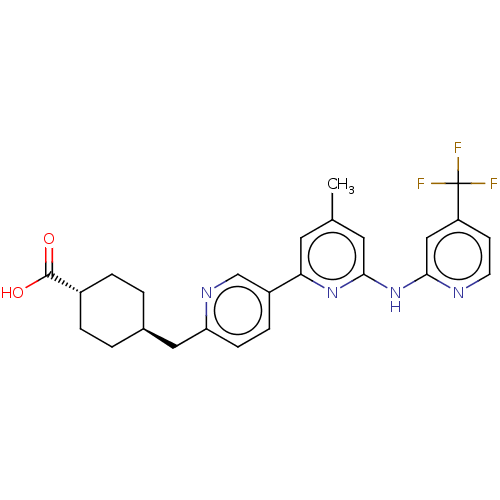 (US9145391, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 730 total ) | Next | Last >> |