 Found 678 hits with Last Name = 'dossetter' and Initial = 'ag'
Found 678 hits with Last Name = 'dossetter' and Initial = 'ag' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50047420
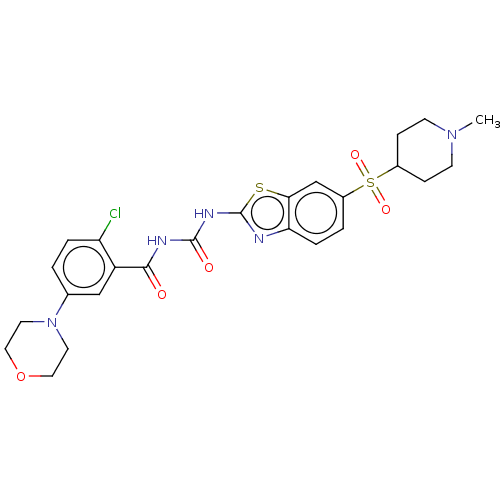
(CHEMBL3319405)Show SMILES CN1CCC(CC1)S(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(ccc3Cl)N3CCOCC3)sc2c1 Show InChI InChI=1S/C25H28ClN5O5S2/c1-30-8-6-17(7-9-30)38(34,35)18-3-5-21-22(15-18)37-25(27-21)29-24(33)28-23(32)19-14-16(2-4-20(19)26)31-10-12-36-13-11-31/h2-5,14-15,17H,6-13H2,1H3,(H2,27,28,29,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of alpha4 nAChR (unknown origin) |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50047420

(CHEMBL3319405)Show SMILES CN1CCC(CC1)S(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(ccc3Cl)N3CCOCC3)sc2c1 Show InChI InChI=1S/C25H28ClN5O5S2/c1-30-8-6-17(7-9-30)38(34,35)18-3-5-21-22(15-18)37-25(27-21)29-24(33)28-23(32)19-14-16(2-4-20(19)26)31-10-12-36-13-11-31/h2-5,14-15,17H,6-13H2,1H3,(H2,27,28,29,32,33) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of DAT (unknown origin) |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047485
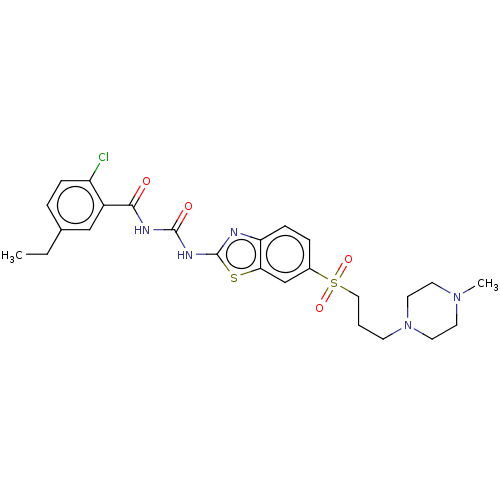
(CHEMBL3319217)Show SMILES CCc1ccc(Cl)c(c1)C(=O)NC(=O)Nc1nc2ccc(cc2s1)S(=O)(=O)CCCN1CCN(C)CC1 Show InChI InChI=1S/C25H30ClN5O4S2/c1-3-17-5-7-20(26)19(15-17)23(32)28-24(33)29-25-27-21-8-6-18(16-22(21)36-25)37(34,35)14-4-9-31-12-10-30(2)11-13-31/h5-8,15-16H,3-4,9-14H2,1-2H3,(H2,27,28,29,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047481

(CHEMBL3319221)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)-n4cccc4)sc3c2)CC1 Show InChI InChI=1S/C27H29ClN6O4S2/c1-32-12-14-33(15-13-32)9-4-16-40(37,38)20-6-8-23-24(18-20)39-27(29-23)31-26(36)30-25(35)21-17-19(5-7-22(21)28)34-10-2-3-11-34/h2-3,5-8,10-11,17-18H,4,9,12-16H2,1H3,(H2,29,30,31,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047429
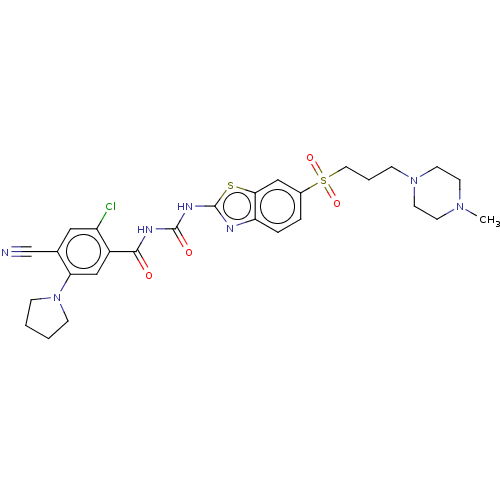
(CHEMBL3319398)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(N5CCCC5)c(cc4Cl)C#N)sc3c2)CC1 Show InChI InChI=1S/C28H32ClN7O4S2/c1-34-10-12-35(13-11-34)7-4-14-42(39,40)20-5-6-23-25(16-20)41-28(31-23)33-27(38)32-26(37)21-17-24(36-8-2-3-9-36)19(18-30)15-22(21)29/h5-6,15-17H,2-4,7-14H2,1H3,(H2,31,32,33,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047480
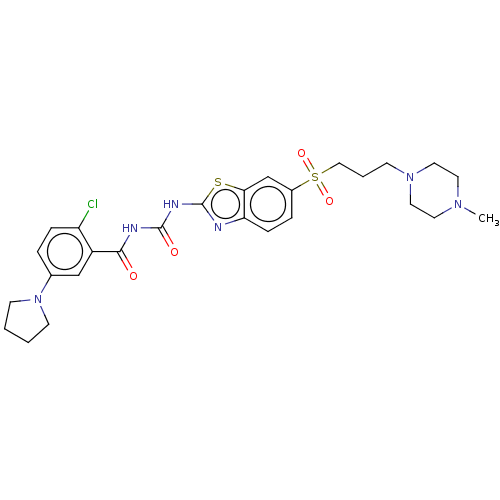
(CHEMBL3319222)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)N4CCCC4)sc3c2)CC1 Show InChI InChI=1S/C27H33ClN6O4S2/c1-32-12-14-33(15-13-32)9-4-16-40(37,38)20-6-8-23-24(18-20)39-27(29-23)31-26(36)30-25(35)21-17-19(5-7-22(21)28)34-10-2-3-11-34/h5-8,17-18H,2-4,9-16H2,1H3,(H2,29,30,31,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047482

(CHEMBL3319220)Show SMILES CCOc1ccc(Cl)c(c1)C(=O)NC(=O)Nc1nc2ccc(cc2s1)S(=O)(=O)CCCN1CCN(C)CC1 Show InChI InChI=1S/C25H30ClN5O5S2/c1-3-36-17-5-7-20(26)19(15-17)23(32)28-24(33)29-25-27-21-8-6-18(16-22(21)37-25)38(34,35)14-4-9-31-12-10-30(2)11-13-31/h5-8,15-16H,3-4,9-14H2,1-2H3,(H2,27,28,29,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047431
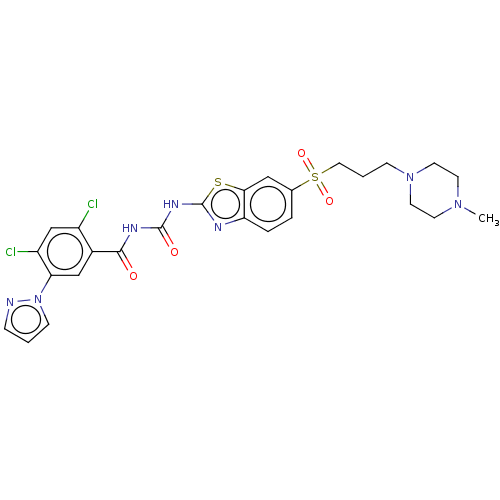
(CHEMBL3319397)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(c(Cl)cc4Cl)-n4cccn4)sc3c2)CC1 Show InChI InChI=1S/C26H27Cl2N7O4S2/c1-33-9-11-34(12-10-33)7-3-13-41(38,39)17-4-5-21-23(14-17)40-26(30-21)32-25(37)31-24(36)18-15-22(20(28)16-19(18)27)35-8-2-6-29-35/h2,4-6,8,14-16H,3,7,9-13H2,1H3,(H2,30,31,32,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047483

(CHEMBL3319219)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)C4CC4)sc3c2)CC1 Show InChI InChI=1S/C26H30ClN5O4S2/c1-31-10-12-32(13-11-31)9-2-14-38(35,36)19-6-8-22-23(16-19)37-26(28-22)30-25(34)29-24(33)20-15-18(17-3-4-17)5-7-21(20)27/h5-8,15-17H,2-4,9-14H2,1H3,(H2,28,29,30,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047688

(CHEMBL3319407)Show SMILES CN1CCC(CC1)S(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(N4CCCC4)c(cc3Cl)C#N)sc2c1 Show InChI InChI=1S/C26H27ClN6O4S2/c1-32-10-6-17(7-11-32)39(36,37)18-4-5-21-23(13-18)38-26(29-21)31-25(35)30-24(34)19-14-22(33-8-2-3-9-33)16(15-28)12-20(19)27/h4-5,12-14,17H,2-3,6-11H2,1H3,(H2,29,30,31,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448706
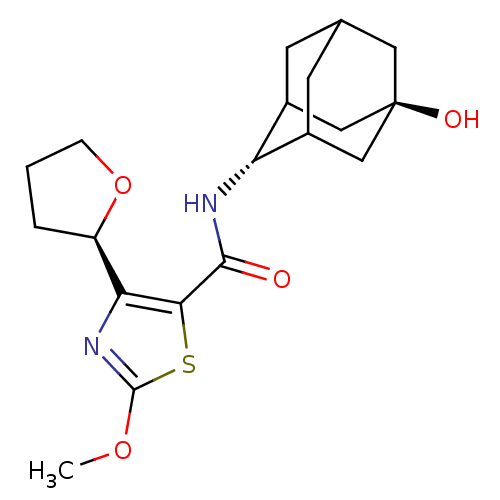
(CHEMBL3127854)Show SMILES COc1nc([C@H]2CCCO2)c(s1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:22.25,wD:15.16,5.4,TLB:14:15:19:21.22.24,25:22:15.16.17:19,THB:25:16:19:21.22.24,24:22:15:17.18.19,24:18:15:21.25.22,23:22:15:17.18.19,23:22:15.16.17:19,(.87,-30.81,;2.03,-29.8,;3.48,-30.3,;3.94,-31.77,;5.48,-31.79,;6.36,-33.05,;5.87,-34.5,;7.1,-35.43,;8.36,-34.54,;7.91,-33.07,;5.97,-30.33,;4.74,-29.41,;7.31,-29.56,;7.3,-28.02,;8.64,-30.32,;9.97,-29.55,;11.47,-29.16,;11.5,-27.57,;12.56,-26.36,;11.2,-26.81,;11.19,-28.3,;12.5,-28.81,;13.91,-28.49,;15.45,-28.45,;13.95,-26.96,;12.87,-29.75,)| Show InChI InChI=1S/C19H26N2O4S/c1-24-18-21-15(13-3-2-4-25-13)16(26-18)17(22)20-14-11-5-10-6-12(14)9-19(23,7-10)8-11/h10-14,23H,2-9H2,1H3,(H,20,22)/t10?,11?,12?,13-,14-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448693
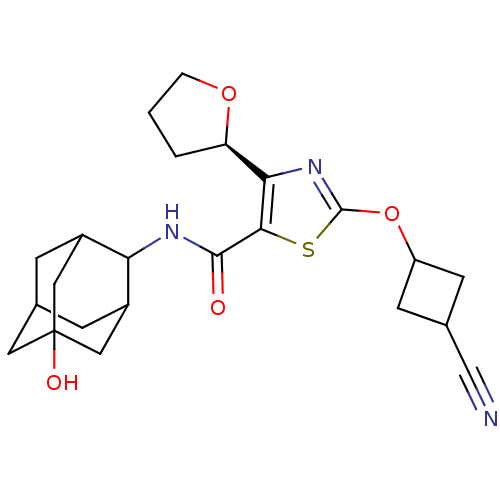
(CHEMBL3127857)Show SMILES OC12CC3CC(C1)C(NC(=O)c1sc(OC4CC(C4)C#N)nc1[C@H]1CCCO1)C(C3)C2 |r,wD:23.25,TLB:8:7:29:30.1.2,6:1:7.5.4:29,THB:6:5:29:30.1.2,2:1:7:4.3.29,2:3:7:30.6.1,0:1:7:4.3.29,0:1:7.5.4:29,(20.14,-39,;18.6,-39.04,;18.64,-37.51,;17.25,-36.9,;16.19,-38.12,;16.16,-39.71,;17.56,-40.3,;14.66,-40.1,;13.33,-40.87,;12,-40.11,;11.99,-38.57,;10.66,-40.88,;9.43,-39.96,;8.17,-40.84,;6.72,-40.34,;5.61,-41.41,;4.08,-41.4,;4.06,-42.94,;5.6,-42.96,;2.95,-44.01,;1.85,-45.09,;8.63,-42.31,;10.17,-42.34,;11.05,-43.6,;10.56,-45.05,;11.79,-45.98,;13.05,-45.09,;12.6,-43.62,;15.88,-38.85,;15.89,-37.35,;17.19,-39.36,)| Show InChI InChI=1S/C23H29N3O4S/c24-11-13-6-16(7-13)30-22-26-19(17-2-1-3-29-17)20(31-22)21(27)25-18-14-4-12-5-15(18)10-23(28,8-12)9-14/h12-18,28H,1-10H2,(H,25,27)/t12?,13?,14?,15?,16?,17-,18?,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047426
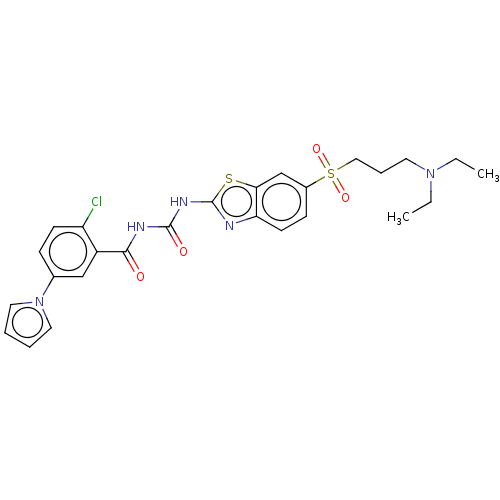
(CHEMBL3319400)Show SMILES CCN(CC)CCCS(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(ccc3Cl)-n3cccc3)sc2c1 Show InChI InChI=1S/C26H28ClN5O4S2/c1-3-31(4-2)12-7-15-38(35,36)19-9-11-22-23(17-19)37-26(28-22)30-25(34)29-24(33)20-16-18(8-10-21(20)27)32-13-5-6-14-32/h5-6,8-11,13-14,16-17H,3-4,7,12,15H2,1-2H3,(H2,28,29,30,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448731
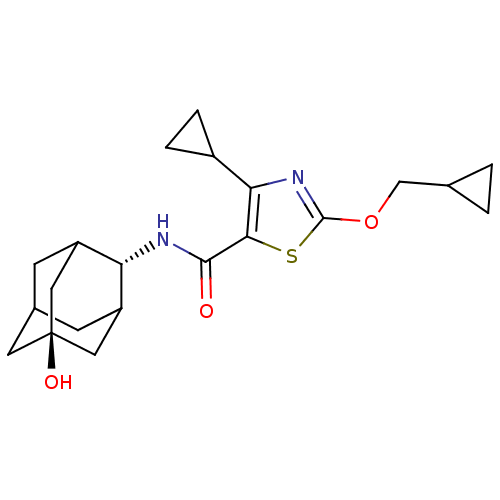
(CHEMBL3127868)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1sc(OCC4CC4)nc1C1CC1)C(C3)C2 |r,wU:1.0,wD:7.8,TLB:8:7:25:26.1.2,6:1:7.5.4:25,THB:6:5:25:26.1.2,2:1:7:4.3.25,2:3:7:26.6.1,0:1:7:4.3.25,0:1:7.5.4:25,(19.29,-27.92,;17.75,-27.95,;17.79,-26.42,;16.4,-25.82,;15.34,-27.03,;15.31,-28.62,;16.72,-29.21,;13.81,-29.01,;12.48,-29.79,;11.15,-29.02,;11.14,-27.48,;9.82,-29.8,;8.59,-28.87,;7.33,-29.76,;5.87,-29.26,;4.71,-30.27,;3.25,-29.77,;2.23,-28.61,;1.73,-30.06,;7.78,-31.23,;9.32,-31.26,;10.21,-32.51,;10.34,-34.04,;11.6,-33.16,;15.03,-27.76,;15.04,-26.27,;16.35,-28.27,)| Show InChI InChI=1S/C21H28N2O3S/c24-19(22-16-14-5-12-6-15(16)9-21(25,7-12)8-14)18-17(13-3-4-13)23-20(27-18)26-10-11-1-2-11/h11-16,25H,1-10H2,(H,22,24)/t12?,14?,15?,16-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448704
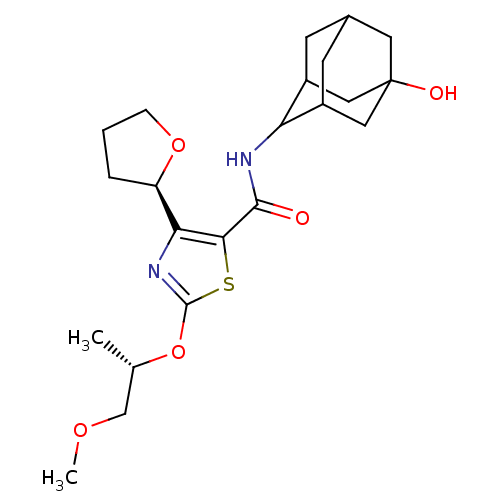
(CHEMBL3127856)Show SMILES COC[C@H](C)Oc1nc([C@H]2CCCO2)c(s1)C(=O)NC1C2CC3CC1CC(O)(C3)C2 |r,wU:3.3,wD:9.8,TLB:18:19:23:25.26.28,29:26:19.20.21:23,THB:29:20:23:25.26.28,28:26:19:21.22.23,28:22:19:25.29.26,27:26:19:21.22.23,27:26:19.20.21:23,(31.36,-30.47,;32.82,-30.97,;33.98,-29.96,;35.44,-30.46,;35.73,-31.97,;36.6,-29.45,;38.06,-29.95,;38.51,-31.42,;40.05,-31.44,;40.93,-32.7,;40.44,-34.15,;41.67,-35.08,;42.93,-34.19,;42.48,-32.72,;40.55,-29.98,;39.32,-29.06,;41.88,-29.21,;41.87,-27.67,;43.21,-29.97,;44.54,-29.2,;46.04,-28.81,;46.07,-27.22,;47.13,-26.01,;45.78,-26.46,;45.76,-27.95,;47.08,-28.46,;48.48,-28.14,;50.02,-28.1,;48.52,-26.61,;47.45,-29.4,)| Show InChI InChI=1S/C22H32N2O5S/c1-12(11-27-2)29-21-24-18(16-4-3-5-28-16)19(30-21)20(25)23-17-14-6-13-7-15(17)10-22(26,8-13)9-14/h12-17,26H,3-11H2,1-2H3,(H,23,25)/t12-,13?,14?,15?,16+,17?,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047474

(CHEMBL3319228)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4ccc(cc4Cl)-n4ccc(C)n4)sc3c2)CC1 Show InChI InChI=1S/C27H30ClN7O4S2/c1-18-8-10-35(32-18)19-4-6-21(22(28)16-19)25(36)30-26(37)31-27-29-23-7-5-20(17-24(23)40-27)41(38,39)15-3-9-34-13-11-33(2)12-14-34/h4-8,10,16-17H,3,9,11-15H2,1-2H3,(H2,29,30,31,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448705
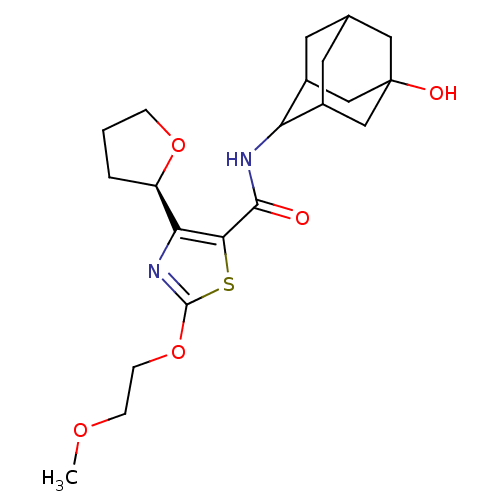
(CHEMBL3127855)Show SMILES COCCOc1nc([C@H]2CCCO2)c(s1)C(=O)NC1C2CC3CC1CC(O)(C3)C2 |r,wD:8.7,TLB:17:18:22:24.25.27,28:25:18.19.20:22,THB:28:19:22:24.25.27,27:25:18:20.21.22,27:21:18:24.28.25,26:25:18:20.21.22,26:25:18.19.20:22,(13.52,-30.59,;14.98,-31.09,;16.14,-30.08,;17.6,-30.58,;18.76,-29.57,;20.21,-30.07,;20.66,-31.54,;22.2,-31.57,;23.09,-32.83,;22.6,-34.28,;23.83,-35.2,;25.09,-34.32,;24.64,-32.85,;22.7,-30.11,;21.47,-29.18,;24.03,-29.33,;24.03,-27.79,;25.37,-30.1,;26.7,-29.32,;28.2,-28.94,;28.22,-27.34,;29.29,-26.13,;27.93,-26.58,;27.92,-28.07,;29.23,-28.58,;30.64,-28.26,;32.18,-28.23,;30.68,-26.73,;29.6,-29.52,)| Show InChI InChI=1S/C21H30N2O5S/c1-26-5-6-28-20-23-17(15-3-2-4-27-15)18(29-20)19(24)22-16-13-7-12-8-14(16)11-21(25,9-12)10-13/h12-16,25H,2-11H2,1H3,(H,22,24)/t12?,13?,14?,15-,16?,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047475
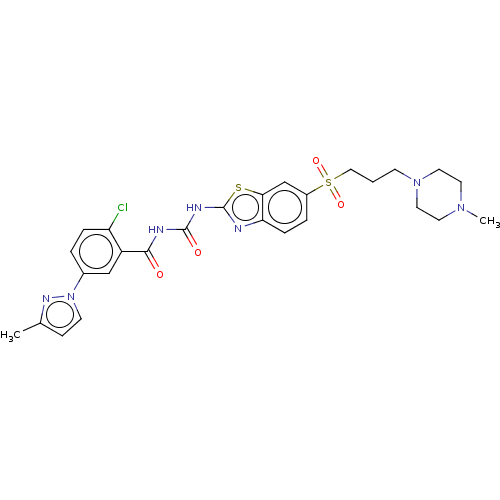
(CHEMBL3319227)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)-n4ccc(C)n4)sc3c2)CC1 Show InChI InChI=1S/C27H30ClN7O4S2/c1-18-8-10-35(32-18)19-4-6-22(28)21(16-19)25(36)30-26(37)31-27-29-23-7-5-20(17-24(23)40-27)41(38,39)15-3-9-34-13-11-33(2)12-14-34/h4-8,10,16-17H,3,9,11-15H2,1-2H3,(H2,29,30,31,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047428
(CHEMBL3319399)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc5n(C)ccc5cc4Cl)sc3c2)CC1 Show InChI InChI=1S/C26H29ClN6O4S2/c1-31-9-11-33(12-10-31)7-3-13-39(36,37)18-4-5-21-23(15-18)38-26(28-21)30-25(35)29-24(34)19-16-22-17(14-20(19)27)6-8-32(22)2/h4-6,8,14-16H,3,7,9-13H2,1-2H3,(H2,28,29,30,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047432

(CHEMBL3319396)Show SMILES COc1cc(Cl)c(cc1-n1cccn1)C(=O)NC(=O)Nc1nc2ccc(cc2s1)S(=O)(=O)CCCN1CCN(C)CC1 Show InChI InChI=1S/C27H30ClN7O5S2/c1-33-10-12-34(13-11-33)8-4-14-42(38,39)18-5-6-21-24(15-18)41-27(30-21)32-26(37)31-25(36)19-16-22(35-9-3-7-29-35)23(40-2)17-20(19)28/h3,5-7,9,15-17H,4,8,10-14H2,1-2H3,(H2,30,31,32,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047478

(CHEMBL3319225)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)-n4cccn4)sc3c2)CC1 Show InChI InChI=1S/C26H28ClN7O4S2/c1-32-11-13-33(14-12-32)9-3-15-40(37,38)19-5-7-22-23(17-19)39-26(29-22)31-25(36)30-24(35)20-16-18(4-6-21(20)27)34-10-2-8-28-34/h2,4-8,10,16-17H,3,9,11-15H2,1H3,(H2,29,30,31,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047479
(CHEMBL3319224)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)-c4ccccn4)sc3c2)CC1 Show InChI InChI=1S/C28H29ClN6O4S2/c1-34-12-14-35(15-13-34)11-4-16-41(38,39)20-7-9-24-25(18-20)40-28(31-24)33-27(37)32-26(36)21-17-19(6-8-22(21)29)23-5-2-3-10-30-23/h2-3,5-10,17-18H,4,11-16H2,1H3,(H2,31,32,33,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047418
(CHEMBL3319223)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)N4CCOCC4)sc3c2)CC1 Show InChI InChI=1S/C27H33ClN6O5S2/c1-32-8-10-33(11-9-32)7-2-16-41(37,38)20-4-6-23-24(18-20)40-27(29-23)31-26(36)30-25(35)21-17-19(3-5-22(21)28)34-12-14-39-15-13-34/h3-6,17-18H,2,7-16H2,1H3,(H2,29,30,31,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Canis familiaris) | BDBM50395256
(CHEMBL2163587)Show SMILES COc1cccc2c3CN(CCc3[nH]c12)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H28N4O3/c1-31-20-8-4-7-15-18-13-28(12-9-19(18)26-21(15)20)23(30)17-6-3-2-5-16(17)22(29)27-24(14-25)10-11-24/h4,7-8,16-17,26H,2-3,5-6,9-13H2,1H3,(H,27,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of dog recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047687
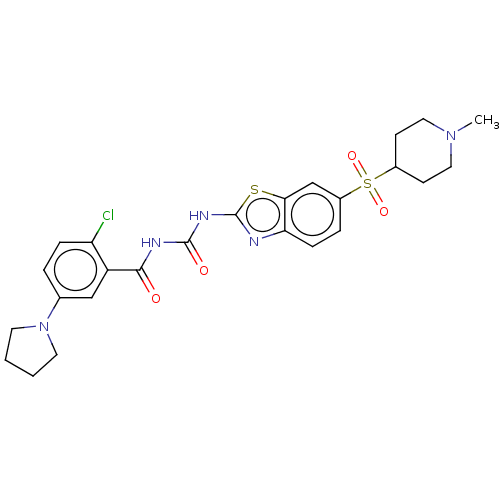
(CHEMBL3319406)Show SMILES CN1CCC(CC1)S(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(ccc3Cl)N3CCCC3)sc2c1 Show InChI InChI=1S/C25H28ClN5O4S2/c1-30-12-8-17(9-13-30)37(34,35)18-5-7-21-22(15-18)36-25(27-21)29-24(33)28-23(32)19-14-16(4-6-20(19)26)31-10-2-3-11-31/h4-7,14-15,17H,2-3,8-13H2,1H3,(H2,27,28,29,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50225079

(CHEMBL250569 | N-((S)-2-(2-(1-(7-aza-bicyclo[2.2.1...)Show SMILES C[C@H](CNC(=O)N1CCC(C1)c1ccccc1)c1c([nH]c2sc(cc12)C(C)(C)C(=O)N1C2CCC1CC2)-c1cc(C)cc(C)c1 |THB:28:30:32.33:35.36| Show InChI InChI=1S/C38H46N4O2S/c1-23-17-24(2)19-28(18-23)34-33(25(3)21-39-37(44)41-16-15-27(22-41)26-9-7-6-8-10-26)31-20-32(45-35(31)40-34)38(4,5)36(43)42-29-11-12-30(42)14-13-29/h6-10,17-20,25,27,29-30,40H,11-16,21-22H2,1-5H3,(H,39,44)/t25-,27?,29?,30?/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Displacement of [125I]D-Trp from rat GnRH receptor |
Bioorg Med Chem Lett 17: 6448-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.099
BindingDB Entry DOI: 10.7270/Q26D5SQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395236
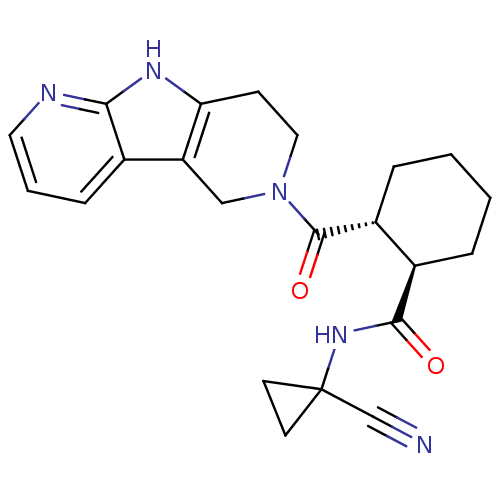
(CHEMBL2163360)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCc2[nH]c3ncccc3c2C1 |r| Show InChI InChI=1S/C22H25N5O2/c23-13-22(8-9-22)26-20(28)15-4-1-2-5-16(15)21(29)27-11-7-18-17(12-27)14-6-3-10-24-19(14)25-18/h3,6,10,15-16H,1-2,4-5,7-9,11-12H2,(H,24,25)(H,26,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395235

(CHEMBL2164670)Show SMILES COc1ccccc1C(NC(=O)[C@@H]1CCCC[C@H]1C(=O)N1CCN(Cc2ccc(F)cc2)CC1)C#N |r| Show InChI InChI=1S/C28H33FN4O3/c1-36-26-9-5-4-8-24(26)25(18-30)31-27(34)22-6-2-3-7-23(22)28(35)33-16-14-32(15-17-33)19-20-10-12-21(29)13-11-20/h4-5,8-13,22-23,25H,2-3,6-7,14-17,19H2,1H3,(H,31,34)/t22-,23-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395254
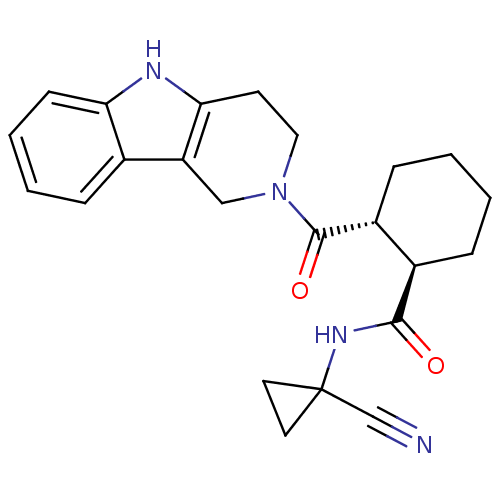
(CHEMBL2164682)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCc2[nH]c3ccccc3c2C1 |r| Show InChI InChI=1S/C23H26N4O2/c24-14-23(10-11-23)26-21(28)16-6-1-2-7-17(16)22(29)27-12-9-20-18(13-27)15-5-3-4-8-19(15)25-20/h3-5,8,16-17,25H,1-2,6-7,9-13H2,(H,26,28)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047416
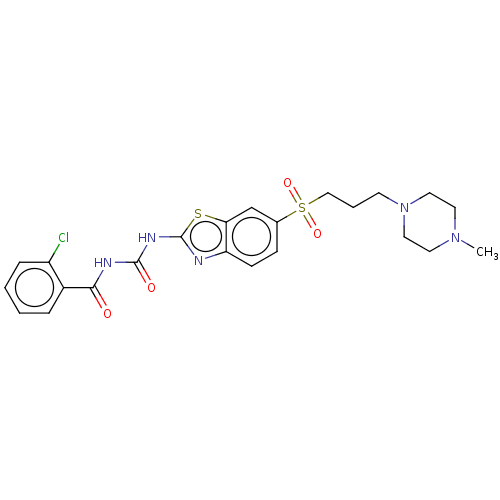
(CHEMBL3319215)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4ccccc4Cl)sc3c2)CC1 Show InChI InChI=1S/C23H26ClN5O4S2/c1-28-10-12-29(13-11-28)9-4-14-35(32,33)16-7-8-19-20(15-16)34-23(25-19)27-22(31)26-21(30)17-5-2-3-6-18(17)24/h2-3,5-8,15H,4,9-14H2,1H3,(H2,25,26,27,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047434
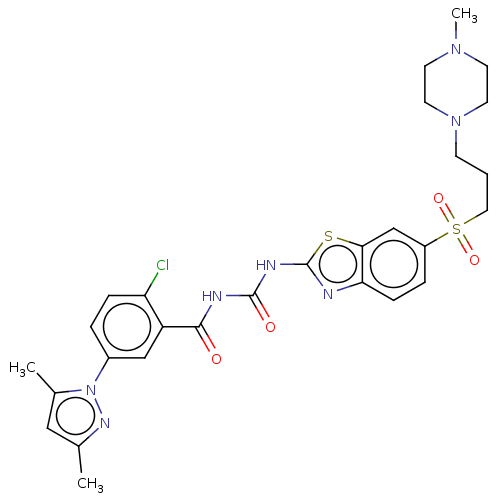
(CHEMBL3319229)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)-n4nc(C)cc4C)sc3c2)CC1 Show InChI InChI=1S/C28H32ClN7O4S2/c1-18-15-19(2)36(33-18)20-5-7-23(29)22(16-20)26(37)31-27(38)32-28-30-24-8-6-21(17-25(24)41-28)42(39,40)14-4-9-35-12-10-34(3)11-13-35/h5-8,15-17H,4,9-14H2,1-3H3,(H2,30,31,32,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448734

(CHEMBL3127865)Show SMILES COC[C@H](C)Oc1nc(C2CC2)c(s1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:24.27,3.3,wD:17.18,TLB:16:17:21:23.24.26,27:24:17.18.19:21,THB:27:18:21:23.24.26,26:24:17:19.20.21,26:20:17:23.27.24,25:24:17:19.20.21,25:24:17.18.19:21,(1.68,-19.21,;3.13,-19.71,;4.29,-18.7,;5.75,-19.2,;6.05,-20.71,;6.91,-18.18,;8.37,-18.68,;8.82,-20.16,;10.36,-20.18,;11.25,-21.43,;11.39,-22.97,;12.65,-22.08,;10.86,-18.72,;9.63,-17.8,;12.19,-17.95,;12.18,-16.41,;13.53,-18.71,;14.86,-17.94,;16.36,-17.55,;16.38,-15.96,;17.44,-14.74,;16.09,-15.2,;16.07,-16.69,;17.39,-17.2,;18.8,-16.88,;20.34,-16.84,;18.84,-15.35,;17.76,-18.14,)| Show InChI InChI=1S/C21H30N2O4S/c1-11(10-26-2)27-20-23-17(13-3-4-13)18(28-20)19(24)22-16-14-5-12-6-15(16)9-21(25,7-12)8-14/h11-16,25H,3-10H2,1-2H3,(H,22,24)/t11-,12?,14?,15?,16-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448733
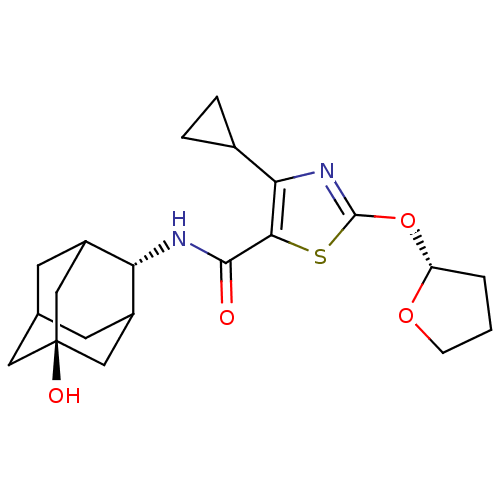
(CHEMBL3127866)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1sc(O[C@@H]4CCCO4)nc1C1CC1)C(C3)C2 |r,wU:1.0,wD:7.8,15.15,TLB:8:7:26:27.1.2,6:1:7.5.4:26,THB:6:5:26:27.1.2,2:1:7:4.3.26,2:3:7:27.6.1,0:1:7:4.3.26,0:1:7.5.4:26,(39.73,-16.8,;38.19,-16.84,;38.23,-15.31,;36.84,-14.7,;35.78,-15.92,;35.75,-17.51,;37.16,-18.1,;34.25,-17.9,;32.92,-18.67,;31.59,-17.91,;31.58,-16.37,;30.26,-18.68,;29.03,-17.76,;27.77,-18.64,;26.31,-18.14,;25.07,-19.06,;25.08,-20.61,;23.62,-21.09,;22.71,-19.85,;23.6,-18.6,;28.22,-20.12,;29.76,-20.14,;30.65,-21.39,;30.78,-22.93,;32.04,-22.04,;35.47,-16.65,;35.49,-15.16,;36.79,-17.16,)| Show InChI InChI=1S/C21H28N2O4S/c24-19(22-16-13-6-11-7-14(16)10-21(25,8-11)9-13)18-17(12-3-4-12)23-20(28-18)27-15-2-1-5-26-15/h11-16,25H,1-10H2,(H,22,24)/t11?,13?,14?,15-,16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448701
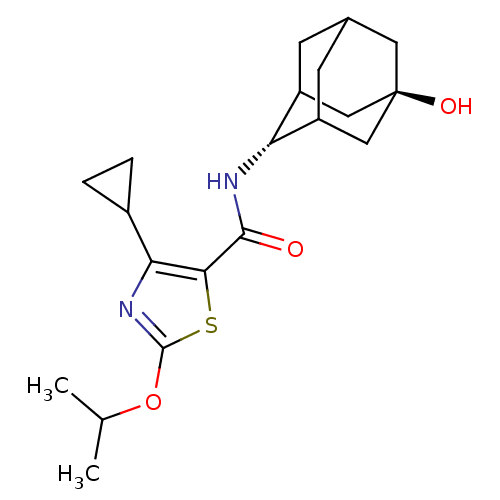
(CHEMBL3127861)Show SMILES CC(C)Oc1nc(C2CC2)c(s1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:22.25,wD:15.16,TLB:14:15:19:21.22.24,25:22:15.16.17:19,THB:25:16:19:21.22.24,24:22:15:17.18.19,24:18:15:21.25.22,23:22:15:17.18.19,23:22:15.16.17:19,(39.67,-54.36,;41.13,-54.86,;41.43,-56.37,;42.29,-53.85,;43.75,-54.35,;44.2,-55.82,;45.74,-55.85,;46.63,-57.1,;46.77,-58.63,;48.03,-57.75,;46.24,-54.39,;45.01,-53.47,;47.57,-53.61,;47.56,-52.07,;48.91,-54.38,;50.24,-53.6,;51.74,-53.22,;51.76,-51.63,;52.82,-50.41,;51.47,-50.86,;51.45,-52.35,;52.77,-52.87,;54.18,-52.54,;55.71,-52.51,;54.21,-51.01,;53.14,-53.8,)| Show InChI InChI=1S/C20H28N2O3S/c1-10(2)25-19-22-16(12-3-4-12)17(26-19)18(23)21-15-13-5-11-6-14(15)9-20(24,7-11)8-13/h10-15,24H,3-9H2,1-2H3,(H,21,23)/t11?,13?,14?,15-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448697

(CHEMBL3127858)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1sc(OC4CCOCC4)nc1[C@H]1CCCO1)C(C3)C2 |r,wU:1.0,wD:7.8,23.25,TLB:8:7:29:30.1.2,6:1:7.5.4:29,THB:6:5:29:30.1.2,2:1:7:4.3.29,2:3:7:30.6.1,0:1:7:4.3.29,0:1:7.5.4:29,(41.98,-39.31,;40.44,-39.34,;40.47,-37.81,;39.08,-37.21,;38.02,-38.42,;38,-40.01,;39.4,-40.6,;36.5,-40.4,;35.17,-41.18,;33.83,-40.41,;33.82,-38.87,;32.5,-41.19,;31.27,-40.26,;30.01,-41.15,;28.55,-40.65,;27.22,-41.43,;25.89,-40.66,;24.57,-41.43,;24.57,-42.97,;25.91,-43.74,;27.24,-42.97,;30.46,-42.62,;32,-42.64,;32.88,-43.9,;32.39,-45.36,;33.62,-46.28,;34.88,-45.4,;34.43,-43.92,;37.71,-39.15,;37.73,-37.66,;39.03,-39.66,)| Show InChI InChI=1S/C23H32N2O5S/c26-21(24-18-14-8-13-9-15(18)12-23(27,10-13)11-14)20-19(17-2-1-5-29-17)25-22(31-20)30-16-3-6-28-7-4-16/h13-18,27H,1-12H2,(H,24,26)/t13?,14?,15?,17-,18-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448702
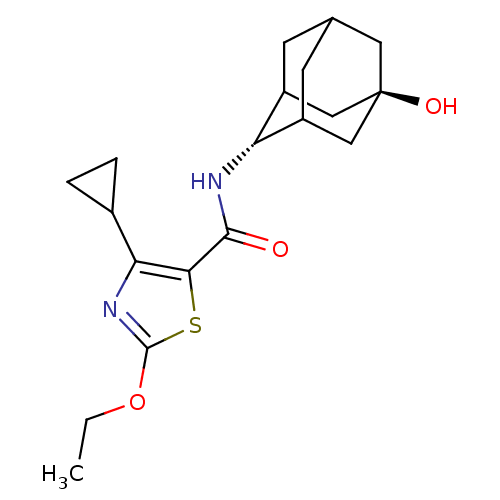
(CHEMBL3127860)Show SMILES CCOc1nc(C2CC2)c(s1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.24,wD:14.15,TLB:13:14:18:20.21.23,24:21:14.15.16:18,THB:24:15:18:20.21.23,23:21:14:16.17.18,23:17:14:20.24.21,22:21:14:16.17.18,22:21:14.15.16:18,(18.99,-53.98,;20.45,-54.48,;21.61,-53.47,;23.07,-53.97,;23.52,-55.44,;25.06,-55.47,;25.95,-56.72,;26.09,-58.25,;27.35,-57.37,;25.56,-54.01,;24.33,-53.09,;26.89,-53.23,;26.88,-51.69,;28.23,-54,;29.56,-53.22,;31.06,-52.84,;31.08,-51.25,;32.14,-50.03,;30.79,-50.48,;30.77,-51.97,;32.09,-52.49,;33.5,-52.16,;35.04,-52.13,;33.53,-50.63,;32.46,-53.42,)| Show InChI InChI=1S/C19H26N2O3S/c1-2-24-18-21-15(11-3-4-11)16(25-18)17(22)20-14-12-5-10-6-13(14)9-19(23,7-10)8-12/h10-14,23H,2-9H2,1H3,(H,20,22)/t10?,12?,13?,14-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395238
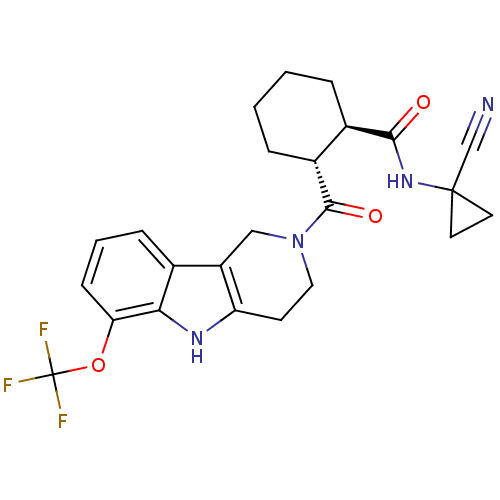
(CHEMBL2163589)Show SMILES FC(F)(F)Oc1cccc2c3CN(CCc3[nH]c12)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H25F3N4O3/c25-24(26,27)34-19-7-3-6-14-17-12-31(11-8-18(17)29-20(14)19)22(33)16-5-2-1-4-15(16)21(32)30-23(13-28)9-10-23/h3,6-7,15-16,29H,1-2,4-5,8-12H2,(H,30,32)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395241
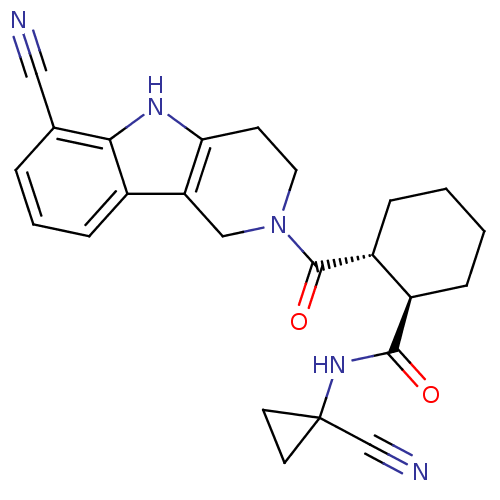
(CHEMBL2163585)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCc2[nH]c3c(cccc3c2C1)C#N |r| Show InChI InChI=1S/C24H25N5O2/c25-12-15-4-3-7-16-19-13-29(11-8-20(19)27-21(15)16)23(31)18-6-2-1-5-17(18)22(30)28-24(14-26)9-10-24/h3-4,7,17-18,27H,1-2,5-6,8-11,13H2,(H,28,30)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395244
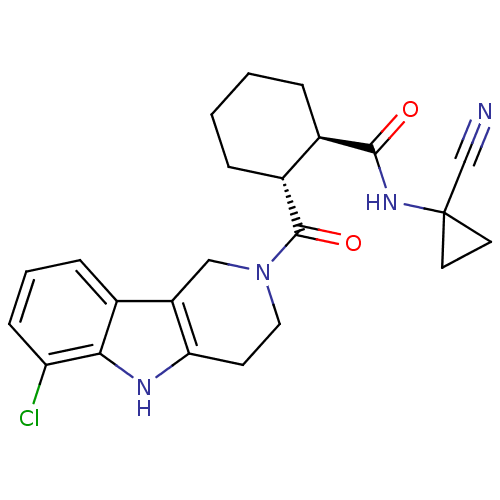
(CHEMBL2163582)Show SMILES Clc1cccc2c3CN(CCc3[nH]c12)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H25ClN4O2/c24-18-7-3-6-14-17-12-28(11-8-19(17)26-20(14)18)22(30)16-5-2-1-4-15(16)21(29)27-23(13-25)9-10-23/h3,6-7,15-16,26H,1-2,4-5,8-12H2,(H,27,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395228
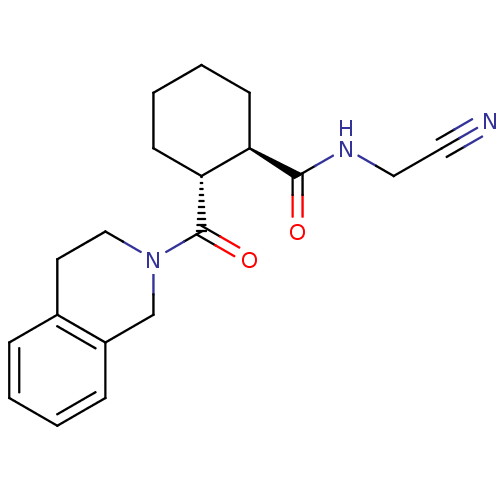
(CHEMBL2164674)Show SMILES O=C(NCC#N)[C@@H]1CCCC[C@H]1C(=O)N1CCc2ccccc2C1 |r| Show InChI InChI=1S/C19H23N3O2/c20-10-11-21-18(23)16-7-3-4-8-17(16)19(24)22-12-9-14-5-1-2-6-15(14)13-22/h1-2,5-6,16-17H,3-4,7-9,11-13H2,(H,21,23)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50225079

(CHEMBL250569 | N-((S)-2-(2-(1-(7-aza-bicyclo[2.2.1...)Show SMILES C[C@H](CNC(=O)N1CCC(C1)c1ccccc1)c1c([nH]c2sc(cc12)C(C)(C)C(=O)N1C2CCC1CC2)-c1cc(C)cc(C)c1 |THB:28:30:32.33:35.36| Show InChI InChI=1S/C38H46N4O2S/c1-23-17-24(2)19-28(18-23)34-33(25(3)21-39-37(44)41-16-15-27(22-41)26-9-7-6-8-10-26)31-20-32(45-35(31)40-34)38(4,5)36(43)42-29-11-12-30(42)14-13-29/h6-10,17-20,25,27,29-30,40H,11-16,21-22H2,1-5H3,(H,39,44)/t25-,27?,29?,30?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Displacement of [125I]D-Trp from human GnRH receptor expressed in HEK cells |
Bioorg Med Chem Lett 17: 6448-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.099
BindingDB Entry DOI: 10.7270/Q26D5SQF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448699
(CHEMBL3127863)Show SMILES COCCOc1nc(C2CC2)c(s1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:23.26,wD:16.17,TLB:15:16:20:22.23.25,26:23:16.17.18:20,THB:26:17:20:22.23.25,25:23:16:18.19.20,25:19:16:22.26.23,24:23:16:18.19.20,24:23:16.17.18:20,(21.81,-7.27,;23.26,-7.77,;24.42,-6.76,;25.88,-7.26,;27.04,-6.24,;28.5,-6.74,;28.95,-8.22,;30.49,-8.24,;31.38,-9.49,;31.52,-11.03,;32.78,-10.14,;30.99,-6.78,;29.76,-5.86,;32.32,-6.01,;32.31,-4.47,;33.66,-6.77,;34.99,-6,;36.49,-5.61,;36.51,-4.02,;37.57,-2.8,;36.22,-3.26,;36.2,-4.75,;37.52,-5.26,;38.93,-4.94,;40.47,-4.9,;38.96,-3.41,;37.89,-6.2,)| Show InChI InChI=1S/C20H28N2O4S/c1-25-4-5-26-19-22-16(12-2-3-12)17(27-19)18(23)21-15-13-6-11-7-14(15)10-20(24,8-11)9-13/h11-15,24H,2-10H2,1H3,(H,21,23)/t11?,13?,14?,15-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047423

(CHEMBL3319402)Show SMILES CCN(CC)CCCS(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(ccc3Cl)N3CCOCC3)sc2c1 Show InChI InChI=1S/C26H32ClN5O5S2/c1-3-31(4-2)10-5-15-39(35,36)19-7-9-22-23(17-19)38-26(28-22)30-25(34)29-24(33)20-16-18(6-8-21(20)27)32-11-13-37-14-12-32/h6-9,16-17H,3-5,10-15H2,1-2H3,(H2,28,29,30,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50397138

(CHEMBL2172003)Show SMILES COc1cc(ccc1C1CC1)N1CCN([C@H](C)C1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H36N4O3/c1-18-16-30(20-9-10-21(19-7-8-19)24(15-20)34-2)13-14-31(18)26(33)23-6-4-3-5-22(23)25(32)29-27(17-28)11-12-27/h9-10,15,18-19,22-23H,3-8,11-14,16H2,1-2H3,(H,29,32)/t18-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin-k using Z-Phe-Arg-AMC as substrate preincubated for 15 mins measured after 1 hr by QFRET assay |
J Med Chem 55: 8827-37 (2012)
Article DOI: 10.1021/jm301119s
BindingDB Entry DOI: 10.7270/Q2HX1DS7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395250

(CHEMBL2164687)Show SMILES Clc1ccc2[nH]c3CCN(Cc3c2c1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H25ClN4O2/c24-14-5-6-19-17(11-14)18-12-28(10-7-20(18)26-19)22(30)16-4-2-1-3-15(16)21(29)27-23(13-25)8-9-23/h5-6,11,15-16,26H,1-4,7-10,12H2,(H,27,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395251
(CHEMBL2164686)Show SMILES Cn1c2CCN(Cc2c2c(F)c(F)ccc12)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H26F2N4O2/c1-29-18-8-11-30(12-16(18)20-19(29)7-6-17(25)21(20)26)23(32)15-5-3-2-4-14(15)22(31)28-24(13-27)9-10-24/h6-7,14-15H,2-5,8-12H2,1H3,(H,28,31)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50225090

(CHEMBL399856 | isopropyl ((S)-2-(2-(1-(7-aza-bicyc...)Show SMILES CC(C)OC(=O)N=C(NC[C@@H](C)c1c([nH]c2sc(cc12)C(C)(C)C(=O)N1C2CCC1CC2)-c1cc(C)cc(C)c1)N1CCC(C1)c1ccncc1 |w:6.5,THB:23:25:27.28:30.31| Show InChI InChI=1S/C41H52N6O3S/c1-24(2)50-40(49)45-39(46-17-14-29(23-46)28-12-15-42-16-13-28)43-22-27(5)35-33-21-34(41(6,7)38(48)47-31-8-9-32(47)11-10-31)51-37(33)44-36(35)30-19-25(3)18-26(4)20-30/h12-13,15-16,18-21,24,27,29,31-32,44H,8-11,14,17,22-23H2,1-7H3,(H,43,45,49)/t27-,29?,31?,32?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Antagonist activity at GnRH receptor expressed in AP Han Wistar rat primary pitutary cells assessed as inhibition of GnRH stimulated LH release after... |
Bioorg Med Chem Lett 17: 6448-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.099
BindingDB Entry DOI: 10.7270/Q26D5SQF |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50225078

(CHEMBL400739 | methyl ((S)-2-(2-(1-(7-aza-bicyclo[...)Show SMILES COC(=O)N=C(NC[C@@H](C)c1c([nH]c2sc(cc12)C(C)(C)C(=O)N1C2CCC1CC2)-c1cc(C)cc(C)c1)N1CCC(C1)c1ccncc1 |w:4.3,THB:21:23:25.26:28.29| Show InChI InChI=1S/C39H48N6O3S/c1-23-17-24(2)19-28(18-23)34-33(31-20-32(49-35(31)42-34)39(4,5)36(46)45-29-7-8-30(45)10-9-29)25(3)21-41-37(43-38(47)48-6)44-16-13-27(22-44)26-11-14-40-15-12-26/h11-12,14-15,17-20,25,27,29-30,42H,7-10,13,16,21-22H2,1-6H3,(H,41,43,47)/t25-,27?,29?,30?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Antagonist activity at GnRH receptor expressed in AP Han Wistar rat primary pitutary cells assessed as inhibition of GnRH stimulated LH release after... |
Bioorg Med Chem Lett 17: 6448-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.099
BindingDB Entry DOI: 10.7270/Q26D5SQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395243

(CHEMBL2163583)Show SMILES Cc1cccc2c3CN(CCc3[nH]c12)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H28N4O2/c1-15-5-4-8-16-19-13-28(12-9-20(19)26-21(15)16)23(30)18-7-3-2-6-17(18)22(29)27-24(14-25)10-11-24/h4-5,8,17-18,26H,2-3,6-7,9-13H2,1H3,(H,27,29)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50395234

(CHEMBL2164669)Show SMILES Fc1ccc(cc1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC(C#N)c1ccccc1 |r| Show InChI InChI=1S/C26H29FN4O2/c27-20-10-12-21(13-11-20)30-14-16-31(17-15-30)26(33)23-9-5-4-8-22(23)25(32)29-24(18-28)19-6-2-1-3-7-19/h1-3,6-7,10-13,22-24H,4-5,8-9,14-17H2,(H,29,32)/t22-,23-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay |
J Med Chem 55: 6363-74 (2012)
Article DOI: 10.1021/jm3007257
BindingDB Entry DOI: 10.7270/Q2833T5F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data