 Found 50 hits with Last Name = 'dracínský' and Initial = 'm'
Found 50 hits with Last Name = 'dracínský' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439836
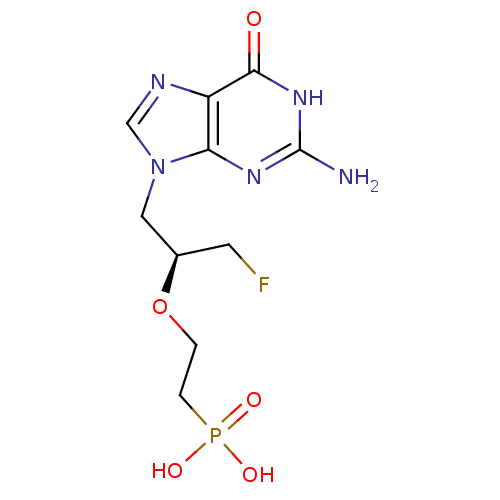
(CHEMBL2420072)Show SMILES Nc1nc2n(C[C@@H](CF)OCCP(O)(O)=O)cnc2c(=O)[nH]1 |r| Show InChI InChI=1S/C10H15FN5O5P/c11-3-6(21-1-2-22(18,19)20)4-16-5-13-7-8(16)14-10(12)15-9(7)17/h5-6H,1-4H2,(H2,18,19,20)(H3,12,14,15,17)/t6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HGPRT expressed in Escherichia coli Sphi606 cells by spectrophotometric analysis |
Eur J Med Chem 67: 81-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.032
BindingDB Entry DOI: 10.7270/Q20Z74Q0 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50049966
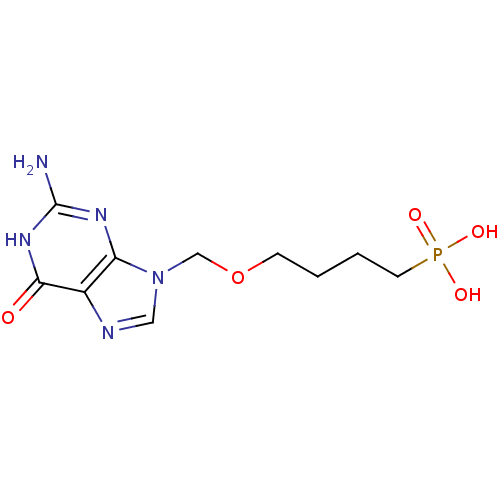
(CHEMBL177948 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C10H16N5O5P/c11-10-13-8-7(9(16)14-10)12-5-15(8)6-20-3-1-2-4-21(17,18)19/h5H,1-4,6H2,(H2,17,18,19)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360117
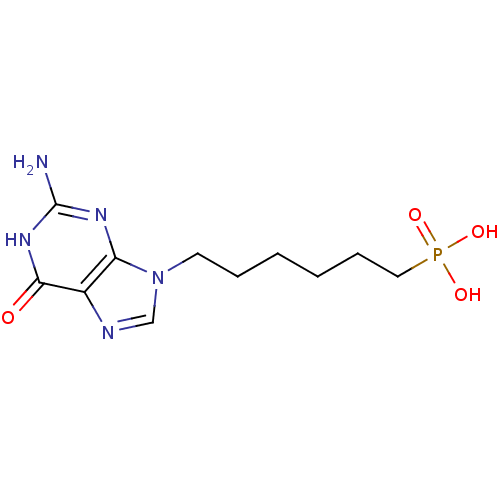
(CHEMBL1928784)Show InChI InChI=1S/C11H18N5O4P/c12-11-14-9-8(10(17)15-11)13-7-16(9)5-3-1-2-4-6-21(18,19)20/h7H,1-6H2,(H2,18,19,20)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50039559
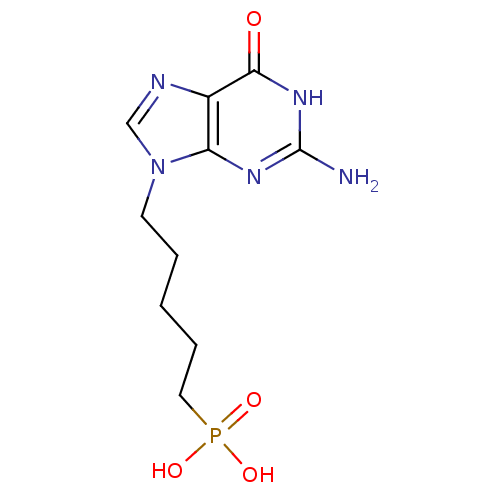
(CHEMBL267803 | [5-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C10H16N5O4P/c11-10-13-8-7(9(16)14-10)12-6-15(8)4-2-1-3-5-20(17,18)19/h6H,1-5H2,(H2,17,18,19)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439833
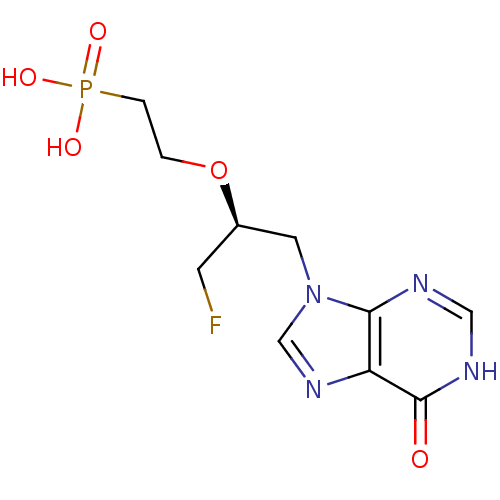
(CHEMBL2420080)Show SMILES OP(O)(=O)CCO[C@H](CF)Cn1cnc2c1nc[nH]c2=O |r| Show InChI InChI=1S/C10H14FN4O5P/c11-3-7(20-1-2-21(17,18)19)4-15-6-14-8-9(15)12-5-13-10(8)16/h5-7H,1-4H2,(H,12,13,16)(H2,17,18,19)/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HGPRT expressed in Escherichia coli Sphi606 cells by spectrophotometric analysis |
Eur J Med Chem 67: 81-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.032
BindingDB Entry DOI: 10.7270/Q20Z74Q0 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360122
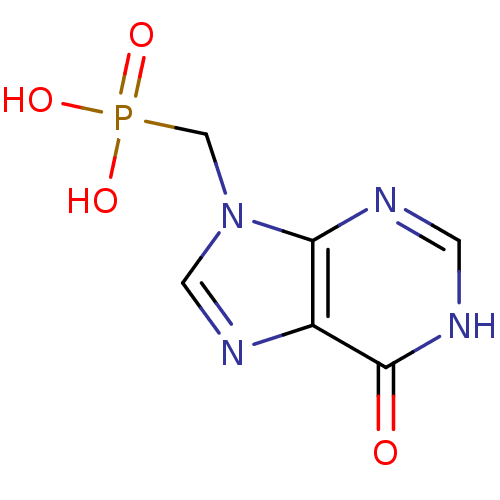
(CHEMBL1928777)Show InChI InChI=1S/C6H7N4O4P/c11-6-4-5(7-1-8-6)10(2-9-4)3-15(12,13)14/h1-2H,3H2,(H,7,8,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360116
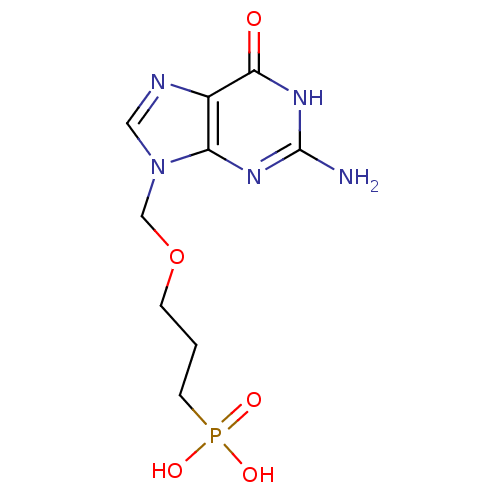
(CHEMBL1928783)Show InChI InChI=1S/C9H14N5O5P/c10-9-12-7-6(8(15)13-9)11-4-14(7)5-19-2-1-3-20(16,17)18/h4H,1-3,5H2,(H2,16,17,18)(H3,10,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360109
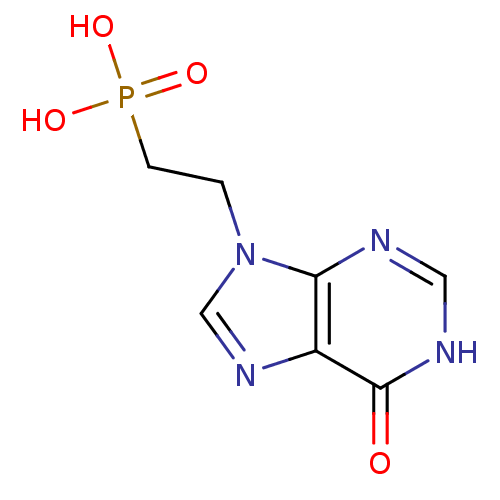
(CHEMBL1928778)Show InChI InChI=1S/C7H9N4O4P/c12-7-5-6(8-3-9-7)11(4-10-5)1-2-16(13,14)15/h3-4H,1-2H2,(H,8,9,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439834
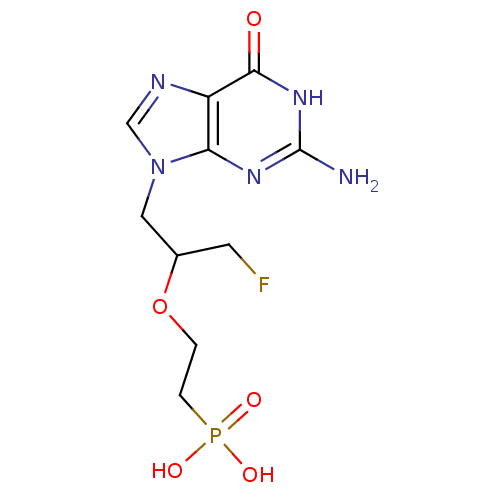
(CHEMBL2420081)Show InChI InChI=1S/C10H15FN5O5P/c11-3-6(21-1-2-22(18,19)20)4-16-5-13-7-8(16)14-10(12)15-9(7)17/h5-6H,1-4H2,(H2,18,19,20)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HGPRT expressed in Escherichia coli Sphi606 cells by spectrophotometric analysis |
Eur J Med Chem 67: 81-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.032
BindingDB Entry DOI: 10.7270/Q20Z74Q0 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50361442
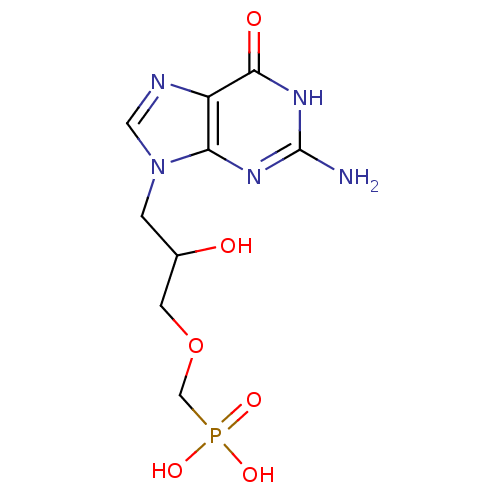
(CHEMBL1934407)Show InChI InChI=1S/C9H14N5O6P/c10-9-12-7-6(8(16)13-9)11-3-14(7)1-5(15)2-20-4-21(17,18)19/h3,5,15H,1-2,4H2,(H2,17,18,19)(H3,10,12,13,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT by spectrophotometric analysis in absence of PPi |
Bioorg Med Chem 20: 1222-30 (2012)
Article DOI: 10.1016/j.bmc.2011.12.034
BindingDB Entry DOI: 10.7270/Q25M665C |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439835
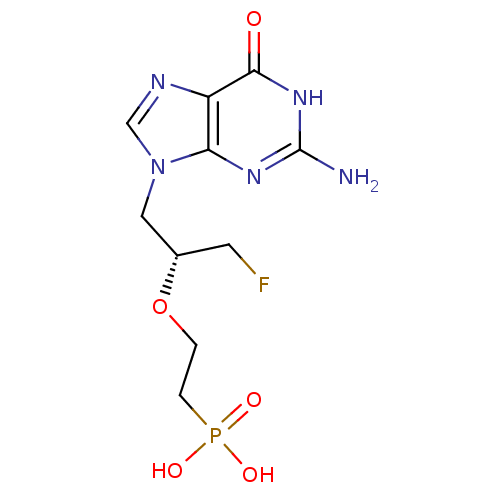
(CHEMBL2420071)Show SMILES Nc1nc2n(C[C@H](CF)OCCP(O)(O)=O)cnc2c(=O)[nH]1 |r| Show InChI InChI=1S/C10H15FN5O5P/c11-3-6(21-1-2-22(18,19)20)4-16-5-13-7-8(16)14-10(12)15-9(7)17/h5-6H,1-4H2,(H2,18,19,20)(H3,12,14,15,17)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HGPRT expressed in Escherichia coli Sphi606 cells by spectrophotometric analysis |
Eur J Med Chem 67: 81-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.032
BindingDB Entry DOI: 10.7270/Q20Z74Q0 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50361443
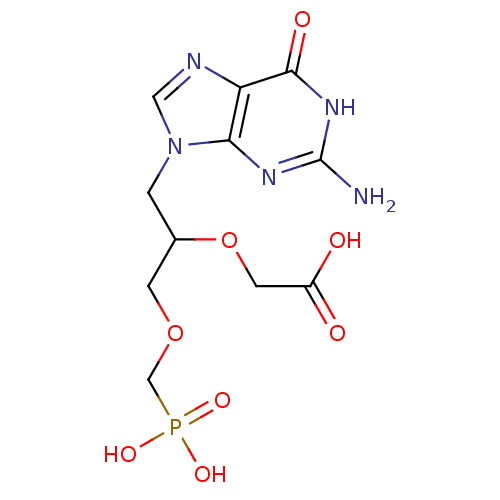
(CHEMBL1934409)Show SMILES Nc1nc2n(CC(COCP(O)(O)=O)OCC(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C11H16N5O8P/c12-11-14-9-8(10(19)15-11)13-4-16(9)1-6(24-3-7(17)18)2-23-5-25(20,21)22/h4,6H,1-3,5H2,(H,17,18)(H2,20,21,22)(H3,12,14,15,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT by spectrophotometric analysis in absence of PPi |
Bioorg Med Chem 20: 1222-30 (2012)
Article DOI: 10.1016/j.bmc.2011.12.034
BindingDB Entry DOI: 10.7270/Q25M665C |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50049962
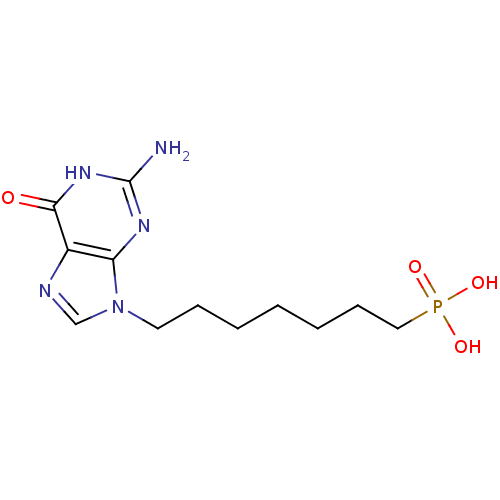
(CHEMBL172844 | [7-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C12H20N5O4P/c13-12-15-10-9(11(18)16-12)14-8-17(10)6-4-2-1-3-5-7-22(19,20)21/h8H,1-7H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439832
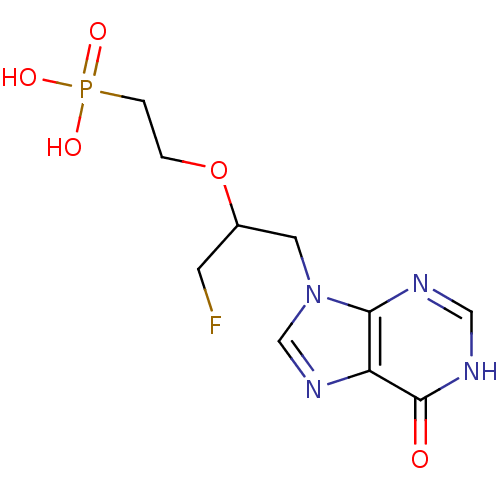
(CHEMBL2420078)Show InChI InChI=1S/C10H14FN4O5P/c11-3-7(20-1-2-21(17,18)19)4-15-6-14-8-9(15)12-5-13-10(8)16/h5-7H,1-4H2,(H,12,13,16)(H2,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HGPRT expressed in Escherichia coli Sphi606 cells by spectrophotometric analysis |
Eur J Med Chem 67: 81-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.032
BindingDB Entry DOI: 10.7270/Q20Z74Q0 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360112

(CHEMBL19784)Show InChI InChI=1S/C9H14N5O4P/c10-9-12-7-6(8(15)13-9)11-5-14(7)3-1-2-4-19(16,17)18/h5H,1-4H2,(H2,16,17,18)(H3,10,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360119
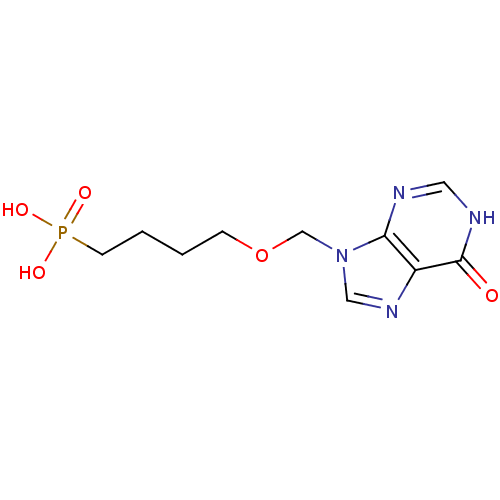
(CHEMBL1928930)Show InChI InChI=1S/C10H15N4O5P/c15-10-8-9(11-5-12-10)14(6-13-8)7-19-3-1-2-4-20(16,17)18/h5-6H,1-4,7H2,(H,11,12,15)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360114
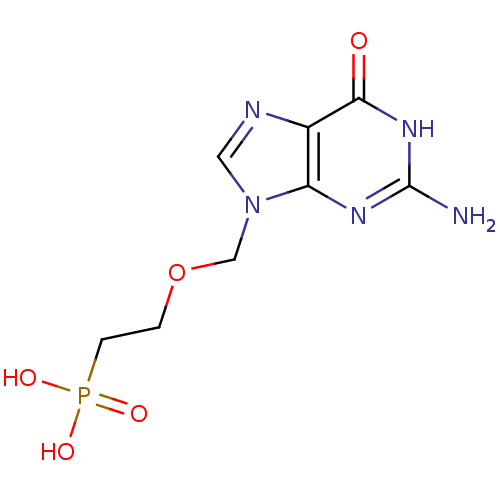
(CHEMBL20283)Show InChI InChI=1S/C8H12N5O5P/c9-8-11-6-5(7(14)12-8)10-3-13(6)4-18-1-2-19(15,16)17/h3H,1-2,4H2,(H2,15,16,17)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360115
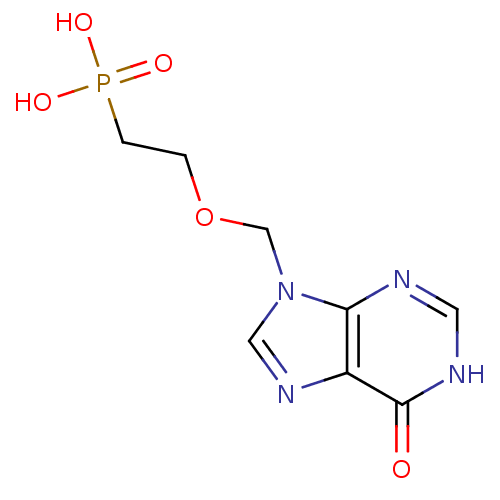
(CHEMBL1928782)Show InChI InChI=1S/C8H11N4O5P/c13-8-6-7(9-3-10-8)12(4-11-6)5-17-1-2-18(14,15)16/h3-4H,1-2,5H2,(H,9,10,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
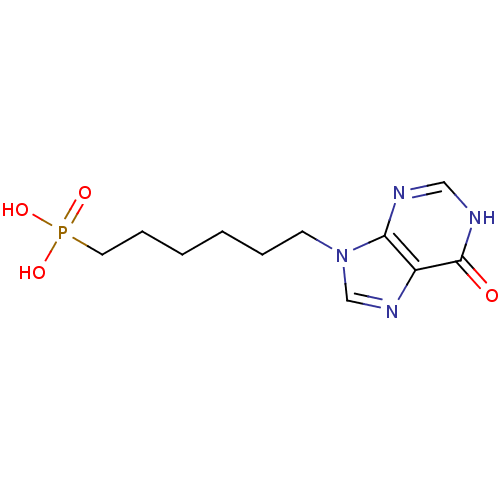
(Homo sapiens (Human)) | BDBM50360118
(CHEMBL1928785)Show InChI InChI=1S/C11H17N4O4P/c16-11-9-10(12-7-13-11)15(8-14-9)5-3-1-2-4-6-20(17,18)19/h7-8H,1-6H2,(H,12,13,16)(H2,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360121

(CHEMBL1928776)Show InChI InChI=1S/C6H8N5O4P/c7-6-9-4-3(5(12)10-6)8-1-11(4)2-16(13,14)15/h1H,2H2,(H2,13,14,15)(H3,7,9,10,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
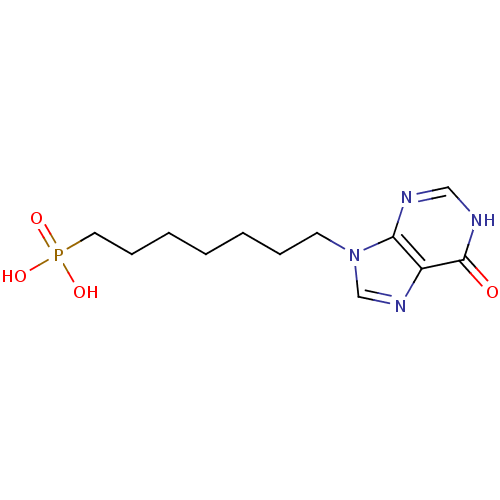
(Homo sapiens (Human)) | BDBM50360120
(CHEMBL1928931)Show InChI InChI=1S/C12H19N4O4P/c17-12-10-11(13-8-14-12)16(9-15-10)6-4-2-1-3-5-7-21(18,19)20/h8-9H,1-7H2,(H,13,14,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
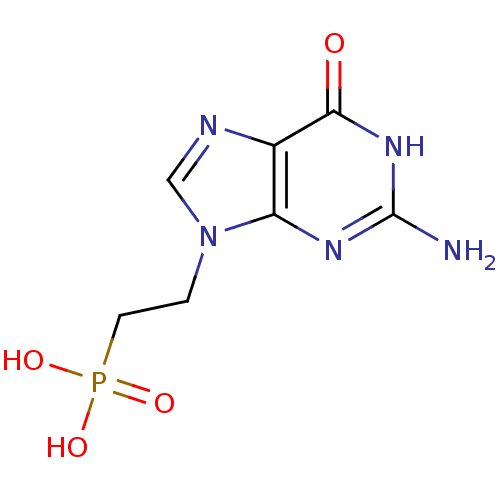
(Homo sapiens (Human)) | BDBM50360108
(CHEMBL1928775)Show InChI InChI=1S/C7H10N5O4P/c8-7-10-5-4(6(13)11-7)9-3-12(5)1-2-17(14,15)16/h3H,1-2H2,(H2,14,15,16)(H3,8,10,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50039555
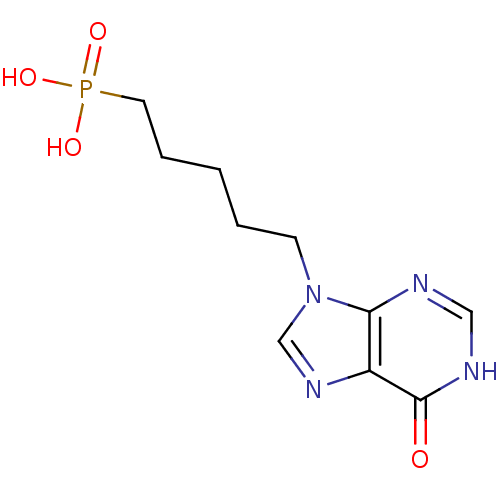
(CHEMBL13130 | [5-(6-Oxo-1,6-dihydro-purin-9-yl)-pe...)Show InChI InChI=1S/C10H15N4O4P/c15-10-8-9(11-6-12-10)14(7-13-8)4-2-1-3-5-19(16,17)18/h6-7H,1-5H2,(H,11,12,15)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360110
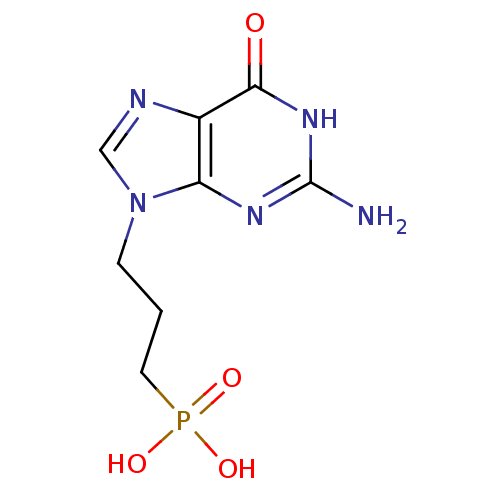
(CHEMBL1928779)Show InChI InChI=1S/C8H12N5O4P/c9-8-11-6-5(7(14)12-8)10-4-13(6)2-1-3-18(15,16)17/h4H,1-3H2,(H2,15,16,17)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360111
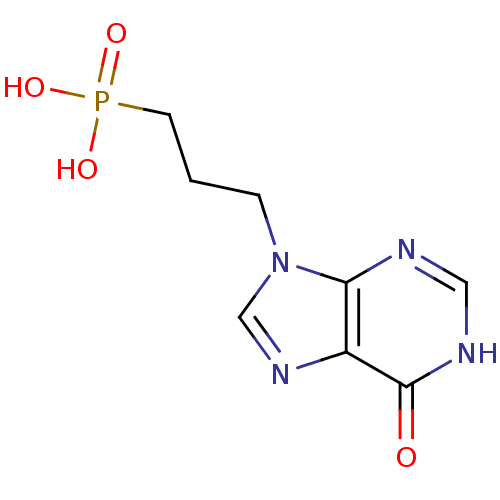
(CHEMBL1928780)Show InChI InChI=1S/C8H11N4O4P/c13-8-6-7(9-4-10-8)12(5-11-6)2-1-3-17(14,15)16/h4-5H,1-3H2,(H,9,10,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50360113
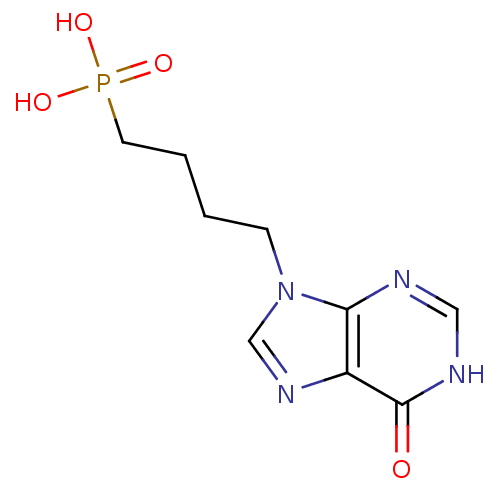
(CHEMBL1928781)Show InChI InChI=1S/C9H13N4O4P/c14-9-7-8(10-5-11-9)13(6-12-7)3-1-2-4-18(15,16)17/h5-6H,1-4H2,(H,10,11,14)(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
Bioorg Med Chem 20: 1076-89 (2012)
Article DOI: 10.1016/j.bmc.2011.11.034
BindingDB Entry DOI: 10.7270/Q2RV0P44 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Antagonist activity at glucocorticoid receptor-LBD in human U2OS cells transfected with Gal4-DBD assessed as inhibition of transactivation activity a... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM19214
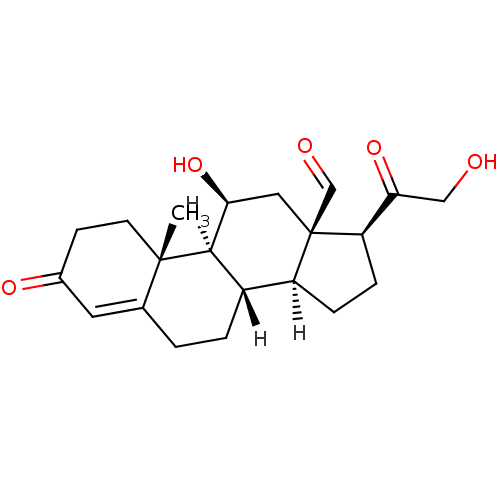
((1S,2R,10S,11S,14S,15R,17S)-17-hydroxy-14-(2-hydro...)Show SMILES [H][C@@]12CC[C@H](C(=O)CO)[C@]1(C[C@H](O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C)C=O |t:21| Show InChI InChI=1S/C21H28O5/c1-20-7-6-13(24)8-12(20)2-3-14-15-4-5-16(18(26)10-22)21(15,11-23)9-17(25)19(14)20/h8,11,14-17,19,22,25H,2-7,9-10H2,1H3/t14-,15-,16+,17-,19+,20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Antagonist activity at mineralocorticoid receptor-LBD in human U2OS cells transfected with Gal4-DBD assessed as inhibition of transactivation activit... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Antagonist activity at ERbeta-LBD in human U2OS cells transfected with Gal4-DBD assessed as inhibition of transactivation activity after 18 hrs by lu... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 21.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha-LBD in human U2OS cells transfected with Gal4-DBD assessed as inhibition of transactivation activity after 18 hrs by l... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417023
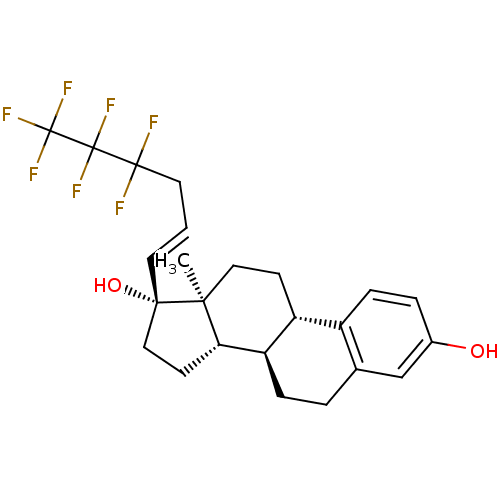
(CHEMBL1258901)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)\C=C\CC(F)(F)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C24H27F7O2/c1-20-11-7-17-16-6-4-15(32)13-14(16)3-5-18(17)19(20)8-12-21(20,33)9-2-10-22(25,26)23(27,28)24(29,30)31/h2,4,6,9,13,17-19,32-33H,3,5,7-8,10-12H2,1H3/b9-2+/t17-,18-,19+,20+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Antagonist activity at glucocorticoid receptor-LBD in human U2OS cells transfected with Gal4-DBD assessed as inhibition of transactivation activity a... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50417023

(CHEMBL1258901)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)\C=C\CC(F)(F)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C24H27F7O2/c1-20-11-7-17-16-6-4-15(32)13-14(16)3-5-18(17)19(20)8-12-21(20,33)9-2-10-22(25,26)23(27,28)24(29,30)31/h2,4,6,9,13,17-19,32-33H,3,5,7-8,10-12H2,1H3/b9-2+/t17-,18-,19+,20+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Antagonist activity at mineralocorticoid receptor-LBD in human U2OS cells transfected with Gal4-DBD assessed as inhibition of transactivation activit... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50417030
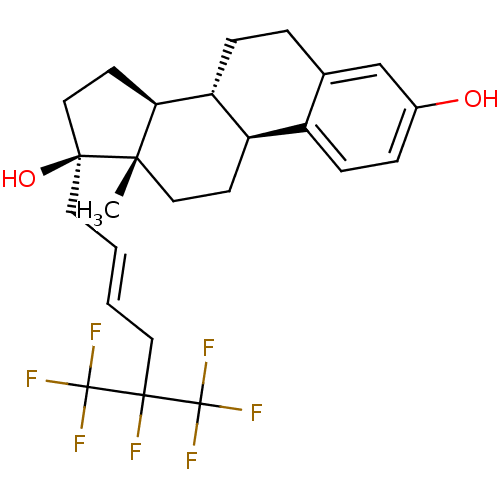
(CHEMBL1257167)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C\C=C\CC(F)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H29F7O2/c1-21-12-8-18-17-7-5-16(33)14-15(17)4-6-19(18)20(21)9-13-22(21,34)10-2-3-11-23(26,24(27,28)29)25(30,31)32/h2-3,5,7,14,18-20,33-34H,4,6,8-13H2,1H3/b3-2+/t18-,19-,20+,21+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Antagonist activity at mineralocorticoid receptor-LBD in human U2OS cells transfected with Gal4-DBD assessed as inhibition of transactivation activit... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417022

(CHEMBL1258783)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)\C=C\CC(F)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H27F7O2/c1-20-11-7-17-16-6-4-15(32)13-14(16)3-5-18(17)19(20)8-12-21(20,33)9-2-10-22(25,23(26,27)28)24(29,30)31/h2,4,6,9,13,17-19,32-33H,3,5,7-8,10-12H2,1H3/b9-2+/t17-,18-,19+,20+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Antagonist activity at glucocorticoid receptor-LBD in human U2OS cells transfected with Gal4-DBD assessed as inhibition of transactivation activity a... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.77 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucife... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50417022

(CHEMBL1258783)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)\C=C\CC(F)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H27F7O2/c1-20-11-7-17-16-6-4-15(32)13-14(16)3-5-18(17)19(20)8-12-21(20,33)9-2-10-22(25,23(26,27)28)24(29,30)31/h2,4,6,9,13,17-19,32-33H,3,5,7-8,10-12H2,1H3/b9-2+/t17-,18-,19+,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.95E+3 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucife... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50417027
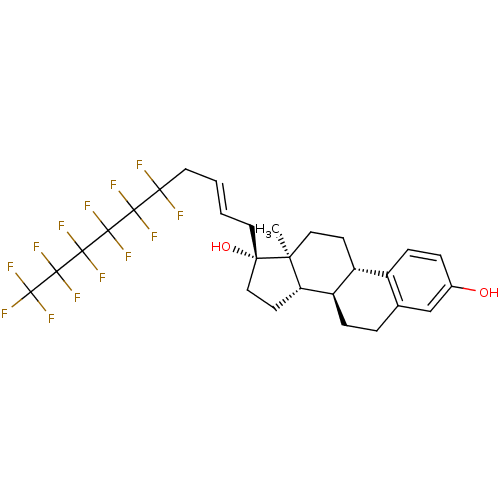
(CHEMBL1256103)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C\C=C\CC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C28H29F13O2/c1-21-12-8-18-17-7-5-16(42)14-15(17)4-6-19(18)20(21)9-13-22(21,43)10-2-3-11-23(29,30)24(31,32)25(33,34)26(35,36)27(37,38)28(39,40)41/h2-3,5,7,14,18-20,42-43H,4,6,8-13H2,1H3/b3-2+/t18-,19-,20+,21+,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucife... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50417029
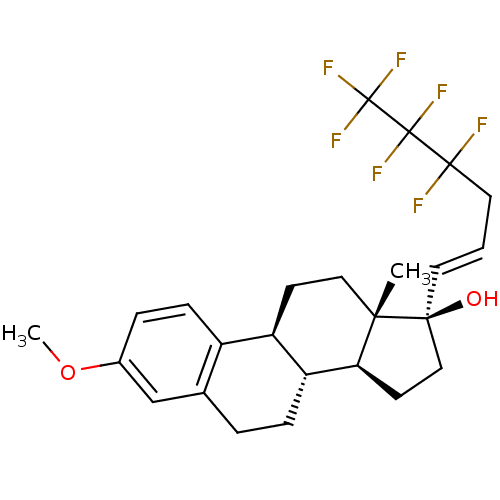
(CHEMBL1257168)Show SMILES COc1ccc2[C@H]3CC[C@@]4(C)[C@@H](CC[C@@]4(O)\C=C\CC(F)(F)C(F)(F)C(F)(F)F)[C@@H]3CCc2c1 |r| Show InChI InChI=1S/C25H29F7O2/c1-21-12-8-18-17-7-5-16(34-2)14-15(17)4-6-19(18)20(21)9-13-22(21,33)10-3-11-23(26,27)24(28,29)25(30,31)32/h3,5,7,10,14,18-20,33H,4,6,8-9,11-13H2,1-2H3/b10-3+/t18-,19-,20+,21+,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.03E+3 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucife... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50417025
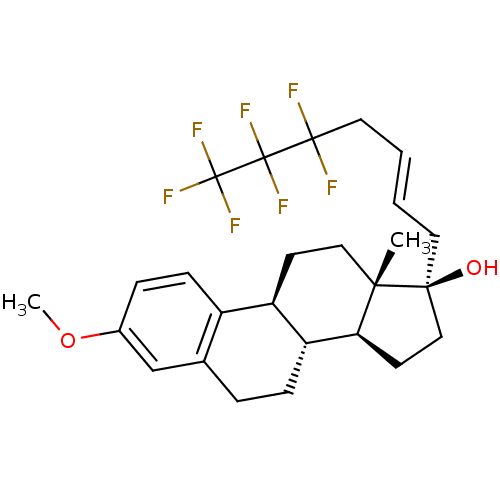
(CHEMBL1257284)Show SMILES COc1ccc2[C@H]3CC[C@@]4(C)[C@@H](CC[C@@]4(O)C\C=C\CC(F)(F)C(F)(F)C(F)(F)F)[C@@H]3CCc2c1 |r| Show InChI InChI=1S/C26H31F7O2/c1-22-13-9-19-18-8-6-17(35-2)15-16(18)5-7-20(19)21(22)10-14-23(22,34)11-3-4-12-24(27,28)25(29,30)26(31,32)33/h3-4,6,8,15,19-21,34H,5,7,9-14H2,1-2H3/b4-3+/t19-,20-,21+,22+,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucife... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM19214

((1S,2R,10S,11S,14S,15R,17S)-17-hydroxy-14-(2-hydro...)Show SMILES [H][C@@]12CC[C@H](C(=O)CO)[C@]1(C[C@H](O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C)C=O |t:21| Show InChI InChI=1S/C21H28O5/c1-20-7-6-13(24)8-12(20)2-3-14-15-4-5-16(18(26)10-22)21(15,11-23)9-17(25)19(14)20/h8,11,14-17,19,22,25H,2-7,9-10H2,1H3/t14-,15-,16+,17-,19+,20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.338 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at mineralocorticoid receptor-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity aft... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50417024

(CHEMBL1256102)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)\C=C\CC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C27H27F13O2/c1-20-11-7-17-16-6-4-15(41)13-14(16)3-5-18(17)19(20)8-12-21(20,42)9-2-10-22(28,29)23(30,31)24(32,33)25(34,35)26(36,37)27(38,39)40/h2,4,6,9,13,17-19,41-42H,3,5,7-8,10-12H2,1H3/b9-2+/t17-,18-,19+,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucife... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.458 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucifer... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50417028
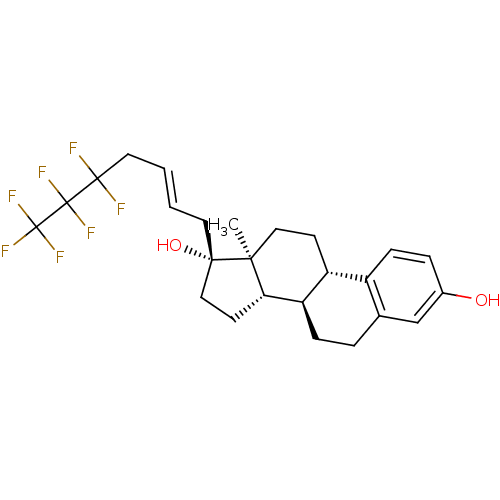
(CHEMBL1258902)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C\C=C\CC(F)(F)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C25H29F7O2/c1-21-12-8-18-17-7-5-16(33)14-15(17)4-6-19(18)20(21)9-13-22(21,34)10-2-3-11-23(26,27)24(28,29)25(30,31)32/h2-3,5,7,14,18-20,33-34H,4,6,8-13H2,1H3/b3-2+/t18-,19-,20+,21+,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 485 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucife... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50417030

(CHEMBL1257167)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C\C=C\CC(F)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H29F7O2/c1-21-12-8-18-17-7-5-16(33)14-15(17)4-6-19(18)20(21)9-13-22(21,34)10-2-3-11-23(26,24(27,28)29)25(30,31)32/h2-3,5,7,14,18-20,33-34H,4,6,8-13H2,1H3/b3-2+/t18-,19-,20+,21+,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 403 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucife... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50417023

(CHEMBL1258901)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)\C=C\CC(F)(F)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C24H27F7O2/c1-20-11-7-17-16-6-4-15(32)13-14(16)3-5-18(17)19(20)8-12-21(20,33)9-2-10-22(25,26)23(27,28)24(29,30)31/h2,4,6,9,13,17-19,32-33H,3,5,7-8,10-12H2,1H3/b9-2+/t17-,18-,19+,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 35.5 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucife... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50417029

(CHEMBL1257168)Show SMILES COc1ccc2[C@H]3CC[C@@]4(C)[C@@H](CC[C@@]4(O)\C=C\CC(F)(F)C(F)(F)C(F)(F)F)[C@@H]3CCc2c1 |r| Show InChI InChI=1S/C25H29F7O2/c1-21-12-8-18-17-7-5-16(34-2)14-15(17)4-6-19(18)20(21)9-13-22(21,33)10-3-11-23(26,27)24(28,29)25(30,31)32/h3,5,7,10,14,18-20,33H,4,6,8-9,11-13H2,1-2H3/b10-3+/t18-,19-,20+,21+,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.19E+3 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucifer... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50417028

(CHEMBL1258902)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C\C=C\CC(F)(F)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C25H29F7O2/c1-21-12-8-18-17-7-5-16(33)14-15(17)4-6-19(18)20(21)9-13-22(21,34)10-2-3-11-23(26,27)24(28,29)25(30,31)32/h2-3,5,7,14,18-20,33-34H,4,6,8-13H2,1H3/b3-2+/t18-,19-,20+,21+,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucifer... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50417027

(CHEMBL1256103)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C\C=C\CC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C28H29F13O2/c1-21-12-8-18-17-7-5-16(42)14-15(17)4-6-19(18)20(21)9-13-22(21,43)10-2-3-11-23(29,30)24(31,32)25(33,34)26(35,36)27(37,38)28(39,40)41/h2-3,5,7,14,18-20,42-43H,4,6,8-13H2,1H3/b3-2+/t18-,19-,20+,21+,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucifer... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417026
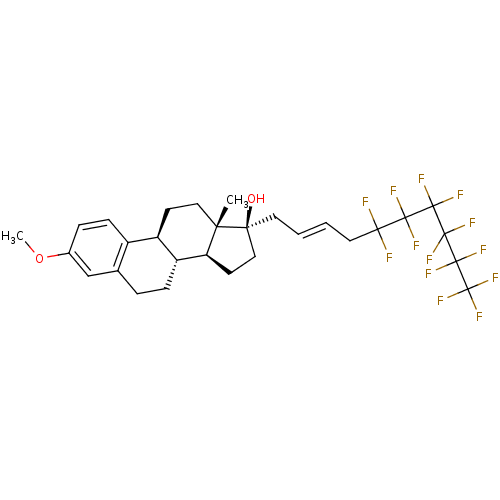
(CHEMBL1256105)Show SMILES COc1ccc2[C@H]3CC[C@@]4(C)[C@@H](CC[C@@]4(O)C\C=C\CC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)[C@@H]3CCc2c1 |r| Show InChI InChI=1S/C29H31F13O2/c1-22-13-9-19-18-8-6-17(44-2)15-16(18)5-7-20(19)21(22)10-14-23(22,43)11-3-4-12-24(30,31)25(32,33)26(34,35)27(36,37)28(38,39)29(40,41)42/h3-4,6,8,15,19-21,43H,5,7,9-14H2,1-2H3/b4-3+/t19-,20-,21+,22+,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.73 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after ... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50417023

(CHEMBL1258901)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)\C=C\CC(F)(F)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C24H27F7O2/c1-20-11-7-17-16-6-4-15(32)13-14(16)3-5-18(17)19(20)8-12-21(20,33)9-2-10-22(25,26)23(27,28)24(29,30)31/h2,4,6,9,13,17-19,32-33H,3,5,7-8,10-12H2,1H3/b9-2+/t17-,18-,19+,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 129 | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucifer... |
J Med Chem 53: 6947-53 (2010)
Article DOI: 10.1021/jm100563h
BindingDB Entry DOI: 10.7270/Q2PC33M2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data