 Found 1103 hits with Last Name = 'ebetino' and Initial = 'fh'
Found 1103 hits with Last Name = 'ebetino' and Initial = 'fh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50131251
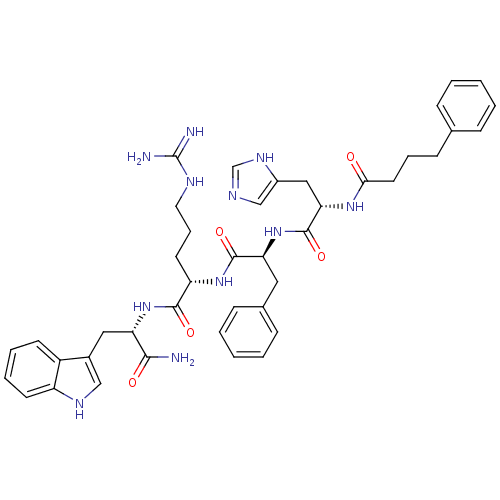
((S)-5-Guanidino-2-{(S)-2-[(S)-3-(3H-imidazol-4-yl)...)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CCCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C42H51N11O5/c43-38(55)34(22-29-24-48-32-17-8-7-16-31(29)32)52-39(56)33(18-10-20-47-42(44)45)51-40(57)35(21-28-13-5-2-6-14-28)53-41(58)36(23-30-25-46-26-49-30)50-37(54)19-9-15-27-11-3-1-4-12-27/h1-8,11-14,16-17,24-26,33-36,48H,9-10,15,18-23H2,(H2,43,55)(H,46,49)(H,50,54)(H,51,57)(H,52,56)(H,53,58)(H4,44,45,47)/t33-,34-,35-,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Binding affinity towards human Melanocortin 1 receptor (hMC1R) |
Bioorg Med Chem Lett 13: 2647-50 (2003)
BindingDB Entry DOI: 10.7270/Q2474BD9 |
More data for this
Ligand-Target Pair | |
Putative farnesyl pyrophosphate synthase
(Cryptosporidium parvum) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0500 | -61.2 | 30.1 | n/a | n/a | n/a | n/a | 7.7 | 37 |
University of Toronto
| Assay Description
Enzymatic assay using CpNPPPS was assayed using Reed and Rilling method with some modification. |
Chem Biol 15: 1296-306 (2008)
Article DOI: 10.1016/j.chembiol.2008.10.017
BindingDB Entry DOI: 10.7270/Q25B00XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Putative farnesyl pyrophosphate synthase
(Cryptosporidium parvum) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.380 | -55.9 | 45.7 | n/a | n/a | n/a | n/a | 7.7 | 37 |
University of Toronto
| Assay Description
Enzymatic assay using CpNPPPS was assayed using Reed and Rilling method with some modification. |
Chem Biol 15: 1296-306 (2008)
Article DOI: 10.1016/j.chembiol.2008.10.017
BindingDB Entry DOI: 10.7270/Q25B00XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50189013
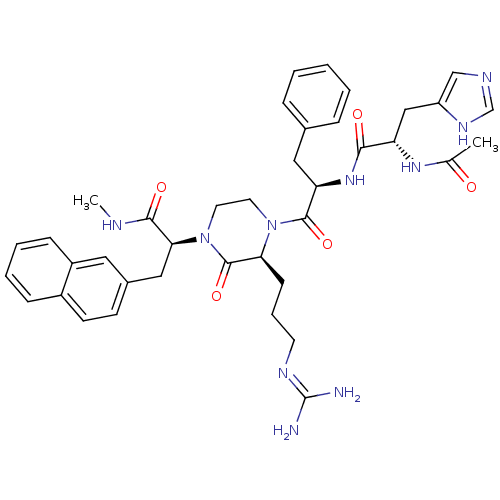
((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCN=C(N)N)C1=O)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O |wU:20.22,4.17,32.43,43.54,(1.84,-9.34,;3.18,-8.56,;4.51,-9.34,;4.51,-10.88,;5.85,-8.58,;7.18,-9.35,;7.18,-10.89,;5.84,-11.66,;5.83,-13.19,;7.17,-13.96,;7.17,-15.49,;8.51,-16.27,;9.84,-15.49,;9.83,-13.95,;8.51,-13.19,;8.51,-11.65,;5.85,-7.03,;4.52,-6.26,;4.52,-4.73,;5.86,-3.95,;7.19,-4.72,;8.53,-3.94,;9.85,-4.71,;11.19,-3.94,;12.52,-4.71,;13.86,-3.94,;15.2,-4.7,;13.86,-2.4,;7.19,-6.27,;8.52,-7.04,;5.85,-2.41,;7.19,-1.65,;4.52,-1.65,;3.18,-2.41,;1.84,-1.66,;.51,-2.41,;-.83,-1.66,;-.82,-.11,;.5,.67,;1.84,-.11,;4.52,-.11,;5.85,.66,;7.2,-.11,;5.85,2.21,;7.2,2.98,;8.54,2.21,;8.7,.67,;10.21,.35,;10.99,1.69,;9.95,2.84,;4.52,2.98,;4.51,4.52,;3.18,5.29,;5.85,5.29,)| Show InChI InChI=1S/C39H48N10O5/c1-25(50)46-31(22-30-23-43-24-45-30)35(51)47-32(20-26-9-4-3-5-10-26)37(53)48-17-18-49(38(54)33(48)13-8-16-44-39(40)41)34(36(52)42-2)21-27-14-15-28-11-6-7-12-29(28)19-27/h3-7,9-12,14-15,19,23-24,31-34H,8,13,16-18,20-22H2,1-2H3,(H,42,52)(H,43,45)(H,46,50)(H,47,51)(H4,40,41,44)/t31-,32+,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC1R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50189024
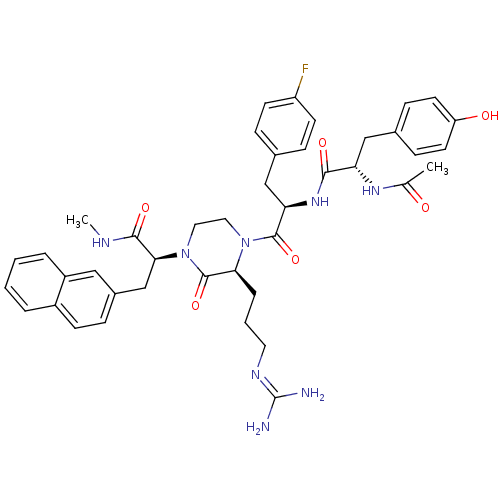
((S)-2-[(S)-4-[(R)-2-[(S)-2-acetylamino-3-(4-hydrox...)Show SMILES [#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-1-[#6]-[#6]-[#7](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]-1=O)-[#6](=O)-[#6@@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](-[#6])=O Show InChI InChI=1S/C42H49FN8O6/c1-26(52)48-34(23-28-12-17-33(53)18-13-28)38(54)49-35(24-27-10-15-32(43)16-11-27)40(56)50-20-21-51(41(57)36(50)8-5-19-47-42(44)45)37(39(55)46-2)25-29-9-14-30-6-3-4-7-31(30)22-29/h3-4,6-7,9-18,22,34-37,53H,5,8,19-21,23-25H2,1-2H3,(H,46,55)(H,48,52)(H,49,54)(H4,44,45,47)/t34-,35+,36-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM82411
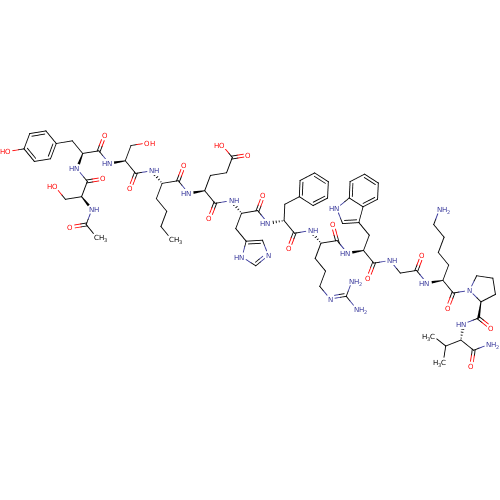
(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Binding affinity towards human Melanocortin 1 receptor (hMC1R) |
Bioorg Med Chem Lett 13: 2647-50 (2003)
BindingDB Entry DOI: 10.7270/Q2474BD9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50189010
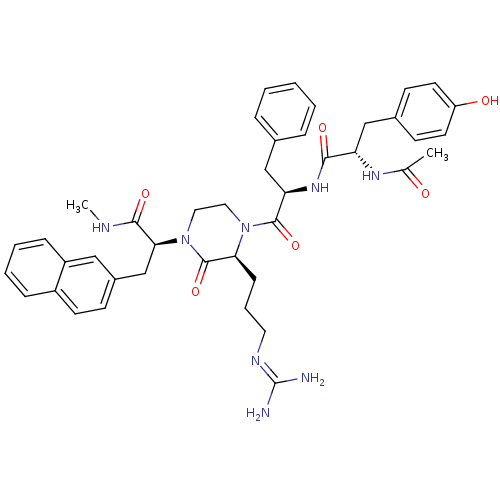
((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...)Show SMILES [#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-1-[#6]-[#6]-[#7](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]-1=O)-[#6](=O)-[#6@@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](-[#6])=O Show InChI InChI=1S/C42H50N8O6/c1-27(51)47-34(24-29-15-18-33(52)19-16-29)38(53)48-35(25-28-9-4-3-5-10-28)40(55)49-21-22-50(41(56)36(49)13-8-20-46-42(43)44)37(39(54)45-2)26-30-14-17-31-11-6-7-12-32(31)23-30/h3-7,9-12,14-19,23,34-37,52H,8,13,20-22,24-26H2,1-2H3,(H,45,54)(H,47,51)(H,48,53)(H4,43,44,46)/t34-,35+,36-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191554
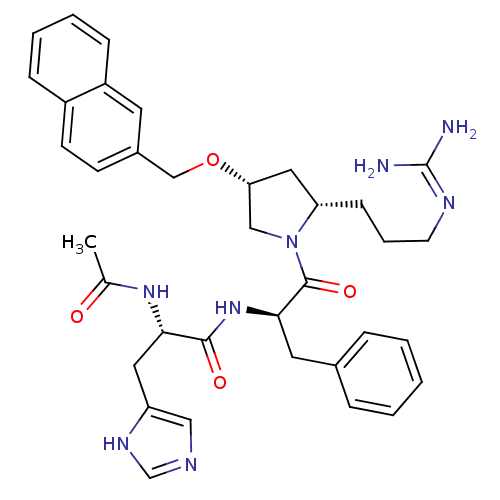
((S)-2-acetamido-N-((R)-1-((2S,4R)-2-(3-guanidinopr...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@@H]1CCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:28.31,14.14,26.38,wD:4.3,(17.84,3.85,;16.5,3.08,;16.5,1.54,;15.17,3.85,;13.84,3.08,;12.5,3.85,;12.5,5.39,;11.25,6.28,;11.72,7.75,;13.26,7.75,;13.74,6.28,;13.84,1.54,;15.17,.77,;12.5,.77,;12.51,-.77,;11.17,-1.54,;9.84,-.77,;8.5,-1.55,;7.16,-.77,;7.17,.77,;8.49,1.54,;9.83,.78,;13.84,-1.54,;15.17,-.77,;13.84,-3.08,;12.59,-3.99,;13.07,-5.45,;14.61,-5.45,;15.09,-3.98,;16.43,-3.24,;17.75,-4.03,;19.1,-3.28,;20.42,-4.07,;21.77,-3.32,;23.09,-4.11,;21.79,-1.78,;12.17,-6.7,;12.8,-8.1,;12.03,-9.44,;12.8,-10.76,;12.04,-12.1,;10.49,-12.11,;9.73,-13.43,;8.2,-13.44,;7.43,-12.1,;8.2,-10.78,;9.73,-10.78,;10.49,-9.44,)| Show InChI InChI=1S/C36H44N8O4/c1-24(45)42-32(18-29-20-39-23-41-29)34(46)43-33(17-25-8-3-2-4-9-25)35(47)44-21-31(19-30(44)12-7-15-40-36(37)38)48-22-26-13-14-27-10-5-6-11-28(27)16-26/h2-6,8-11,13-14,16,20,23,30-33H,7,12,15,17-19,21-22H2,1H3,(H,39,41)(H,42,45)(H,43,46)(H4,37,38,40)/t30-,31+,32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM82411

(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Binding affinity towards human Melanocortin 3 receptor (hMC3R) |
Bioorg Med Chem Lett 13: 2647-50 (2003)
BindingDB Entry DOI: 10.7270/Q2474BD9 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098378
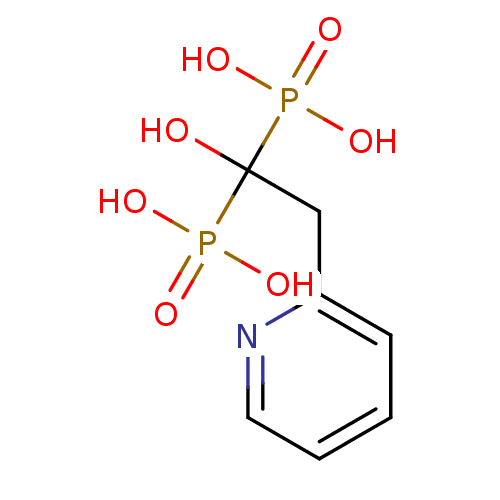
((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)5-6-3-1-2-4-8-6/h1-4,9H,5H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50189013

((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCN=C(N)N)C1=O)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O |wU:20.22,4.17,32.43,43.54,(1.84,-9.34,;3.18,-8.56,;4.51,-9.34,;4.51,-10.88,;5.85,-8.58,;7.18,-9.35,;7.18,-10.89,;5.84,-11.66,;5.83,-13.19,;7.17,-13.96,;7.17,-15.49,;8.51,-16.27,;9.84,-15.49,;9.83,-13.95,;8.51,-13.19,;8.51,-11.65,;5.85,-7.03,;4.52,-6.26,;4.52,-4.73,;5.86,-3.95,;7.19,-4.72,;8.53,-3.94,;9.85,-4.71,;11.19,-3.94,;12.52,-4.71,;13.86,-3.94,;15.2,-4.7,;13.86,-2.4,;7.19,-6.27,;8.52,-7.04,;5.85,-2.41,;7.19,-1.65,;4.52,-1.65,;3.18,-2.41,;1.84,-1.66,;.51,-2.41,;-.83,-1.66,;-.82,-.11,;.5,.67,;1.84,-.11,;4.52,-.11,;5.85,.66,;7.2,-.11,;5.85,2.21,;7.2,2.98,;8.54,2.21,;8.7,.67,;10.21,.35,;10.99,1.69,;9.95,2.84,;4.52,2.98,;4.51,4.52,;3.18,5.29,;5.85,5.29,)| Show InChI InChI=1S/C39H48N10O5/c1-25(50)46-31(22-30-23-43-24-45-30)35(51)47-32(20-26-9-4-3-5-10-26)37(53)48-17-18-49(38(54)33(48)13-8-16-44-39(40)41)34(36(52)42-2)21-27-14-15-28-11-6-7-12-29(28)19-27/h3-7,9-12,14-15,19,23-24,31-34H,8,13,16-18,20-22H2,1-2H3,(H,42,52)(H,43,45)(H,46,50)(H,47,51)(H4,40,41,44)/t31-,32+,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM82411

(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin 4 receptor (hMC4R) |
Bioorg Med Chem Lett 13: 2647-50 (2003)
BindingDB Entry DOI: 10.7270/Q2474BD9 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373094
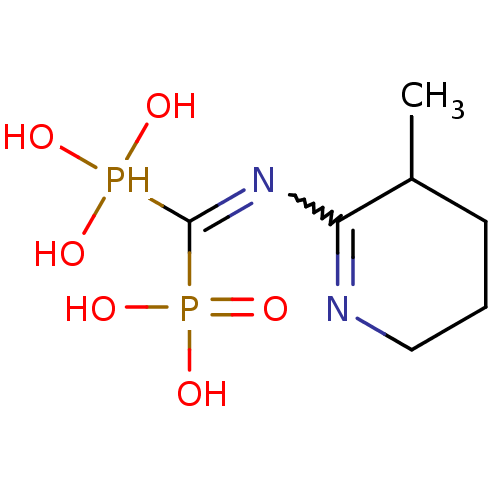
(CHEMBL408608)Show SMILES CC1CCCN=C1N=C(P(O)(O)O)P(O)(O)=O |w:7.7,c:5| Show InChI InChI=1S/C7H16N2O6P2/c1-5-3-2-4-8-6(5)9-7(16(10,11)12)17(13,14)15/h5,10-12,16H,2-4H2,1H3,(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50189010

((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...)Show SMILES [#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-1-[#6]-[#6]-[#7](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]-1=O)-[#6](=O)-[#6@@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](-[#6])=O Show InChI InChI=1S/C42H50N8O6/c1-27(51)47-34(24-29-15-18-33(52)19-16-29)38(53)48-35(25-28-9-4-3-5-10-28)40(55)49-21-22-50(41(56)36(49)13-8-20-46-42(43)44)37(39(54)45-2)26-30-14-17-31-11-6-7-12-32(31)23-30/h3-7,9-12,14-19,23,34-37,52H,8,13,20-22,24-26H2,1-2H3,(H,45,54)(H,47,51)(H,48,53)(H4,43,44,46)/t34-,35+,36-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC1R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50189010

((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...)Show SMILES [#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-1-[#6]-[#6]-[#7](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]-1=O)-[#6](=O)-[#6@@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](-[#6])=O Show InChI InChI=1S/C42H50N8O6/c1-27(51)47-34(24-29-15-18-33(52)19-16-29)38(53)48-35(25-28-9-4-3-5-10-28)40(55)49-21-22-50(41(56)36(49)13-8-20-46-42(43)44)37(39(54)45-2)26-30-14-17-31-11-6-7-12-32(31)23-30/h3-7,9-12,14-19,23,34-37,52H,8,13,20-22,24-26H2,1-2H3,(H,45,54)(H,47,51)(H,48,53)(H4,43,44,46)/t34-,35+,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC3R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50189013

((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCN=C(N)N)C1=O)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O |wU:20.22,4.17,32.43,43.54,(1.84,-9.34,;3.18,-8.56,;4.51,-9.34,;4.51,-10.88,;5.85,-8.58,;7.18,-9.35,;7.18,-10.89,;5.84,-11.66,;5.83,-13.19,;7.17,-13.96,;7.17,-15.49,;8.51,-16.27,;9.84,-15.49,;9.83,-13.95,;8.51,-13.19,;8.51,-11.65,;5.85,-7.03,;4.52,-6.26,;4.52,-4.73,;5.86,-3.95,;7.19,-4.72,;8.53,-3.94,;9.85,-4.71,;11.19,-3.94,;12.52,-4.71,;13.86,-3.94,;15.2,-4.7,;13.86,-2.4,;7.19,-6.27,;8.52,-7.04,;5.85,-2.41,;7.19,-1.65,;4.52,-1.65,;3.18,-2.41,;1.84,-1.66,;.51,-2.41,;-.83,-1.66,;-.82,-.11,;.5,.67,;1.84,-.11,;4.52,-.11,;5.85,.66,;7.2,-.11,;5.85,2.21,;7.2,2.98,;8.54,2.21,;8.7,.67,;10.21,.35,;10.99,1.69,;9.95,2.84,;4.52,2.98,;4.51,4.52,;3.18,5.29,;5.85,5.29,)| Show InChI InChI=1S/C39H48N10O5/c1-25(50)46-31(22-30-23-43-24-45-30)35(51)47-32(20-26-9-4-3-5-10-26)37(53)48-17-18-49(38(54)33(48)13-8-16-44-39(40)41)34(36(52)42-2)21-27-14-15-28-11-6-7-12-29(28)19-27/h3-7,9-12,14-15,19,23-24,31-34H,8,13,16-18,20-22H2,1-2H3,(H,42,52)(H,43,45)(H,46,50)(H,47,51)(H4,40,41,44)/t31-,32+,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC3R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098390
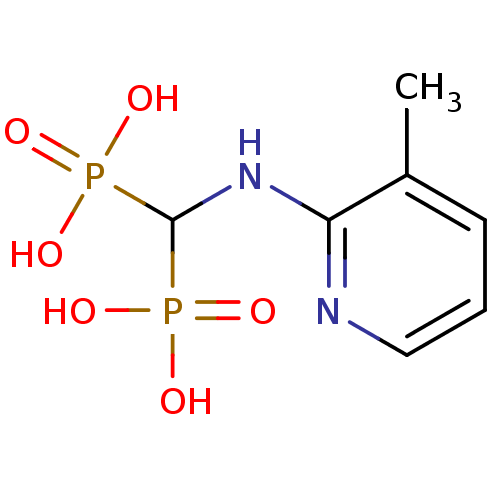
((3-methylpyridin-2-ylamino)methylenediphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c1-5-3-2-4-8-6(5)9-7(16(10,11)12)17(13,14)15/h2-4,7H,1H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50189024

((S)-2-[(S)-4-[(R)-2-[(S)-2-acetylamino-3-(4-hydrox...)Show SMILES [#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-1-[#6]-[#6]-[#7](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]-1=O)-[#6](=O)-[#6@@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](-[#6])=O Show InChI InChI=1S/C42H49FN8O6/c1-26(52)48-34(23-28-12-17-33(53)18-13-28)38(54)49-35(24-27-10-15-32(43)16-11-27)40(56)50-20-21-51(41(57)36(50)8-5-19-47-42(44)45)37(39(55)46-2)25-29-9-14-30-6-3-4-7-31(30)22-29/h3-4,6-7,9-18,22,34-37,53H,5,8,19-21,23-25H2,1-2H3,(H,46,55)(H,48,52)(H,49,54)(H4,44,45,47)/t34-,35+,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC3R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50189025

((S)-2-acetamido-N1-((R)-3-(4-fluorophenyl)-1-((S)-...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCNC(N)=N)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@H](CCC(N)=O)NC(C)=O Show InChI InChI=1S/C38H48FN9O6/c1-23(49)45-29(15-16-33(40)50)34(51)46-30(21-24-10-13-28(39)14-11-24)36(53)47-18-19-48(37(54)31(47)8-5-17-44-38(41)42)32(35(52)43-2)22-25-9-12-26-6-3-4-7-27(26)20-25/h3-4,6-7,9-14,20,29-32H,5,8,15-19,21-22H2,1-2H3,(H2,40,50)(H,43,52)(H,45,49)(H,46,51)(H4,41,42,44)/t29-,30+,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50189014

((S)-2-((S)-4-((R)-2-acetamido-3-(4-fluorophenyl)pr...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCNC(N)=N)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(C)=O Show InChI InChI=1S/C33H40FN7O4/c1-21(42)39-27(19-22-10-13-26(34)14-11-22)31(44)40-16-17-41(32(45)28(40)8-5-15-38-33(35)36)29(30(43)37-2)20-23-9-12-24-6-3-4-7-25(24)18-23/h3-4,6-7,9-14,18,27-29H,5,8,15-17,19-20H2,1-2H3,(H,37,43)(H,39,42)(H4,35,36,38)/t27-,28+,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191569
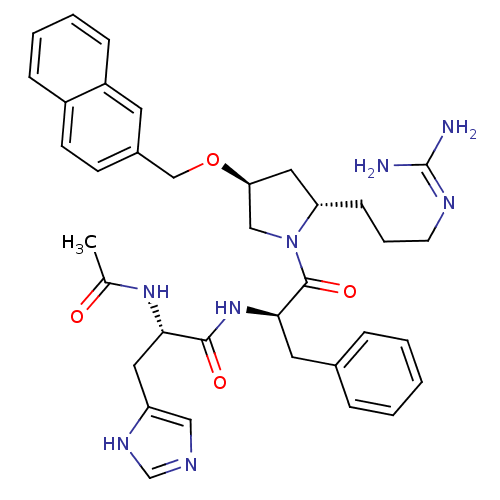
((S)-2-Acetamido-N-((R)-1-((2S,4S)-2-(3-guanidinopr...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@H](C[C@@H]1CCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:28.31,14.14,4.3,wD:26.38,(12.66,3.78,;13.99,3.01,;13.99,1.47,;15.32,3.78,;16.66,3.01,;17.99,3.78,;17.99,5.32,;16.73,6.23,;17.21,7.69,;18.75,7.7,;19.23,6.23,;16.66,1.47,;17.99,.7,;15.33,.7,;15.33,-.84,;14,-1.61,;12.66,-.84,;11.32,-1.61,;9.99,-.84,;9.99,.7,;11.31,1.47,;12.66,.71,;16.66,-1.61,;18,-.84,;16.66,-3.15,;15.42,-4.06,;15.9,-5.52,;17.44,-5.52,;17.91,-4.05,;19.26,-3.3,;20.58,-4.1,;21.92,-3.35,;23.24,-4.14,;24.59,-3.39,;25.91,-4.18,;24.62,-1.85,;14.99,-6.77,;15.62,-8.17,;14.85,-9.51,;15.63,-10.83,;14.87,-12.17,;13.32,-12.18,;12.56,-13.5,;11.03,-13.51,;10.25,-12.17,;11.02,-10.84,;12.55,-10.84,;13.31,-9.51,)| Show InChI InChI=1S/C36H44N8O4/c1-24(45)42-32(18-29-20-39-23-41-29)34(46)43-33(17-25-8-3-2-4-9-25)35(47)44-21-31(19-30(44)12-7-15-40-36(37)38)48-22-26-13-14-27-10-5-6-11-28(27)16-26/h2-6,8-11,13-14,16,20,23,30-33H,7,12,15,17-19,21-22H2,1H3,(H,39,41)(H,42,45)(H,43,46)(H4,37,38,40)/t30-,31-,32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50189023
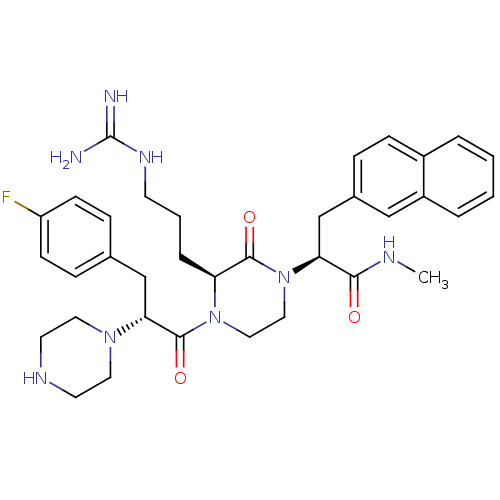
((S)-2-((S)-4-((R)-3-(4-fluorophenyl)-2-(piperazin-...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCNC(N)=N)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)N1CCNCC1 Show InChI InChI=1S/C35H45FN8O3/c1-39-32(45)30(23-25-8-11-26-5-2-3-6-27(26)21-25)44-20-19-43(29(33(44)46)7-4-14-41-35(37)38)34(47)31(42-17-15-40-16-18-42)22-24-9-12-28(36)13-10-24/h2-3,5-6,8-13,21,29-31,40H,4,7,14-20,22-23H2,1H3,(H,39,45)(H4,37,38,41)/t29-,30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50253660
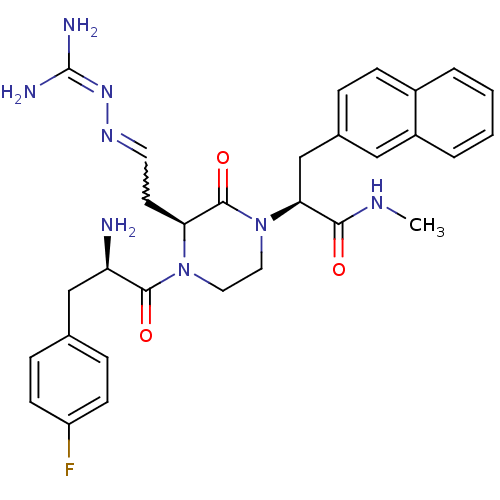
(2-{3-(2-Amino-ethyl-guanidino)-4-[2-amino-3-(4-flu...)Show SMILES [#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-1-[#6]-[#6]-[#7](-[#6@@H](-[#6]-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])-[#6]-1=O)-[#6](=O)-[#6@H](-[#7])-[#6]-c1ccc(F)cc1 |r,w:22.23| Show InChI InChI=1S/C30H35FN8O3/c1-35-27(40)26(18-20-6-9-21-4-2-3-5-22(21)16-20)39-15-14-38(25(29(39)42)12-13-36-37-30(33)34)28(41)24(32)17-19-7-10-23(31)11-8-19/h2-11,13,16,24-26H,12,14-15,17-18,32H2,1H3,(H,35,40)(H4,33,34,37)/t24-,25+,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 51: 6055-66 (2008)
Article DOI: 10.1021/jm800525p
BindingDB Entry DOI: 10.7270/Q2DV1JQC |
More data for this
Ligand-Target Pair | |
Putative farnesyl pyrophosphate synthase
(Cryptosporidium parvum) | BDBM81349
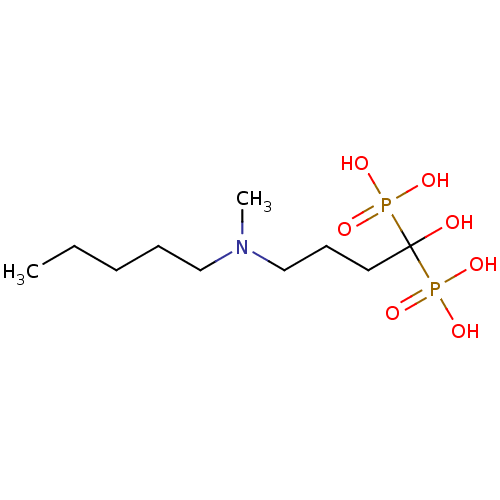
(IBAN)Show InChI InChI=1S/C10H25NO7P2/c1-3-4-5-8-11(2)9-6-7-10(12,19(13,14)15)20(16,17)18/h12H,3-9H2,1-2H3,(H2,13,14,15)(H2,16,17,18) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | -52.2 | 52.7 | n/a | n/a | n/a | n/a | 7.7 | 37 |
University of Toronto
| Assay Description
Enzymatic assay using CpNPPPS was assayed using Reed and Rilling method with some modification. |
Chem Biol 15: 1296-306 (2008)
Article DOI: 10.1016/j.chembiol.2008.10.017
BindingDB Entry DOI: 10.7270/Q25B00XQ |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373098
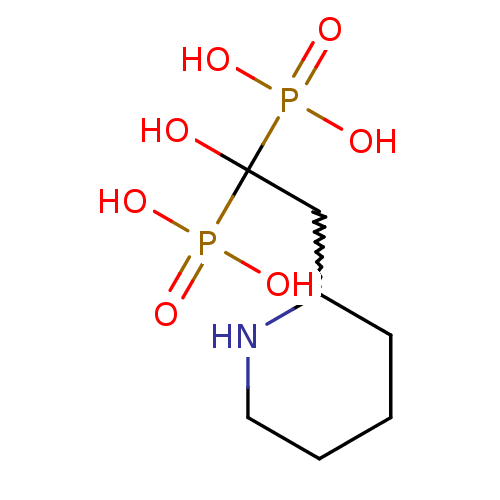
(CHEMBL259729)Show InChI InChI=1S/C7H17NO7P2/c9-7(16(10,11)12,17(13,14)15)5-6-3-1-2-4-8-6/h6,8-9H,1-5H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50189025

((S)-2-acetamido-N1-((R)-3-(4-fluorophenyl)-1-((S)-...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCNC(N)=N)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@H](CCC(N)=O)NC(C)=O Show InChI InChI=1S/C38H48FN9O6/c1-23(49)45-29(15-16-33(40)50)34(51)46-30(21-24-10-13-28(39)14-11-24)36(53)47-18-19-48(37(54)31(47)8-5-17-44-38(41)42)32(35(52)43-2)22-25-9-12-26-6-3-4-7-27(26)20-25/h3-4,6-7,9-14,20,29-32H,5,8,15-19,21-22H2,1-2H3,(H2,40,50)(H,43,52)(H,45,49)(H,46,51)(H4,41,42,44)/t29-,30+,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC1R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50189022
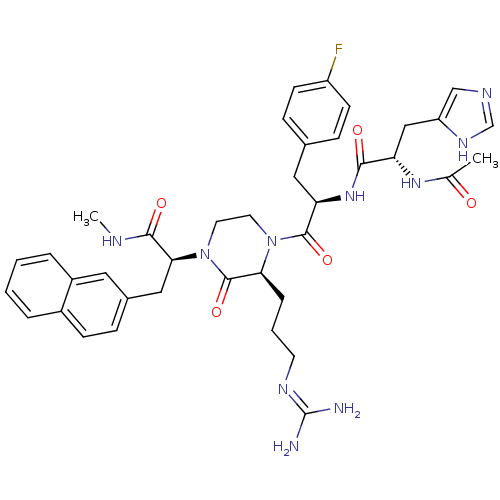
((S)-2-[(S)-4-[(R)-2-[(S)-2-acetylamino-3-(3H-imida...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCN=C(N)N)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O |wU:20.22,4.17,32.44,44.55,(15.61,-28.99,;16.95,-28.22,;18.29,-29,;18.28,-30.54,;19.62,-28.23,;20.95,-29.01,;20.95,-30.55,;19.61,-31.31,;19.61,-32.85,;20.94,-33.61,;20.94,-35.15,;22.28,-35.93,;23.61,-35.14,;23.6,-33.61,;22.28,-32.85,;22.28,-31.31,;19.63,-26.68,;18.29,-25.92,;18.3,-24.38,;19.63,-23.6,;20.97,-24.38,;22.3,-23.6,;23.63,-24.37,;24.96,-23.6,;26.29,-24.37,;27.63,-23.59,;28.97,-24.36,;27.63,-22.05,;20.96,-25.92,;22.29,-26.69,;19.63,-22.06,;20.96,-21.31,;18.29,-21.31,;16.95,-22.07,;15.62,-21.31,;14.28,-22.07,;12.95,-21.31,;12.95,-19.77,;11.61,-19.01,;14.28,-18.99,;15.61,-19.76,;18.29,-19.77,;19.63,-18.99,;20.97,-19.77,;19.63,-17.45,;20.97,-16.68,;22.31,-17.45,;22.47,-18.98,;23.98,-19.31,;24.76,-17.97,;23.72,-16.82,;18.29,-16.68,;18.29,-15.14,;16.95,-14.37,;19.62,-14.36,)| Show InChI InChI=1S/C39H47FN10O5/c1-24(51)47-31(21-30-22-44-23-46-30)35(52)48-32(19-25-10-13-29(40)14-11-25)37(54)49-16-17-50(38(55)33(49)8-5-15-45-39(41)42)34(36(53)43-2)20-26-9-12-27-6-3-4-7-28(27)18-26/h3-4,6-7,9-14,18,22-23,31-34H,5,8,15-17,19-21H2,1-2H3,(H,43,53)(H,44,46)(H,47,51)(H,48,52)(H4,41,42,45)/t31-,32+,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC1R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115104
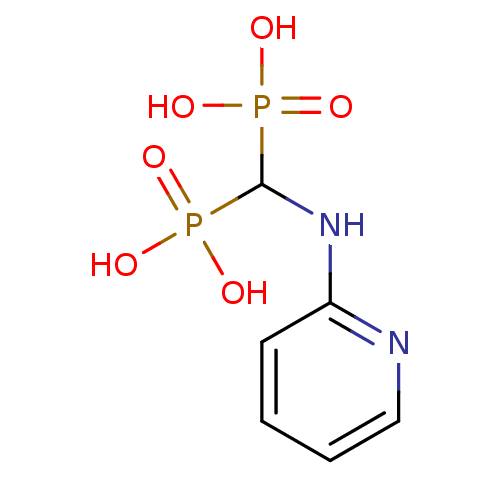
((pyridin-2-ylamino)methylenediphosphonic acid | 2-...)Show InChI InChI=1S/C6H10N2O6P2/c9-15(10,11)6(16(12,13)14)8-5-3-1-2-4-7-5/h1-4,6H,(H,7,8)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115106
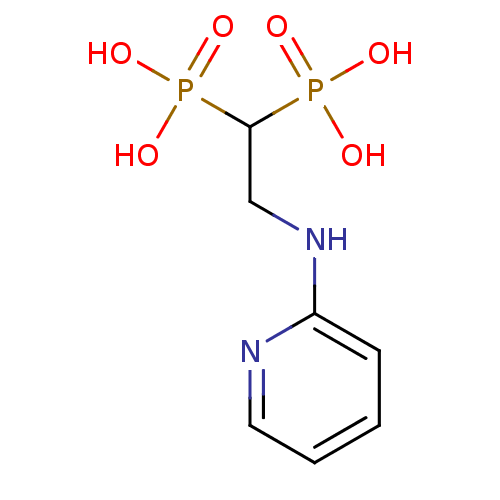
(2-(pyridin-2-ylamino)ethane-1,1-diyldiphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c10-16(11,12)7(17(13,14)15)5-9-6-3-1-2-4-8-6/h1-4,7H,5H2,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50119368

((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...)Show SMILES Clc1ccc(C[C@@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCC(Cn3cncn3)(CC2)C2CCCCC2)cc1 Show InChI InChI=1S/C33H41ClN6O2/c34-28-12-10-24(11-13-28)18-30(38-31(41)29-19-25-6-4-5-7-26(25)20-36-29)32(42)39-16-14-33(15-17-39,21-40-23-35-22-37-40)27-8-2-1-3-9-27/h4-7,10-13,22-23,27,29-30,36H,1-3,8-9,14-21H2,(H,38,41)/t29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC4 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1223-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.109
BindingDB Entry DOI: 10.7270/Q2TM7BZM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373097
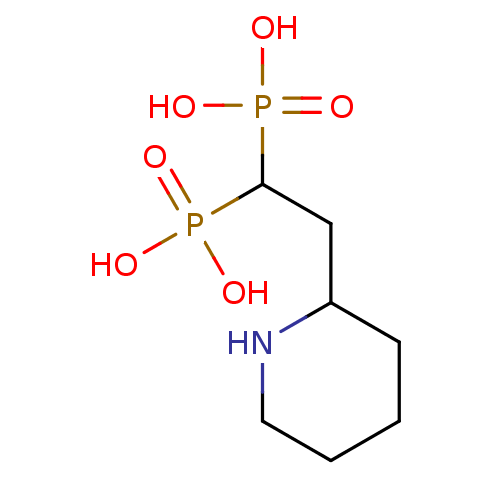
(CHEMBL406820)Show InChI InChI=1S/C7H17NO6P2/c9-15(10,11)7(16(12,13)14)5-6-3-1-2-4-8-6/h6-8H,1-5H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50189025

((S)-2-acetamido-N1-((R)-3-(4-fluorophenyl)-1-((S)-...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCNC(N)=N)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@H](CCC(N)=O)NC(C)=O Show InChI InChI=1S/C38H48FN9O6/c1-23(49)45-29(15-16-33(40)50)34(51)46-30(21-24-10-13-28(39)14-11-24)36(53)47-18-19-48(37(54)31(47)8-5-17-44-38(41)42)32(35(52)43-2)22-25-9-12-26-6-3-4-7-27(26)20-25/h3-4,6-7,9-14,20,29-32H,5,8,15-19,21-22H2,1-2H3,(H2,40,50)(H,43,52)(H,45,49)(H,46,51)(H4,41,42,44)/t29-,30+,31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC3R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50173792

(2-(2-aminophenyl)-1-hydroxyethane-1,1-diyldiphosph...)Show InChI InChI=1S/C8H13NO7P2/c9-7-4-2-1-3-6(7)5-8(10,17(11,12)13)18(14,15)16/h1-4,10H,5,9H2,(H2,11,12,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50131258
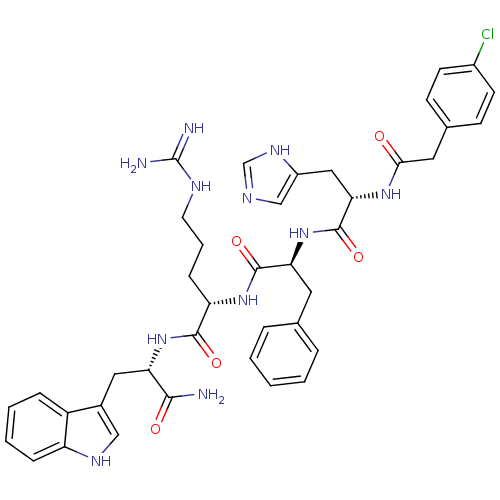
((S)-2-{(S)-2-[(S)-2-[2-(4-Chloro-phenyl)-acetylami...)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)Cc1ccc(Cl)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C40H46ClN11O5/c41-27-14-12-25(13-15-27)18-35(53)49-34(20-28-22-45-23-48-28)39(57)52-33(17-24-7-2-1-3-8-24)38(56)50-31(11-6-16-46-40(43)44)37(55)51-32(36(42)54)19-26-21-47-30-10-5-4-9-29(26)30/h1-5,7-10,12-15,21-23,31-34,47H,6,11,16-20H2,(H2,42,54)(H,45,48)(H,49,53)(H,50,56)(H,51,55)(H,52,57)(H4,43,44,46)/t31-,32-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro agonist potency was evaluated in HEK293 cells transfected with human melanocortin receptor (hMC3R) |
Bioorg Med Chem Lett 13: 2647-50 (2003)
BindingDB Entry DOI: 10.7270/Q2474BD9 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12577
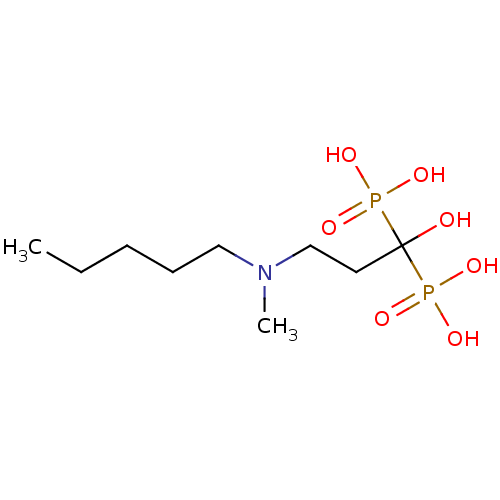
(Bisphosphonate 2 | CHEMBL997 | JMC515594 Compound ...)Show InChI InChI=1S/C9H23NO7P2/c1-3-4-5-7-10(2)8-6-9(11,18(12,13)14)19(15,16)17/h11H,3-8H2,1-2H3,(H2,12,13,14)(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373099

(CHEMBL99553 | NE-10575)Show InChI InChI=1S/C8H13NO7P2/c1-9-4-2-3-7(6-9)5-8(10,17(11,12)13)18(14,15)16/h2-4,6,10H,5H2,1H3,(H3-,11,12,13,14,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50253738
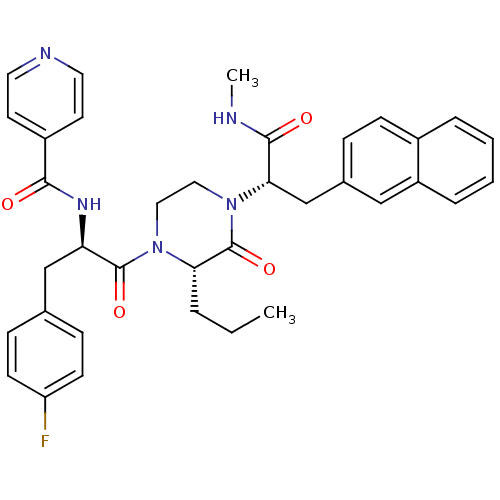
(CHEMBL449131 | N-((R)-3-(4-Fluorophenyl)-1-((S)-4-...)Show SMILES CCC[C@@H]1N(CCN([C@@H](Cc2ccc3ccccc3c2)C(=O)NC)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)c1ccncc1 |r| Show InChI InChI=1S/C36H38FN5O4/c1-3-6-31-36(46)42(32(34(44)38-2)23-25-9-12-26-7-4-5-8-28(26)21-25)20-19-41(31)35(45)30(22-24-10-13-29(37)14-11-24)40-33(43)27-15-17-39-18-16-27/h4-5,7-18,21,30-32H,3,6,19-20,22-23H2,1-2H3,(H,38,44)(H,40,43)/t30-,31+,32+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 51: 6055-66 (2008)
Article DOI: 10.1021/jm800525p
BindingDB Entry DOI: 10.7270/Q2DV1JQC |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50253736
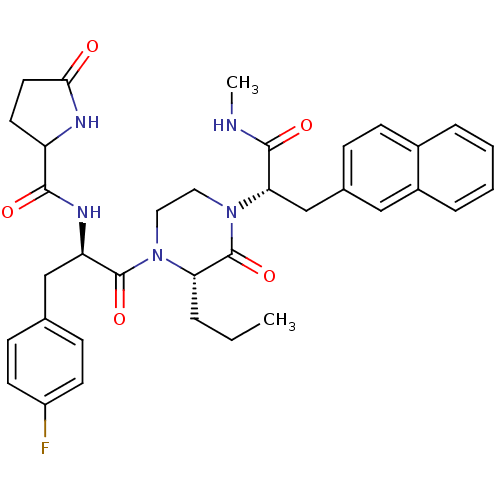
(CHEMBL506272 | N-((R)-3-(4-Fluorophenyl)-1-((S)-4-...)Show SMILES CCC[C@@H]1N(CCN([C@@H](Cc2ccc3ccccc3c2)C(=O)NC)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)C1CCC(=O)N1 |r| Show InChI InChI=1S/C35H40FN5O5/c1-3-6-29-35(46)41(30(33(44)37-2)21-23-9-12-24-7-4-5-8-25(24)19-23)18-17-40(29)34(45)28(20-22-10-13-26(36)14-11-22)39-32(43)27-15-16-31(42)38-27/h4-5,7-14,19,27-30H,3,6,15-18,20-21H2,1-2H3,(H,37,44)(H,38,42)(H,39,43)/t27?,28-,29+,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 51: 6055-66 (2008)
Article DOI: 10.1021/jm800525p
BindingDB Entry DOI: 10.7270/Q2DV1JQC |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50189023

((S)-2-((S)-4-((R)-3-(4-fluorophenyl)-2-(piperazin-...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCNC(N)=N)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)N1CCNCC1 Show InChI InChI=1S/C35H45FN8O3/c1-39-32(45)30(23-25-8-11-26-5-2-3-6-27(26)21-25)44-20-19-43(29(33(44)46)7-4-14-41-35(37)38)34(47)31(42-17-15-40-16-18-42)22-24-9-12-28(36)13-10-24/h2-3,5-6,8-13,21,29-31,40H,4,7,14-20,22-23H2,1H3,(H,39,45)(H4,37,38,41)/t29-,30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC1R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373096

(CHEMBL99369 | Piridronic acid)Show InChI InChI=1S/C7H11NO6P2/c9-15(10,11)7(16(12,13)14)5-6-3-1-2-4-8-6/h1-4,7H,5H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191575

((S)-2-acetamido-N-((R)-1-((2R,4R)-2-(3-guanidinopr...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@H]1CCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:14.14,26.38,4.3,wD:28.31,(10.98,-16.28,;12.31,-17.05,;12.32,-18.59,;13.65,-16.27,;14.98,-17.04,;16.31,-16.27,;16.31,-14.73,;15.06,-13.83,;15.53,-12.36,;17.07,-12.36,;17.55,-13.82,;14.98,-18.58,;16.32,-19.35,;13.65,-19.35,;13.65,-20.89,;12.32,-21.67,;10.98,-20.9,;9.64,-21.67,;8.31,-20.9,;8.31,-19.35,;9.64,-18.58,;10.98,-19.35,;14.99,-21.66,;16.32,-20.89,;14.99,-23.2,;13.74,-24.11,;14.22,-25.57,;15.76,-25.57,;16.23,-24.11,;17.58,-23.36,;18.9,-24.15,;20.25,-23.4,;21.57,-24.19,;22.91,-23.44,;24.24,-24.24,;22.94,-21.9,;13.31,-26.82,;13.94,-28.23,;13.18,-29.56,;13.95,-30.89,;13.19,-32.22,;11.64,-32.23,;10.88,-33.55,;9.35,-33.56,;8.58,-32.23,;9.34,-30.9,;10.88,-30.9,;11.63,-29.56,)| Show InChI InChI=1S/C36H44N8O4/c1-24(45)42-32(18-29-20-39-23-41-29)34(46)43-33(17-25-8-3-2-4-9-25)35(47)44-21-31(19-30(44)12-7-15-40-36(37)38)48-22-26-13-14-27-10-5-6-11-28(27)16-26/h2-6,8-11,13-14,16,20,23,30-33H,7,12,15,17-19,21-22H2,1H3,(H,39,41)(H,42,45)(H,43,46)(H4,37,38,40)/t30-,31-,32+,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50179466
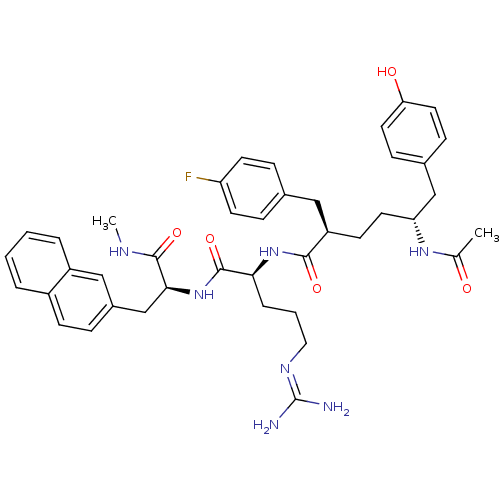
((2R,5R)-5-acetylamino-2-(4-fluoro-benzyl)-6-(4-hyd...)Show SMILES [#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](-[#6])=O)-[#6]-c1ccc(F)cc1 Show InChI InChI=1S/C41H50FN7O5/c1-26(50)47-34(24-28-12-19-35(51)20-13-28)18-15-32(22-27-10-16-33(42)17-11-27)38(52)48-36(8-5-21-46-41(43)44)40(54)49-37(39(53)45-2)25-29-9-14-30-6-3-4-7-31(30)23-29/h3-4,6-7,9-14,16-17,19-20,23,32,34,36-37,51H,5,8,15,18,21-22,24-25H2,1-2H3,(H,45,53)(H,47,50)(H,48,52)(H,49,54)(H4,43,44,46)/t32-,34-,36+,37+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to MC4 receptor |
Bioorg Med Chem Lett 16: 1721-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.005
BindingDB Entry DOI: 10.7270/Q2ZS2W2N |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50131258

((S)-2-{(S)-2-[(S)-2-[2-(4-Chloro-phenyl)-acetylami...)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)Cc1ccc(Cl)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C40H46ClN11O5/c41-27-14-12-25(13-15-27)18-35(53)49-34(20-28-22-45-23-48-28)39(57)52-33(17-24-7-2-1-3-8-24)38(56)50-31(11-6-16-46-40(43)44)37(55)51-32(36(42)54)19-26-21-47-30-10-5-4-9-29(26)30/h1-5,7-10,12-15,21-23,31-34,47H,6,11,16-20H2,(H2,42,54)(H,45,48)(H,49,53)(H,50,56)(H,51,55)(H,52,57)(H4,43,44,46)/t31-,32-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin 4 receptor (hMC4R) |
Bioorg Med Chem Lett 13: 2647-50 (2003)
BindingDB Entry DOI: 10.7270/Q2474BD9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50253745
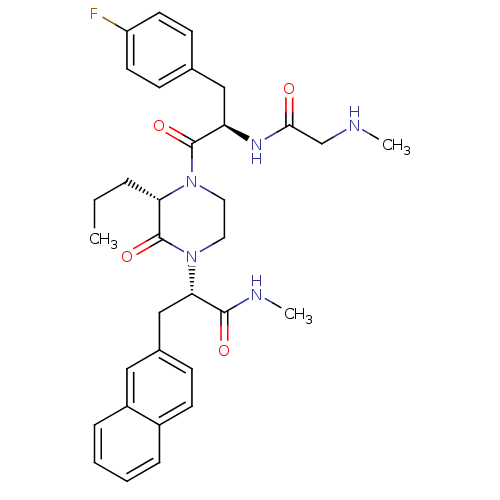
((S)-2-((S)-4-((R)-3-(4-Fluorophenyl)-2-(2-(methyla...)Show SMILES CCC[C@@H]1N(CCN([C@@H](Cc2ccc3ccccc3c2)C(=O)NC)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)CNC |r| Show InChI InChI=1S/C33H40FN5O4/c1-4-7-28-33(43)39(29(31(41)36-3)20-23-10-13-24-8-5-6-9-25(24)18-23)17-16-38(28)32(42)27(37-30(40)21-35-2)19-22-11-14-26(34)15-12-22/h5-6,8-15,18,27-29,35H,4,7,16-17,19-21H2,1-3H3,(H,36,41)(H,37,40)/t27-,28+,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 51: 6055-66 (2008)
Article DOI: 10.1021/jm800525p
BindingDB Entry DOI: 10.7270/Q2DV1JQC |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50253748
((2S)-N-((1R)-1-[(4-Fluorophenyl)methyl]-2-{(2S)-4-...)Show SMILES CCC[C@@H]1N(CCN([C@@H](Cc2ccc3ccccc3c2)C(=O)NC)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@H]1CCCCN1 |r| Show InChI InChI=1S/C36H44FN5O4/c1-3-8-31-36(46)42(32(34(44)38-2)23-25-12-15-26-9-4-5-10-27(26)21-25)20-19-41(31)35(45)30(22-24-13-16-28(37)17-14-24)40-33(43)29-11-6-7-18-39-29/h4-5,9-10,12-17,21,29-32,39H,3,6-8,11,18-20,22-23H2,1-2H3,(H,38,44)(H,40,43)/t29-,30-,31+,32+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 51: 6055-66 (2008)
Article DOI: 10.1021/jm800525p
BindingDB Entry DOI: 10.7270/Q2DV1JQC |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50189022

((S)-2-[(S)-4-[(R)-2-[(S)-2-acetylamino-3-(3H-imida...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCN=C(N)N)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O |wU:20.22,4.17,32.44,44.55,(15.61,-28.99,;16.95,-28.22,;18.29,-29,;18.28,-30.54,;19.62,-28.23,;20.95,-29.01,;20.95,-30.55,;19.61,-31.31,;19.61,-32.85,;20.94,-33.61,;20.94,-35.15,;22.28,-35.93,;23.61,-35.14,;23.6,-33.61,;22.28,-32.85,;22.28,-31.31,;19.63,-26.68,;18.29,-25.92,;18.3,-24.38,;19.63,-23.6,;20.97,-24.38,;22.3,-23.6,;23.63,-24.37,;24.96,-23.6,;26.29,-24.37,;27.63,-23.59,;28.97,-24.36,;27.63,-22.05,;20.96,-25.92,;22.29,-26.69,;19.63,-22.06,;20.96,-21.31,;18.29,-21.31,;16.95,-22.07,;15.62,-21.31,;14.28,-22.07,;12.95,-21.31,;12.95,-19.77,;11.61,-19.01,;14.28,-18.99,;15.61,-19.76,;18.29,-19.77,;19.63,-18.99,;20.97,-19.77,;19.63,-17.45,;20.97,-16.68,;22.31,-17.45,;22.47,-18.98,;23.98,-19.31,;24.76,-17.97,;23.72,-16.82,;18.29,-16.68,;18.29,-15.14,;16.95,-14.37,;19.62,-14.36,)| Show InChI InChI=1S/C39H47FN10O5/c1-24(51)47-31(21-30-22-44-23-46-30)35(52)48-32(19-25-10-13-29(40)14-11-25)37(54)49-16-17-50(38(55)33(49)8-5-15-45-39(41)42)34(36(53)43-2)20-26-9-12-27-6-3-4-7-28(27)18-26/h3-4,6-7,9-14,18,22-23,31-34H,5,8,15-17,19-21H2,1-2H3,(H,43,53)(H,44,46)(H,47,51)(H,48,52)(H4,41,42,45)/t31-,32+,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50253749

(CHEMBL506762 | N-((1R)-1-[(4-Fluorophenyl)methyl]-...)Show SMILES CCC[C@@H]1N(CCN([C@@H](Cc2ccc3ccccc3c2)C(=O)NC)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)C1CCNCC1 |r| Show InChI InChI=1S/C36H44FN5O4/c1-3-6-31-36(46)42(32(34(44)38-2)23-25-9-12-26-7-4-5-8-28(26)21-25)20-19-41(31)35(45)30(22-24-10-13-29(37)14-11-24)40-33(43)27-15-17-39-18-16-27/h4-5,7-14,21,27,30-32,39H,3,6,15-20,22-23H2,1-2H3,(H,38,44)(H,40,43)/t30-,31+,32+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 51: 6055-66 (2008)
Article DOI: 10.1021/jm800525p
BindingDB Entry DOI: 10.7270/Q2DV1JQC |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50189009

((S)-2-((S)-4-(3-(4-fluorophenyl)propanoyl)-3-(3-gu...)Show SMILES [#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-1-[#6]-[#6]-[#7](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]-1=O)-[#6](=O)-[#6]-[#6]-c1ccc(F)cc1 Show InChI InChI=1S/C31H37FN6O3/c1-35-29(40)27(20-22-8-12-23-5-2-3-6-24(23)19-22)38-18-17-37(26(30(38)41)7-4-16-36-31(33)34)28(39)15-11-21-9-13-25(32)14-10-21/h2-3,5-6,8-10,12-14,19,26-27H,4,7,11,15-18,20H2,1H3,(H,35,40)(H4,33,34,36)/t26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data