 Found 3364 hits with Last Name = 'egan' and Initial = 'c'
Found 3364 hits with Last Name = 'egan' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Casein kinase II subunit alpha'/beta
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cylene Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CK2alpha/CK2beta using RRRDDDSDDD peptide as substrate by radiometric assay |
Bioorg Med Chem Lett 22: 45-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.087
BindingDB Entry DOI: 10.7270/Q24Q7XV4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against rat Dopamine receptor D2. |
J Med Chem 41: 5084-93 (1999)
Article DOI: 10.1021/jm980452a
BindingDB Entry DOI: 10.7270/Q2CZ369B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50166450
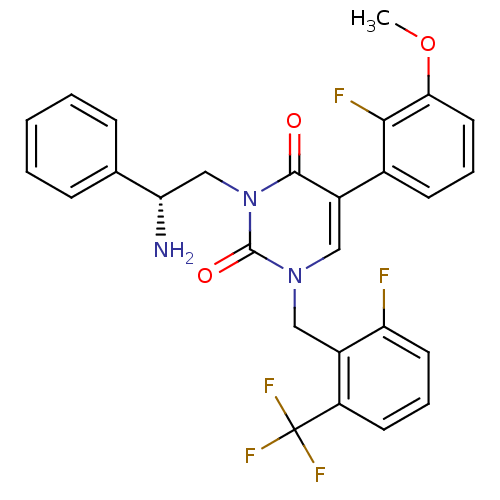
((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...)Show SMILES COc1cccc(c1F)-c1cn(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r| Show InChI InChI=1S/C27H22F5N3O3/c1-38-23-12-5-9-17(24(23)29)18-13-34(14-19-20(27(30,31)32)10-6-11-21(19)28)26(37)35(25(18)36)15-22(33)16-7-3-2-4-8-16/h2-13,22H,14-15,33H2,1H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Pro-N-Et-GnRH from human cloned GnRH receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50068530

(1-Cyclohexyl-8-[4-(4-fluoro-phenyl)-4-oxo-butyl]-1...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)C1CCCCC1 Show InChI InChI=1S/C23H32FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h8-11,20H,1-7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against rat Dopamine receptor D2. |
J Med Chem 41: 5084-93 (1999)
Article DOI: 10.1021/jm980452a
BindingDB Entry DOI: 10.7270/Q2CZ369B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50068530

(1-Cyclohexyl-8-[4-(4-fluoro-phenyl)-4-oxo-butyl]-1...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)C1CCCCC1 Show InChI InChI=1S/C23H32FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h8-11,20H,1-7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-hydroxytryptamine 2A receptor in NIH3T3 cells. |
J Med Chem 41: 5084-93 (1999)
Article DOI: 10.1021/jm980452a
BindingDB Entry DOI: 10.7270/Q2CZ369B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50031942
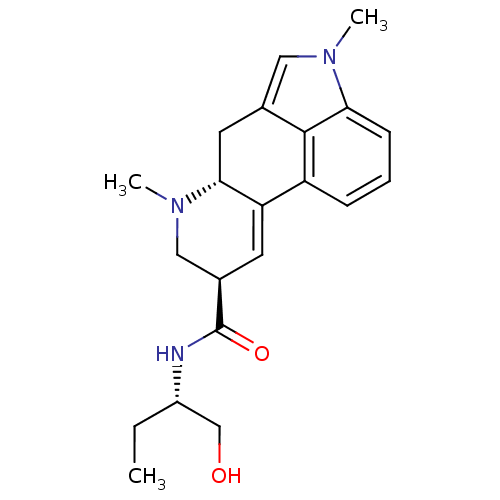
((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |r,c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50272662
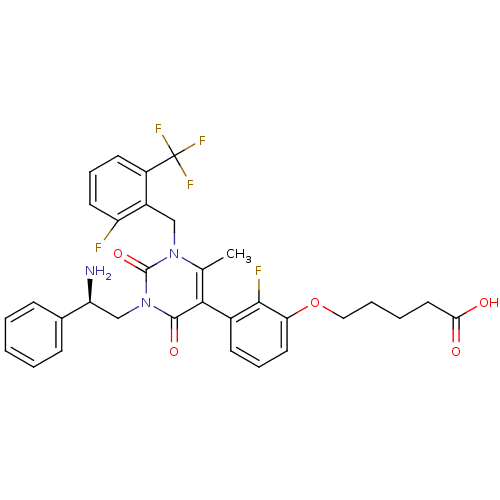
((R)-5-(3-(1-(2-fluoro-6-(trifluoromethyl)benzyl)-3...)Show SMILES Cc1c(-c2cccc(OCCCCC(O)=O)c2F)c(=O)n(C[C@H](N)c2ccccc2)c(=O)n1Cc1c(F)cccc1C(F)(F)F |r,wD:22.23,(32.43,-44.71,;31.09,-43.94,;31.09,-42.4,;32.42,-41.63,;33.75,-42.41,;35.09,-41.65,;35.1,-40.11,;33.75,-39.33,;33.75,-37.79,;35.09,-37.02,;35.09,-35.48,;36.42,-34.71,;36.42,-33.17,;37.75,-32.4,;39.09,-33.17,;37.75,-30.86,;32.42,-40.1,;31.09,-39.33,;29.76,-41.62,;29.76,-40.08,;28.44,-42.4,;27.1,-41.63,;25.77,-42.4,;25.77,-43.94,;24.43,-41.64,;24.44,-40.1,;23.1,-39.33,;21.77,-40.11,;21.78,-41.66,;23.11,-42.42,;28.44,-43.94,;27.1,-44.71,;29.76,-44.7,;29.76,-46.24,;28.98,-47.57,;29.81,-48.85,;31.35,-48.77,;29.12,-50.22,;27.57,-50.3,;26.74,-49.01,;27.44,-47.64,;26.61,-46.34,;25.79,-45.04,;25.31,-47.17,;27.91,-45.51,)| Show InChI InChI=1S/C32H30F5N3O5/c1-19-28(21-11-7-14-26(29(21)34)45-16-6-5-15-27(41)42)30(43)40(18-25(38)20-9-3-2-4-10-20)31(44)39(19)17-22-23(32(35,36)37)12-8-13-24(22)33/h2-4,7-14,25H,5-6,15-18,38H2,1H3,(H,41,42)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr5,D-Leu6,NMeLeu7-GnRH from human cloned GnRH receptor expressed in RBL cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50031942

((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |r,c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50272658
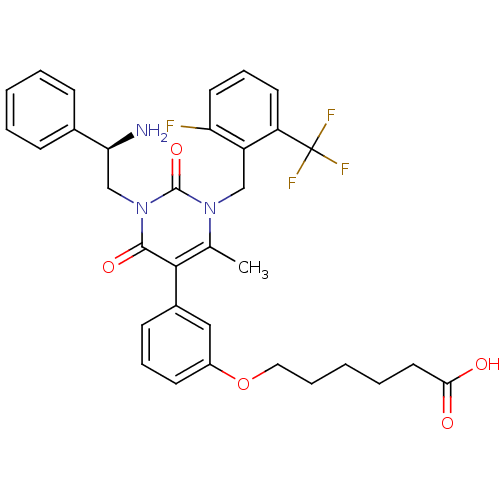
((R)-6-(3-(1-(2-fluoro-6-(trifluoromethyl)benzyl)-3...)Show SMILES Cc1c(-c2cccc(OCCCCCC(O)=O)c2)c(=O)n(C[C@H](N)c2ccccc2)c(=O)n1Cc1c(F)cccc1C(F)(F)F |r| Show InChI InChI=1S/C33H33F4N3O5/c1-21-30(23-12-8-13-24(18-23)45-17-7-3-6-16-29(41)42)31(43)40(20-28(38)22-10-4-2-5-11-22)32(44)39(21)19-25-26(33(35,36)37)14-9-15-27(25)34/h2,4-5,8-15,18,28H,3,6-7,16-17,19-20,38H2,1H3,(H,41,42)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr5,D-Leu6,NMeLeu7-GnRH from human cloned GnRH receptor expressed in RBL cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50272661

((R)-6-(3-(1-(2-fluoro-6-(trifluoromethyl)benzyl)-3...)Show SMILES Cc1c(-c2cccc(OCCCCCC(O)=O)c2F)c(=O)n(C[C@H](N)c2ccccc2)c(=O)n1Cc1c(F)cccc1C(F)(F)F |r,wD:23.24,(33.36,-44.3,;32.03,-43.53,;32.03,-41.99,;33.36,-41.23,;34.69,-42.01,;36.02,-41.24,;36.03,-39.7,;34.69,-38.93,;34.69,-37.39,;36.02,-36.62,;36.02,-35.08,;37.35,-34.31,;37.35,-32.77,;38.68,-31.99,;38.68,-30.45,;40.02,-29.68,;37.35,-29.68,;33.36,-39.7,;32.02,-38.93,;30.7,-41.21,;30.7,-39.67,;29.37,-41.99,;28.03,-41.23,;26.7,-42,;26.71,-43.54,;25.37,-41.23,;25.37,-39.7,;24.03,-38.93,;22.7,-39.7,;22.71,-41.25,;24.05,-42.01,;29.37,-43.53,;28.04,-44.3,;30.7,-44.29,;30.7,-45.83,;29.91,-47.16,;30.75,-48.45,;32.28,-48.37,;30.05,-49.82,;28.5,-49.9,;27.67,-48.6,;28.37,-47.23,;27.54,-45.94,;26.72,-44.63,;26.24,-46.76,;28.84,-45.11,)| Show InChI InChI=1S/C33H32F5N3O5/c1-20-29(22-12-8-15-27(30(22)35)46-17-7-3-6-16-28(42)43)31(44)41(19-26(39)21-10-4-2-5-11-21)32(45)40(20)18-23-24(33(36,37)38)13-9-14-25(23)34/h2,4-5,8-15,26H,3,6-7,16-19,39H2,1H3,(H,42,43)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr5,D-Leu6,NMeLeu7-GnRH from human cloned GnRH receptor expressed in RBL cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50272662

((R)-5-(3-(1-(2-fluoro-6-(trifluoromethyl)benzyl)-3...)Show SMILES Cc1c(-c2cccc(OCCCCC(O)=O)c2F)c(=O)n(C[C@H](N)c2ccccc2)c(=O)n1Cc1c(F)cccc1C(F)(F)F |r,wD:22.23,(32.43,-44.71,;31.09,-43.94,;31.09,-42.4,;32.42,-41.63,;33.75,-42.41,;35.09,-41.65,;35.1,-40.11,;33.75,-39.33,;33.75,-37.79,;35.09,-37.02,;35.09,-35.48,;36.42,-34.71,;36.42,-33.17,;37.75,-32.4,;39.09,-33.17,;37.75,-30.86,;32.42,-40.1,;31.09,-39.33,;29.76,-41.62,;29.76,-40.08,;28.44,-42.4,;27.1,-41.63,;25.77,-42.4,;25.77,-43.94,;24.43,-41.64,;24.44,-40.1,;23.1,-39.33,;21.77,-40.11,;21.78,-41.66,;23.11,-42.42,;28.44,-43.94,;27.1,-44.71,;29.76,-44.7,;29.76,-46.24,;28.98,-47.57,;29.81,-48.85,;31.35,-48.77,;29.12,-50.22,;27.57,-50.3,;26.74,-49.01,;27.44,-47.64,;26.61,-46.34,;25.79,-45.04,;25.31,-47.17,;27.91,-45.51,)| Show InChI InChI=1S/C32H30F5N3O5/c1-19-28(21-11-7-14-26(29(21)34)45-16-6-5-15-27(41)42)30(43)40(18-25(38)20-9-3-2-4-10-20)31(44)39(19)17-22-23(32(35,36)37)12-8-13-24(22)33/h2-4,7-14,25H,5-6,15-18,38H2,1H3,(H,41,42)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Pro-N-Et-GnRH from human cloned GnRH receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50272666
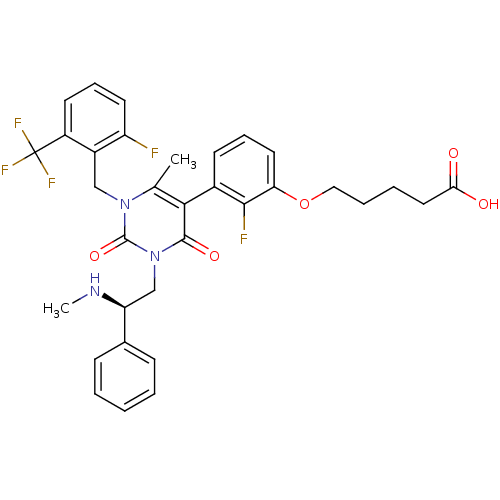
((R)-5-(3-(1-(2-fluoro-6-(trifluoromethyl)benzyl)-6...)Show SMILES CN[C@@H](Cn1c(=O)c(c(C)n(Cc2c(F)cccc2C(F)(F)F)c1=O)-c1cccc(OCCCCC(O)=O)c1F)c1ccccc1 |r,wD:2.1,(29.55,-7.07,;30.88,-6.3,;30.88,-4.76,;32.21,-3.99,;33.55,-4.75,;34.87,-3.97,;34.87,-2.43,;36.2,-4.75,;36.2,-6.29,;37.54,-7.06,;34.87,-7.05,;34.87,-8.59,;34.09,-9.92,;34.92,-11.21,;36.46,-11.13,;34.23,-12.58,;32.68,-12.66,;31.85,-11.36,;32.55,-9.99,;31.72,-8.7,;30.9,-7.39,;30.42,-9.52,;33.02,-7.87,;33.55,-6.29,;32.21,-7.06,;37.53,-3.99,;38.86,-4.77,;40.2,-4,;40.21,-2.46,;38.86,-1.69,;38.86,-.15,;40.2,.62,;40.2,2.16,;41.53,2.93,;41.53,4.47,;42.86,5.25,;44.2,4.48,;42.86,6.79,;37.53,-2.46,;36.2,-1.69,;29.54,-3.99,;29.55,-2.46,;28.21,-1.69,;26.88,-2.46,;26.89,-4.01,;28.22,-4.77,)| Show InChI InChI=1S/C33H32F5N3O5/c1-20-29(22-12-8-15-27(30(22)35)46-17-7-6-16-28(42)43)31(44)41(19-26(39-2)21-10-4-3-5-11-21)32(45)40(20)18-23-24(33(36,37)38)13-9-14-25(23)34/h3-5,8-15,26,39H,6-7,16-19H2,1-2H3,(H,42,43)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Pro-N-Et-GnRH from human cloned GnRH receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50260759
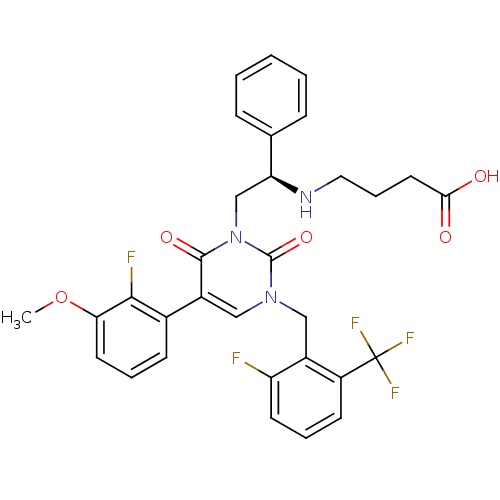
((R)-4-(2-(3-(2-fluoro-6-(trifluoromethyl)benzyl)-5...)Show SMILES COc1cccc(c1F)-c1cn(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](NCCCC(O)=O)c2ccccc2)c1=O |r| Show InChI InChI=1S/C31H28F5N3O5/c1-44-26-13-5-10-20(28(26)33)21-16-38(17-22-23(31(34,35)36)11-6-12-24(22)32)30(43)39(29(21)42)18-25(19-8-3-2-4-9-19)37-15-7-14-27(40)41/h2-6,8-13,16,25,37H,7,14-15,17-18H2,1H3,(H,40,41)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Pro-N-Et-GnRH from human cloned GnRH receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50272661

((R)-6-(3-(1-(2-fluoro-6-(trifluoromethyl)benzyl)-3...)Show SMILES Cc1c(-c2cccc(OCCCCCC(O)=O)c2F)c(=O)n(C[C@H](N)c2ccccc2)c(=O)n1Cc1c(F)cccc1C(F)(F)F |r,wD:23.24,(33.36,-44.3,;32.03,-43.53,;32.03,-41.99,;33.36,-41.23,;34.69,-42.01,;36.02,-41.24,;36.03,-39.7,;34.69,-38.93,;34.69,-37.39,;36.02,-36.62,;36.02,-35.08,;37.35,-34.31,;37.35,-32.77,;38.68,-31.99,;38.68,-30.45,;40.02,-29.68,;37.35,-29.68,;33.36,-39.7,;32.02,-38.93,;30.7,-41.21,;30.7,-39.67,;29.37,-41.99,;28.03,-41.23,;26.7,-42,;26.71,-43.54,;25.37,-41.23,;25.37,-39.7,;24.03,-38.93,;22.7,-39.7,;22.71,-41.25,;24.05,-42.01,;29.37,-43.53,;28.04,-44.3,;30.7,-44.29,;30.7,-45.83,;29.91,-47.16,;30.75,-48.45,;32.28,-48.37,;30.05,-49.82,;28.5,-49.9,;27.67,-48.6,;28.37,-47.23,;27.54,-45.94,;26.72,-44.63,;26.24,-46.76,;28.84,-45.11,)| Show InChI InChI=1S/C33H32F5N3O5/c1-20-29(22-12-8-15-27(30(22)35)46-17-7-3-6-16-28(42)43)31(44)41(19-26(39)21-10-4-2-5-11-21)32(45)40(20)18-23-24(33(36,37)38)13-9-14-25(23)34/h2,4-5,8-15,26H,3,6-7,16-19,39H2,1H3,(H,42,43)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Pro-N-Et-GnRH from human cloned GnRH receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM22867
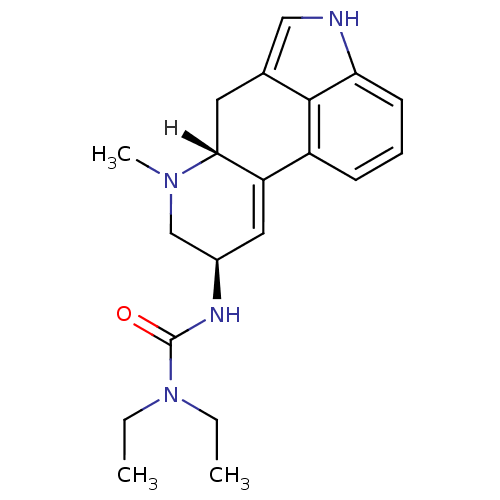
(1,1-diethyl-3-[(8beta)-6-methyl-9,10-didehydroergo...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)NC(=O)N(CC)CC)c34 |r,c:12| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-hydroxytryptamine 2A receptor in NIH3T3 cells. |
J Med Chem 41: 5084-93 (1999)
Article DOI: 10.1021/jm980452a
BindingDB Entry DOI: 10.7270/Q2CZ369B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50168694
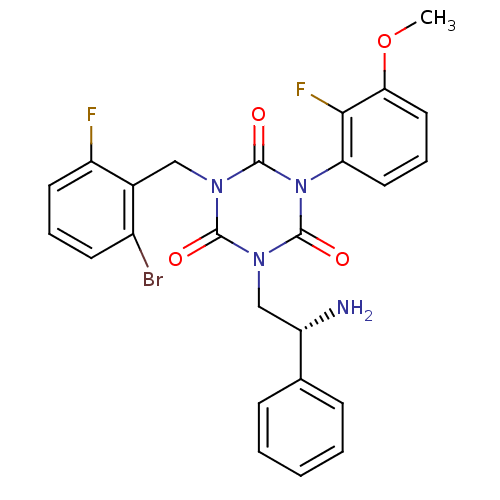
(1-((R)-2-Amino-2-phenyl-ethyl)-3-(2-bromo-6-fluoro...)Show SMILES COc1cccc(c1F)-n1c(=O)n(C[C@H](N)c2ccccc2)c(=O)n(Cc2c(F)cccc2Br)c1=O |wD:14.15,(8.68,1.49,;7.35,.72,;7.36,-.82,;8.7,-1.59,;8.7,-3.13,;7.37,-3.9,;6.04,-3.13,;6.03,-1.61,;4.7,-.85,;4.71,-3.92,;3.37,-3.15,;3.37,-1.61,;2.04,-3.92,;.71,-3.15,;-.62,-3.93,;-1.4,-5.26,;-1.96,-3.16,;-3.31,-3.93,;-4.64,-3.16,;-4.64,-1.61,;-3.31,-.84,;-1.96,-1.61,;2.04,-5.46,;.71,-6.23,;3.38,-6.23,;3.38,-7.77,;4.72,-8.54,;6.04,-7.75,;7.12,-6.66,;7.37,-8.52,;7.38,-10.06,;6.05,-10.83,;4.71,-10.08,;3.38,-10.85,;4.71,-5.46,;6.05,-6.21,)| Show InChI InChI=1S/C25H21BrF2N4O4/c1-36-21-12-6-11-20(22(21)28)32-24(34)30(13-16-17(26)9-5-10-18(16)27)23(33)31(25(32)35)14-19(29)15-7-3-2-4-8-15/h2-12,19H,13-14,29H2,1H3/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit des-Gly[125I-Tyr, DLeu, NMeLeu,Pro-NEt]-GnRH radioligand binding to the cloned human GnRH receptor stably expressed in HE... |
Bioorg Med Chem Lett 15: 3685-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.038
BindingDB Entry DOI: 10.7270/Q2DZ07TN |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50272658

((R)-6-(3-(1-(2-fluoro-6-(trifluoromethyl)benzyl)-3...)Show SMILES Cc1c(-c2cccc(OCCCCCC(O)=O)c2)c(=O)n(C[C@H](N)c2ccccc2)c(=O)n1Cc1c(F)cccc1C(F)(F)F |r| Show InChI InChI=1S/C33H33F4N3O5/c1-21-30(23-12-8-13-24(18-23)45-17-7-3-6-16-29(41)42)31(43)40(20-28(38)22-10-4-2-5-11-22)32(44)39(21)19-25-26(33(35,36)37)14-9-15-27(25)34/h2,4-5,8-15,18,28H,3,6-7,16-17,19-20,38H2,1H3,(H,41,42)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Pro-N-Et-GnRH from human cloned GnRH receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21342
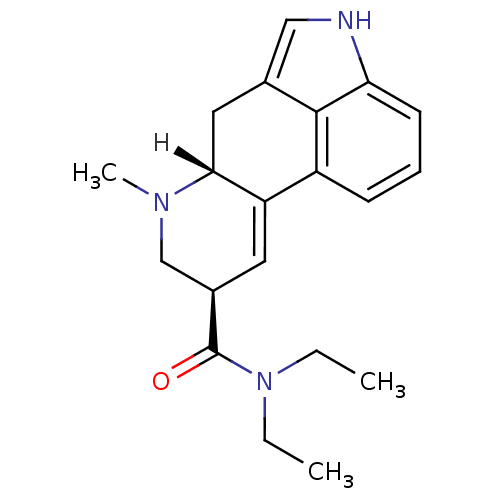
((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM21342

((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50168462

(2-(2,4-Dichloro-phenyl)-4-methyl-6-(1-propyl-butyl...)Show SMILES CCCC(CCC)N1CCc2nn(-c3ccc(Cl)cc3Cl)c3nc(C)cc1c23 Show InChI InChI=1S/C22H26Cl2N4/c1-4-6-16(7-5-2)27-11-10-18-21-20(27)12-14(3)25-22(21)28(26-18)19-9-8-15(23)13-17(19)24/h8-9,12-13,16H,4-7,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of [125I]sauvagine binding to corticotropin releasing factor receptor 1 expressed in LtK- cells |
J Med Chem 48: 4100-10 (2005)
Article DOI: 10.1021/jm050070m
BindingDB Entry DOI: 10.7270/Q2ZK5HGJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50168700

(1-((R)-2-Amino-2-phenyl-ethyl)-3-(2-fluoro-3-metho...)Show SMILES COc1cccc(c1F)-n1c(=O)n(C[C@H](N)c2ccccc2)c(=O)n(Cc2c(F)cccc2C(F)(F)F)c1=O |wD:14.15,(8.68,1.49,;7.35,.72,;7.36,-.82,;8.7,-1.59,;8.7,-3.13,;7.37,-3.9,;6.04,-3.13,;6.03,-1.61,;4.7,-.85,;4.71,-3.92,;3.37,-3.15,;3.37,-1.61,;2.04,-3.92,;.71,-3.15,;-.62,-3.93,;-1.4,-5.26,;-1.96,-3.16,;-1.96,-1.61,;-3.31,-.84,;-4.64,-1.61,;-4.64,-3.16,;-3.31,-3.93,;2.04,-5.46,;.71,-6.23,;3.38,-6.23,;3.38,-7.77,;4.72,-8.54,;6.04,-7.75,;7.12,-6.66,;7.37,-8.52,;7.38,-10.06,;6.05,-10.83,;4.71,-10.08,;3.38,-10.85,;2.04,-10.06,;3.37,-12.39,;2.29,-11.93,;4.71,-5.46,;6.05,-6.21,)| Show InChI InChI=1S/C26H21F5N4O4/c1-39-21-12-6-11-20(22(21)28)35-24(37)33(13-16-17(26(29,30)31)9-5-10-18(16)27)23(36)34(25(35)38)14-19(32)15-7-3-2-4-8-15/h2-12,19H,13-14,32H2,1H3/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit des-Gly[125I-Tyr, DLeu, NMeLeu,Pro-NEt]-GnRH radioligand binding to the cloned human GnRH receptor stably expressed in HE... |
Bioorg Med Chem Lett 15: 3685-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.038
BindingDB Entry DOI: 10.7270/Q2DZ07TN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50091069
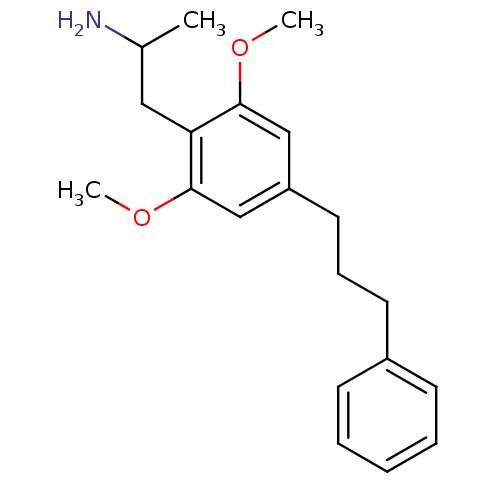
(2-[2,6-Dimethoxy-4-(3-phenyl-propyl)-phenyl]-1-met...)Show InChI InChI=1S/C20H27NO2/c1-15(21)12-18-19(22-2)13-17(14-20(18)23-3)11-7-10-16-8-5-4-6-9-16/h4-6,8-9,13-15H,7,10-12,21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ketanserin from NIH3T3 cells stably expressing rat 5-hydroxytryptamine 2A receptor |
J Med Chem 43: 3074-84 (2000)
BindingDB Entry DOI: 10.7270/Q2NZ86VW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM28582
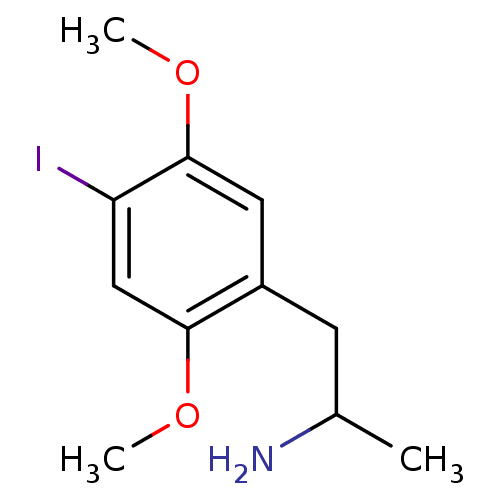
(1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...)Show InChI InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074501
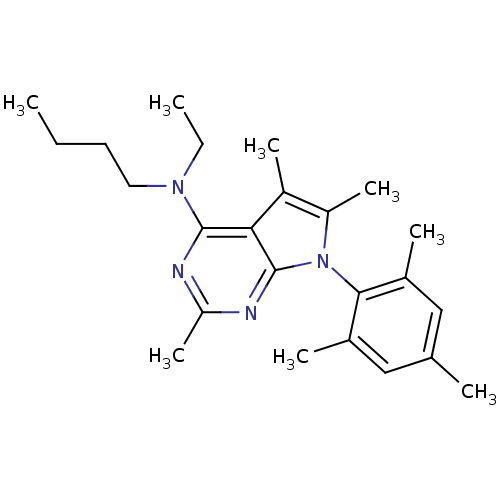
(Butyl-ethyl-[2,5,6-trimethyl-7-(2,4,6-trimethyl-ph...)Show SMILES CCCCN(CC)c1nc(C)nc2n(c(C)c(C)c12)-c1c(C)cc(C)cc1C |(4.9,1.82,;3.57,2.56,;2.24,1.79,;.91,2.55,;-.44,1.78,;-1.76,2.55,;-3.1,1.78,;-.42,.25,;-1.75,-.52,;-1.75,-2.06,;-3.08,-2.83,;-.42,-2.83,;.91,-2.06,;2.38,-2.54,;3.31,-1.29,;4.85,-1.29,;2.38,-.03,;2.86,1.43,;.91,-.52,;2.86,-4.02,;4.37,-4.33,;5.39,-3.17,;4.85,-5.78,;3.82,-6.93,;4.31,-8.4,;2.32,-6.61,;1.84,-5.14,;.33,-4.82,)| Show InChI InChI=1S/C24H34N4/c1-9-11-12-27(10-2)23-21-18(6)19(7)28(24(21)26-20(8)25-23)22-16(4)13-15(3)14-17(22)5/h13-14H,9-12H2,1-8H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of [125I]sauvagine binding to corticotropin releasing factor receptor 1 expressed in LtK- cells |
J Med Chem 48: 4100-10 (2005)
Article DOI: 10.1021/jm050070m
BindingDB Entry DOI: 10.7270/Q2ZK5HGJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50005257
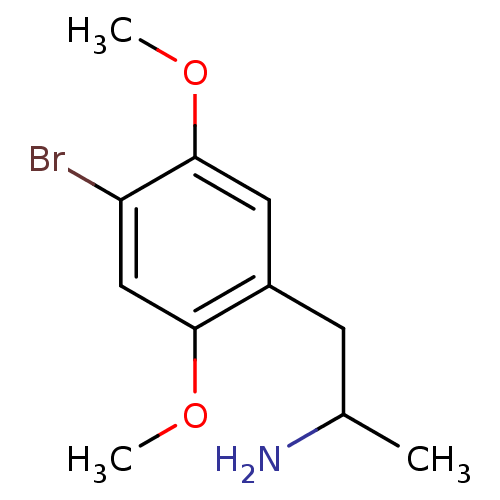
((+)-2-(4-Bromo-2,5-dimethoxy-phenyl)-1-methyl-ethy...)Show InChI InChI=1S/C11H16BrNO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50168475
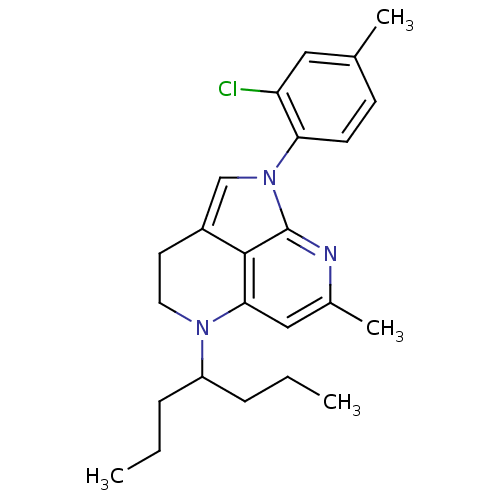
(1-(2-Chloro-4-methyl-phenyl)-7-methyl-5-(1-propyl-...)Show SMILES CCCC(CCC)N1CCc2cn(-c3ccc(C)cc3Cl)c3nc(C)cc1c23 Show InChI InChI=1S/C24H30ClN3/c1-5-7-19(8-6-2)27-12-11-18-15-28(21-10-9-16(3)13-20(21)25)24-23(18)22(27)14-17(4)26-24/h9-10,13-15,19H,5-8,11-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of [125I]sauvagine binding to corticotropin releasing factor receptor 1 expressed in LtK- cells |
J Med Chem 48: 4100-10 (2005)
Article DOI: 10.1021/jm050070m
BindingDB Entry DOI: 10.7270/Q2ZK5HGJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50091079

(2-[3,5-Dimethoxy-4-(3-phenyl-propyl)-phenyl]-1-met...)Show InChI InChI=1S/C20H27NO2/c1-15(21)12-17-13-19(22-2)18(20(14-17)23-3)11-7-10-16-8-5-4-6-9-16/h4-6,8-9,13-15H,7,10-12,21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ketanserin from NIH3T3 cells stably expressing rat 5-hydroxytryptamine 2A receptor |
J Med Chem 43: 3074-84 (2000)
BindingDB Entry DOI: 10.7270/Q2NZ86VW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50091065
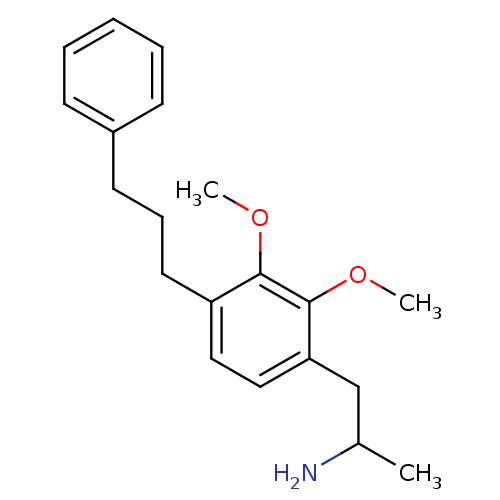
(2-[2,3-Dimethoxy-4-(3-phenyl-propyl)-phenyl]-1-met...)Show InChI InChI=1S/C20H27NO2/c1-15(21)14-18-13-12-17(19(22-2)20(18)23-3)11-7-10-16-8-5-4-6-9-16/h4-6,8-9,12-13,15H,7,10-11,14,21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ketanserin from NIH3T3 cells stably expressing rat 5-hydroxytryptamine 2A receptor |
J Med Chem 43: 3074-84 (2000)
BindingDB Entry DOI: 10.7270/Q2NZ86VW |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50272659
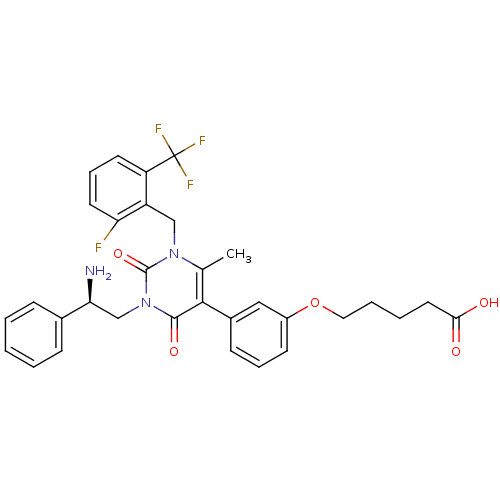
((R)-5-(3-(1-(2-fluoro-6-(trifluoromethyl)benzyl)-3...)Show SMILES Cc1c(-c2cccc(OCCCCC(O)=O)c2)c(=O)n(C[C@H](N)c2ccccc2)c(=O)n1Cc1c(F)cccc1C(F)(F)F |r| Show InChI InChI=1S/C32H31F4N3O5/c1-20-29(22-11-7-12-23(17-22)44-16-6-5-15-28(40)41)30(42)39(19-27(37)21-9-3-2-4-10-21)31(43)38(20)18-24-25(32(34,35)36)13-8-14-26(24)33/h2-4,7-14,17,27H,5-6,15-16,18-19,37H2,1H3,(H,40,41)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Pro-N-Et-GnRH from human cloned GnRH receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50168466
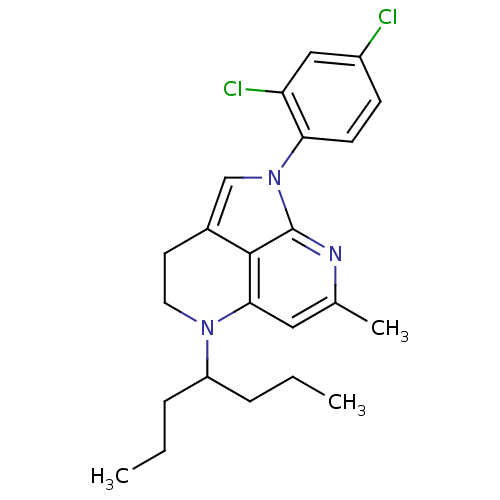
(1-(2,4-Dichloro-phenyl)-7-methyl-5-(1-propyl-butyl...)Show SMILES CCCC(CCC)N1CCc2cn(-c3ccc(Cl)cc3Cl)c3nc(C)cc1c23 Show InChI InChI=1S/C23H27Cl2N3/c1-4-6-18(7-5-2)27-11-10-16-14-28(20-9-8-17(24)13-19(20)25)23-22(16)21(27)12-15(3)26-23/h8-9,12-14,18H,4-7,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of [125I]sauvagine binding to corticotropin releasing factor receptor 1 expressed in LtK- cells |
J Med Chem 48: 4100-10 (2005)
Article DOI: 10.1021/jm050070m
BindingDB Entry DOI: 10.7270/Q2ZK5HGJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50272663

((R)-4-(3-(1-(2-fluoro-6-(trifluoromethyl)benzyl)-3...)Show SMILES Cc1c(-c2cccc(OCCCC(O)=O)c2F)c(=O)n(C[C@H](N)c2ccccc2)c(=O)n1Cc1c(F)cccc1C(F)(F)F |r,wD:21.22,(33.83,-41.56,;32.49,-40.79,;32.49,-39.25,;33.82,-38.48,;35.15,-39.26,;36.49,-38.5,;36.5,-36.96,;35.15,-36.18,;35.15,-34.64,;36.49,-33.87,;36.49,-32.33,;37.82,-31.56,;37.82,-30.02,;39.15,-29.25,;36.49,-29.25,;33.82,-36.95,;32.49,-36.18,;31.16,-38.47,;31.16,-36.93,;29.84,-39.25,;28.5,-38.48,;27.17,-39.25,;27.17,-40.79,;25.83,-38.49,;25.84,-36.95,;24.5,-36.18,;23.17,-36.96,;23.18,-38.51,;24.51,-39.27,;29.84,-40.79,;28.5,-41.56,;31.16,-41.55,;31.16,-43.09,;30.38,-44.42,;31.21,-45.7,;32.75,-45.62,;30.52,-47.07,;28.97,-47.15,;28.14,-45.86,;28.84,-44.49,;28.01,-43.19,;27.19,-41.89,;26.71,-44.02,;29.31,-42.36,)| Show InChI InChI=1S/C31H28F5N3O5/c1-18-27(20-10-5-13-25(28(20)33)44-15-7-14-26(40)41)29(42)39(17-24(37)19-8-3-2-4-9-19)30(43)38(18)16-21-22(31(34,35)36)11-6-12-23(21)32/h2-6,8-13,24H,7,14-17,37H2,1H3,(H,40,41)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr5,D-Leu6,NMeLeu7-GnRH from human cloned GnRH receptor expressed in RBL cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50168722
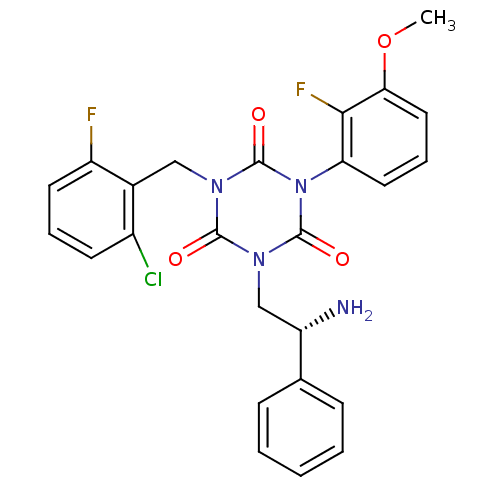
(1-((R)-2-Amino-2-phenyl-ethyl)-3-(2-chloro-6-fluor...)Show SMILES COc1cccc(c1F)-n1c(=O)n(C[C@H](N)c2ccccc2)c(=O)n(Cc2c(F)cccc2Cl)c1=O |wD:14.15,(8.68,1.49,;7.35,.72,;7.36,-.82,;8.7,-1.59,;8.7,-3.13,;7.37,-3.9,;6.04,-3.13,;6.03,-1.61,;4.7,-.85,;4.71,-3.92,;3.37,-3.15,;3.37,-1.61,;2.04,-3.92,;.71,-3.15,;-.62,-3.93,;-1.4,-5.26,;-1.96,-3.16,;-3.31,-3.93,;-4.64,-3.16,;-4.64,-1.61,;-3.31,-.84,;-1.96,-1.61,;2.04,-5.46,;.71,-6.23,;3.38,-6.23,;3.38,-7.77,;4.72,-8.54,;6.04,-7.75,;7.12,-6.66,;7.37,-8.52,;7.38,-10.06,;6.05,-10.83,;4.71,-10.08,;3.38,-10.85,;4.71,-5.46,;6.05,-6.21,)| Show InChI InChI=1S/C25H21ClF2N4O4/c1-36-21-12-6-11-20(22(21)28)32-24(34)30(13-16-17(26)9-5-10-18(16)27)23(33)31(25(32)35)14-19(29)15-7-3-2-4-8-15/h2-12,19H,13-14,29H2,1H3/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit des-Gly[125I-Tyr, DLeu, NMeLeu,Pro-NEt]-GnRH radioligand binding to the cloned human GnRH receptor stably expressed in HE... |
Bioorg Med Chem Lett 15: 3685-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.038
BindingDB Entry DOI: 10.7270/Q2DZ07TN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM22867

(1,1-diethyl-3-[(8beta)-6-methyl-9,10-didehydroergo...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)NC(=O)N(CC)CC)c34 |r,c:12| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21342

((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM22867

(1,1-diethyl-3-[(8beta)-6-methyl-9,10-didehydroergo...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)NC(=O)N(CC)CC)c34 |r,c:12| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50168463

(1-(2,6-Dimethoxy-pyridin-3-yl)-7-methyl-5-(1-propy...)Show SMILES CCCC(CCC)N1CCc2cn(-c3ccc(OC)nc3OC)c3nc(C)cc1c23 Show InChI InChI=1S/C24H32N4O2/c1-6-8-18(9-7-2)27-13-12-17-15-28(23-22(17)20(27)14-16(3)25-23)19-10-11-21(29-4)26-24(19)30-5/h10-11,14-15,18H,6-9,12-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of [125I]sauvagine binding to corticotropin releasing factor receptor 1 expressed in LtK- cells |
J Med Chem 48: 4100-10 (2005)
Article DOI: 10.1021/jm050070m
BindingDB Entry DOI: 10.7270/Q2ZK5HGJ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50168474
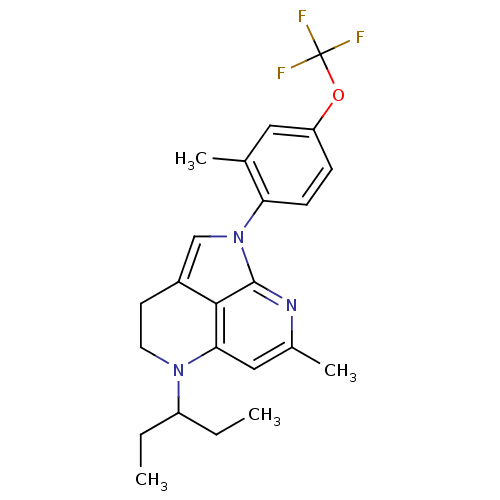
(5-(1-Ethyl-propyl)-7-methyl-1-(2-methyl-4-trifluor...)Show SMILES CCC(CC)N1CCc2cn(-c3ccc(OC(F)(F)F)cc3C)c3nc(C)cc1c23 Show InChI InChI=1S/C23H26F3N3O/c1-5-17(6-2)28-10-9-16-13-29(22-21(16)20(28)12-15(4)27-22)19-8-7-18(11-14(19)3)30-23(24,25)26/h7-8,11-13,17H,5-6,9-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of [125I]sauvagine binding to corticotropin releasing factor receptor 1 expressed in LtK- cells |
J Med Chem 48: 4100-10 (2005)
Article DOI: 10.1021/jm050070m
BindingDB Entry DOI: 10.7270/Q2ZK5HGJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50068525

(4-Cyclohexylamino-1-[4-(4-fluoro-phenyl)-4-oxo-but...)Show SMILES NC(=O)C1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)NC1CCCCC1 Show InChI InChI=1S/C22H32FN3O2/c23-18-10-8-17(9-11-18)20(27)7-4-14-26-15-12-22(13-16-26,21(24)28)25-19-5-2-1-3-6-19/h8-11,19,25H,1-7,12-16H2,(H2,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-hydroxytryptamine 2A receptor in NIH3T3 cells. |
J Med Chem 41: 5084-93 (1999)
Article DOI: 10.1021/jm980452a
BindingDB Entry DOI: 10.7270/Q2CZ369B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50091074
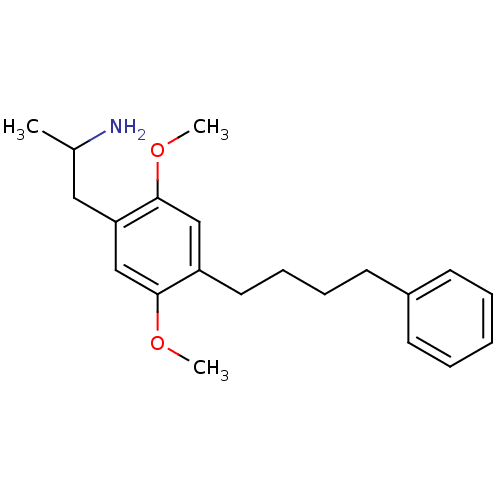
(2-[2,5-Dimethoxy-4-(4-phenyl-butyl)-phenyl]-1-meth...)Show InChI InChI=1S/C21H29NO2/c1-16(22)13-19-15-20(23-2)18(14-21(19)24-3)12-8-7-11-17-9-5-4-6-10-17/h4-6,9-10,14-16H,7-8,11-13,22H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from A9 cells stably expressing rat 5-hydroxytryptamine 2C receptor |
J Med Chem 43: 3074-84 (2000)
BindingDB Entry DOI: 10.7270/Q2NZ86VW |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50168464

(1-(4-Methoxy-2-methyl-phenyl)-7-methyl-5-(1-propyl...)Show SMILES CCCC(CCC)N1CCc2cn(-c3ccc(OC)cc3C)c3nc(C)cc1c23 Show InChI InChI=1S/C25H33N3O/c1-6-8-20(9-7-2)27-13-12-19-16-28(22-11-10-21(29-5)14-17(22)3)25-24(19)23(27)15-18(4)26-25/h10-11,14-16,20H,6-9,12-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of [125I]sauvagine binding to corticotropin releasing factor receptor 1 expressed in LtK- cells |
J Med Chem 48: 4100-10 (2005)
Article DOI: 10.1021/jm050070m
BindingDB Entry DOI: 10.7270/Q2ZK5HGJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM21342

((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50068518
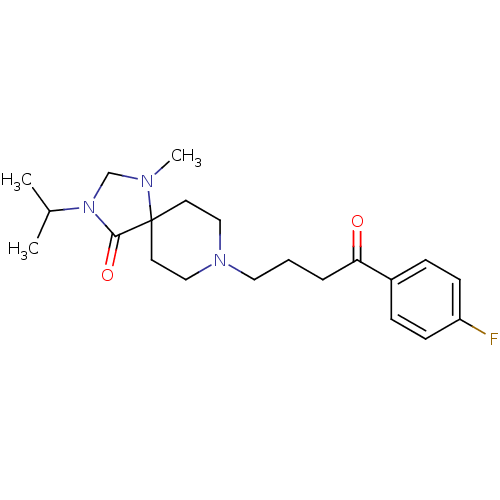
(8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-isopropyl-1-...)Show SMILES CC(C)N1CN(C)C2(CCN(CCCC(=O)c3ccc(F)cc3)CC2)C1=O Show InChI InChI=1S/C21H30FN3O2/c1-16(2)25-15-23(3)21(20(25)27)10-13-24(14-11-21)12-4-5-19(26)17-6-8-18(22)9-7-17/h6-9,16H,4-5,10-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-hydroxytryptamine 2A receptor in NIH3T3 cells. |
J Med Chem 41: 5084-93 (1999)
Article DOI: 10.1021/jm980452a
BindingDB Entry DOI: 10.7270/Q2CZ369B |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50168473

(3-Chloro-4-[7-methyl-5-(1-propyl-butyl)-4,5-dihydr...)Show SMILES CCCC(CCC)N1CCc2cn(-c3ccc(cc3Cl)C#N)c3nc(C)cc1c23 Show InChI InChI=1S/C24H27ClN4/c1-4-6-19(7-5-2)28-11-10-18-15-29(21-9-8-17(14-26)13-20(21)25)24-23(18)22(28)12-16(3)27-24/h8-9,12-13,15,19H,4-7,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of [125I]sauvagine binding to corticotropin releasing factor receptor 1 expressed in LtK- cells |
J Med Chem 48: 4100-10 (2005)
Article DOI: 10.1021/jm050070m
BindingDB Entry DOI: 10.7270/Q2ZK5HGJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50272660

((R)-4-(3-(1-(2-fluoro-6-(trifluoromethyl)benzyl)-3...)Show SMILES Cc1c(-c2cccc(OCCCC(O)=O)c2)c(=O)n(C[C@H](N)c2ccccc2)c(=O)n1Cc1c(F)cccc1C(F)(F)F |r| Show InChI InChI=1S/C31H29F4N3O5/c1-19-28(21-10-5-11-22(16-21)43-15-7-14-27(39)40)29(41)38(18-26(36)20-8-3-2-4-9-20)30(42)37(19)17-23-24(31(33,34)35)12-6-13-25(23)32/h2-6,8-13,16,26H,7,14-15,17-18,36H2,1H3,(H,39,40)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr5,D-Leu6,NMeLeu7-GnRH from human cloned GnRH receptor expressed in RBL cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50116105

(3-(6-(dimethylamino)-4-methylpyridin-3-yl)-2,5-dim...)Show SMILES CCCN(CCC)c1cc(C)nc2c(c(C)nn12)-c1cnc(cc1C)N(C)C |(-1.91,-13.39,;-3.24,-14.16,;-4.58,-13.4,;-5.91,-14.18,;-7.24,-13.41,;-8.57,-14.19,;-9.91,-13.42,;-5.9,-15.72,;-7.22,-16.49,;-7.23,-18.03,;-8.56,-18.8,;-5.9,-18.8,;-4.55,-18.03,;-3.07,-18.5,;-2.16,-17.24,;-.62,-17.23,;-3.09,-15.99,;-4.56,-16.48,;-2.58,-19.96,;-3.61,-21.11,;-3.13,-22.57,;-1.62,-22.88,;-.59,-21.72,;-1.08,-20.27,;-.06,-19.11,;-1.13,-24.34,;.38,-24.65,;-2.15,-25.5,)| Show InChI InChI=1S/C22H32N6/c1-8-10-27(11-9-2)20-13-16(4)24-22-21(17(5)25-28(20)22)18-14-23-19(26(6)7)12-15(18)3/h12-14H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of [125I]sauvagine binding to corticotropin releasing factor receptor 1 expressed in LtK- cells |
J Med Chem 48: 4100-10 (2005)
Article DOI: 10.1021/jm050070m
BindingDB Entry DOI: 10.7270/Q2ZK5HGJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50091067
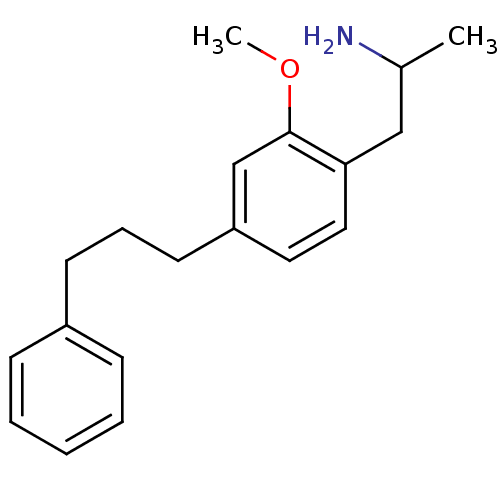
(2-[2-Methoxy-4-(3-phenyl-propyl)-phenyl]-1-methyl-...)Show InChI InChI=1S/C19H25NO/c1-15(20)13-18-12-11-17(14-19(18)21-2)10-6-9-16-7-4-3-5-8-16/h3-5,7-8,11-12,14-15H,6,9-10,13,20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ketanserin from NIH3T3 cells stably expressing rat 5-hydroxytryptamine 2A receptor |
J Med Chem 43: 3074-84 (2000)
BindingDB Entry DOI: 10.7270/Q2NZ86VW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50091074

(2-[2,5-Dimethoxy-4-(4-phenyl-butyl)-phenyl]-1-meth...)Show InChI InChI=1S/C21H29NO2/c1-16(22)13-19-15-20(23-2)18(14-21(19)24-3)12-8-7-11-17-9-5-4-6-10-17/h4-6,9-10,14-16H,7-8,11-13,22H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ketanserin from NIH3T3 cells stably expressing rat 5-hydroxytryptamine 2A receptor |
J Med Chem 43: 3074-84 (2000)
BindingDB Entry DOI: 10.7270/Q2NZ86VW |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50168697

((R)-1-(2,6-difluorobenzyl)-3-(2-amino-2-phenylethy...)Show SMILES COc1cccc(c1F)-n1c(=O)n(C[C@H](N)c2ccccc2)c(=O)n(Cc2c(F)cccc2F)c1=O |r,wD:14.15,(31.35,-22.11,;30.02,-22.89,;30.02,-24.43,;31.37,-25.2,;31.37,-26.74,;30.04,-27.51,;28.71,-26.74,;28.7,-25.2,;27.36,-24.44,;27.38,-27.51,;26.05,-26.73,;26.05,-25.19,;24.72,-27.51,;23.38,-26.74,;22.05,-27.52,;22.05,-29.06,;20.72,-26.76,;20.72,-25.21,;19.38,-24.45,;18.05,-25.22,;18.06,-26.77,;19.39,-27.53,;24.72,-29.05,;23.39,-29.82,;26.06,-29.82,;26.06,-31.36,;27.4,-32.12,;28.72,-31.34,;27.38,-30.57,;30.06,-32.1,;30.07,-33.65,;28.74,-34.42,;27.4,-33.66,;26.07,-34.43,;27.38,-29.05,;28.71,-29.82,)| Show InChI InChI=1S/C25H21F3N4O4/c1-36-21-12-6-11-20(22(21)28)32-24(34)30(13-16-17(26)9-5-10-18(16)27)23(33)31(25(32)35)14-19(29)15-7-3-2-4-8-15/h2-12,19H,13-14,29H2,1H3/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit des-Gly[125I-Tyr, DLeu, NMeLeu,Pro-NEt]-GnRH radioligand binding to the cloned human GnRH receptor stably expressed in HE... |
Bioorg Med Chem Lett 15: 3685-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.038
BindingDB Entry DOI: 10.7270/Q2DZ07TN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM28582

(1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...)Show InChI InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Medical College
Curated by PDSP Ki Database
| |
Synapse 35: 144-50 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K
BindingDB Entry DOI: 10.7270/Q2ST7NDX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data