

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50091753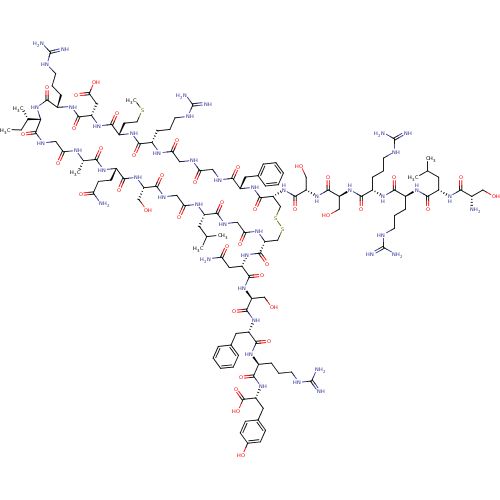 (CHEMBL405854 | H-Ser-Leu-Arg-Arg-Ser-Ser-cyclic(Cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50091751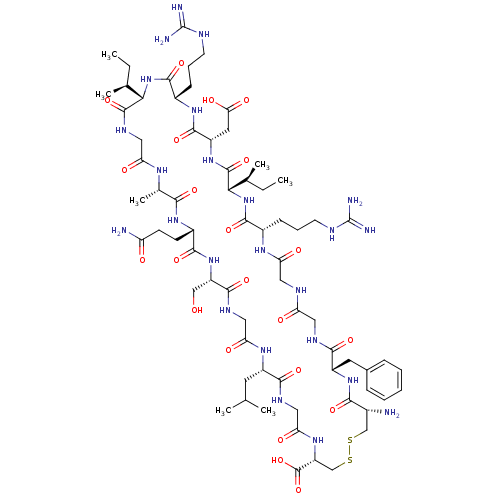 (CHEMBL411542 | Cyclic-(Cys-Phe-Gly-Gly-Arg-Ile-Asp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 1 (Homo sapiens (Human)) | BDBM50091751 (CHEMBL411542 | Cyclic-(Cys-Phe-Gly-Gly-Arg-Ile-Asp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074629 (4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... | Bioorg Med Chem Lett 7: 3017-3022 (1997) Article DOI: 10.1016/S0960-894X(97)10137-8 BindingDB Entry DOI: 10.7270/Q2280843 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50091758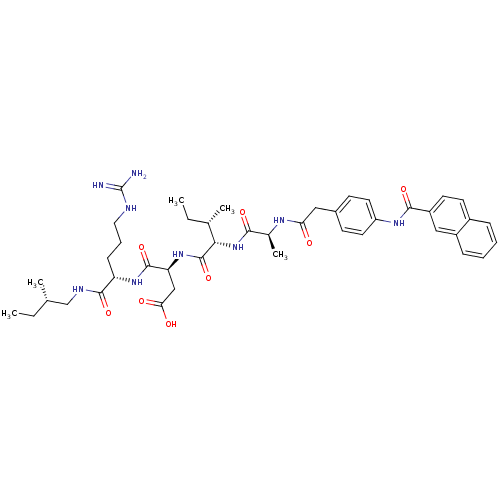 ((S)-N-[(S)-4-Guanidino-1-((S)-2-methyl-butylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22916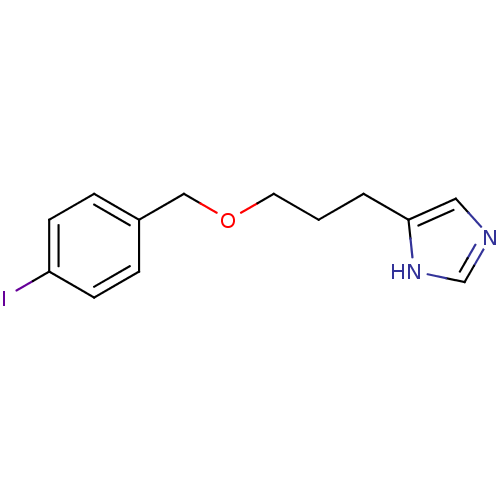 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... | Bioorg Med Chem Lett 7: 3017-3022 (1997) Article DOI: 10.1016/S0960-894X(97)10137-8 BindingDB Entry DOI: 10.7270/Q2280843 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 1 (Homo sapiens (Human)) | BDBM50091758 ((S)-N-[(S)-4-Guanidino-1-((S)-2-methyl-butylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074627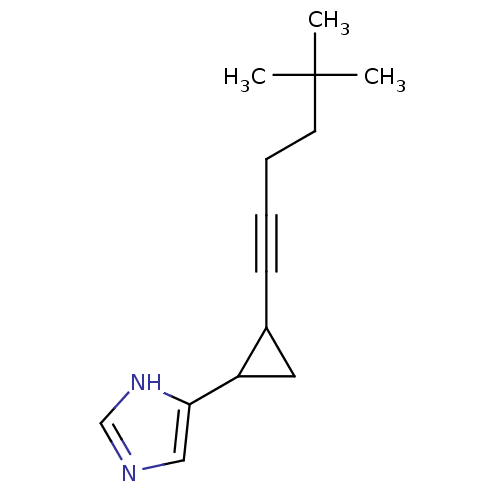 (4-[2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 1 (Homo sapiens (Human)) | BDBM50091752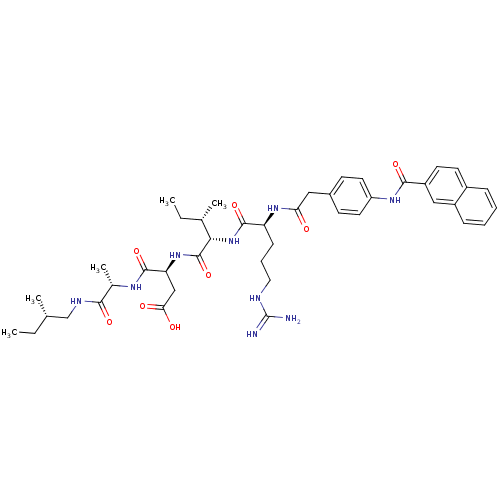 ((S)-3-{(2S,3S)-2-[(S)-5-Guanidino-2-(2-{4-[(naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50091752 ((S)-3-{(2S,3S)-2-[(S)-5-Guanidino-2-(2-{4-[(naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50091761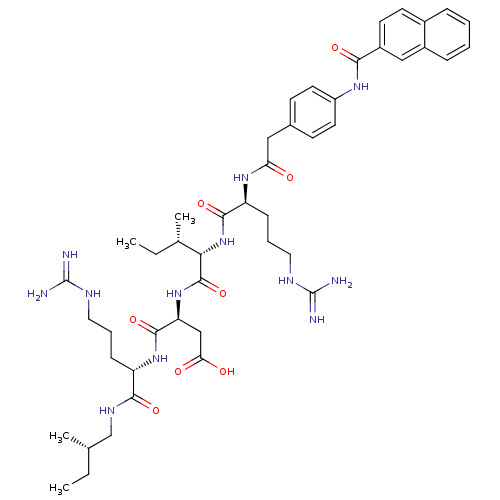 ((S)-N-[(S)-4-Guanidino-1-((S)-2-methyl-butylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50091757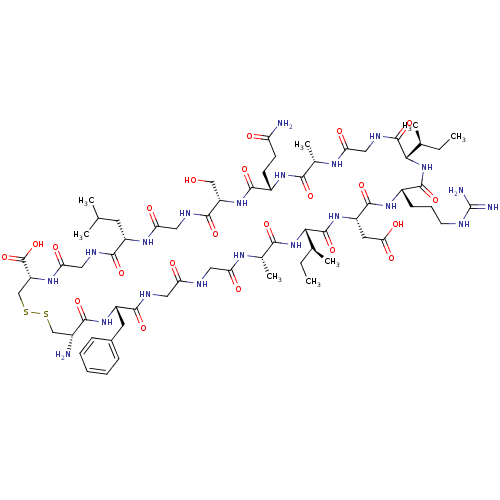 (CHEMBL264744 | Cyclic-(Cys-Phe-Gly-Gly-Ala-Ile-Asp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 1 (Homo sapiens (Human)) | BDBM50091757 (CHEMBL264744 | Cyclic-(Cys-Phe-Gly-Gly-Ala-Ile-Asp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070217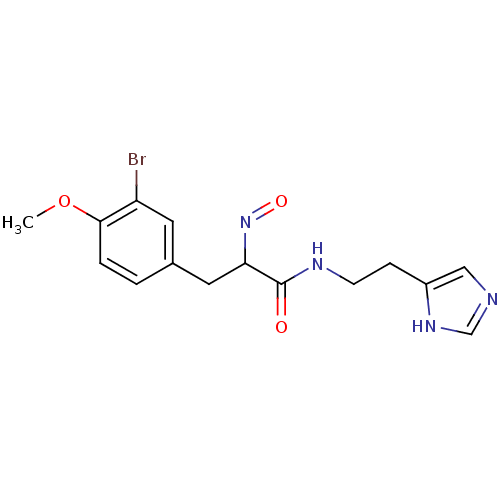 (3-(3-Bromo-4-methoxy-phenyl)-2-[(E)-hydroxyimino]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 1 (Homo sapiens (Human)) | BDBM50091753 (CHEMBL405854 | H-Ser-Leu-Arg-Arg-Ser-Ser-cyclic(Cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070214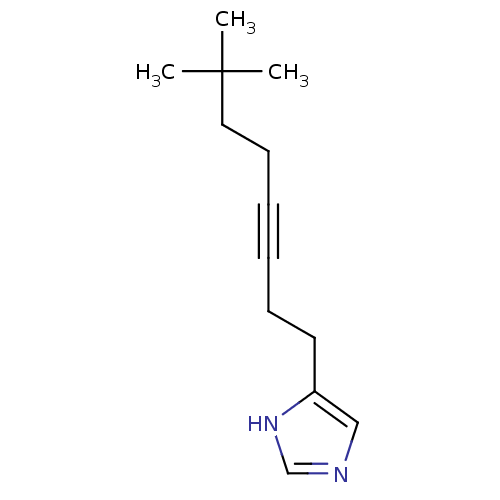 (4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070214 (4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM50070214 (4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070220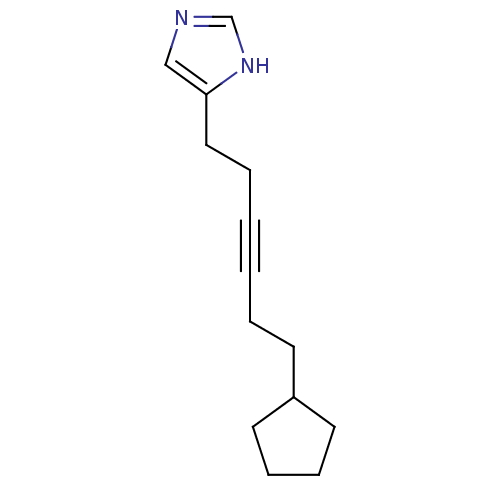 (4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070220 (4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM50070220 (4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM7967 (1-methyl-5-(beta-aminoethyl)-imidazole | 2-(1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM85407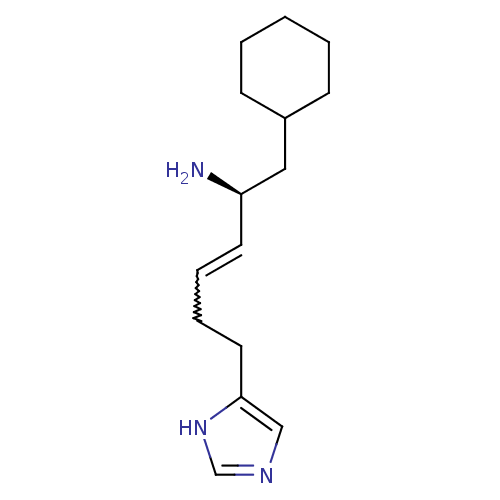 (GT 2231) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074630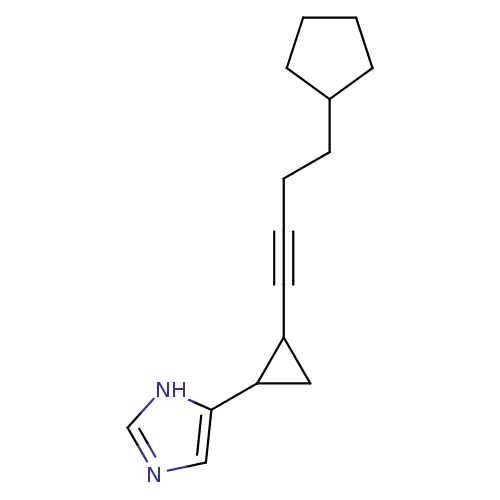 (4-[2-(4-Cyclopentyl-but-1-ynyl)-cyclopropyl]-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Antagonistic activity of the compound was evaluated against histamine H3 receptor using [3H]- N-alpha-methylhistamine as radioligand in experiment 2 | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50091754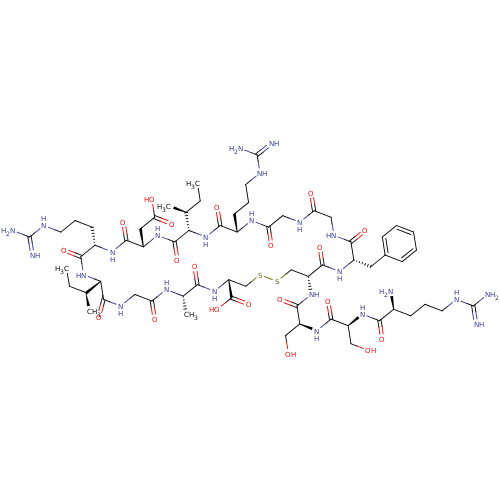 (Arg-Ser-Ser-cyclic-(Cys-Phe-Gly-Gly-Arg-Ile-Asp-Ar...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074624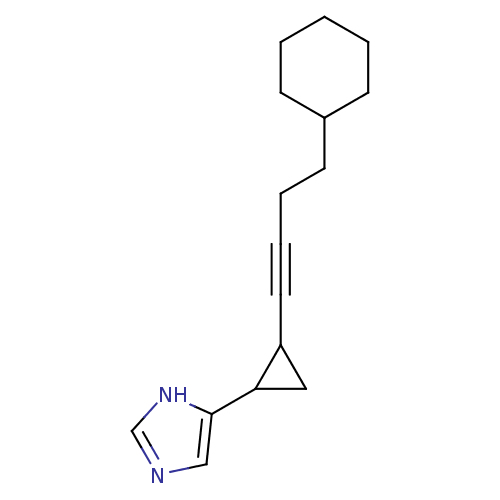 (4-[2-(4-Cyclohexyl-but-1-ynyl)-cyclopropyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech, Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 275: 598-604 (1995) BindingDB Entry DOI: 10.7270/Q21G0JSP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229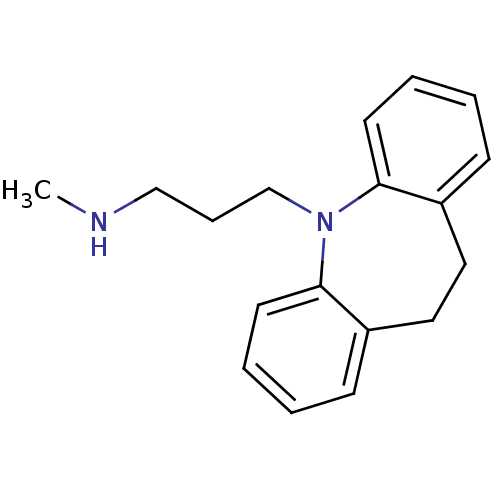 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human norepinephrine transporter expressed in MDCK-Net6 cells | J Med Chem 51: 4038-49 (2008) Article DOI: 10.1021/jm8002262 BindingDB Entry DOI: 10.7270/Q2TB16PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]nisoxetine binding to human NET expressed in MDCK-Net6 cells by plate scintillation counting | J Med Chem 52: 5703-11 (2009) Article DOI: 10.1021/jm900888c BindingDB Entry DOI: 10.7270/Q2CR5V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074628 (4-Undec-3-ynyl-1H-imidazole | CHEMBL170723) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM50070211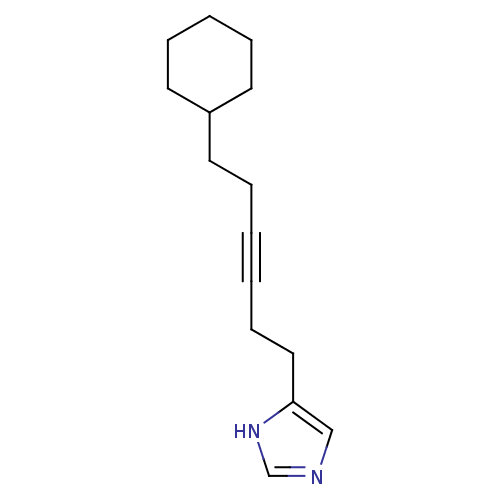 (4-(6-Cyclohexyl-hex-3-ynyl)-1H-imidazole | CHEMBL1...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070211 (4-(6-Cyclohexyl-hex-3-ynyl)-1H-imidazole | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070211 (4-(6-Cyclohexyl-hex-3-ynyl)-1H-imidazole | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055234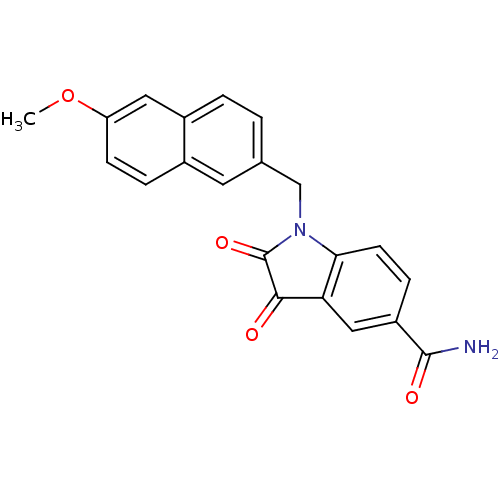 (1-(6-Methoxy-naphthalen-2-ylmethyl)-2,3-dioxo-2,3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM85405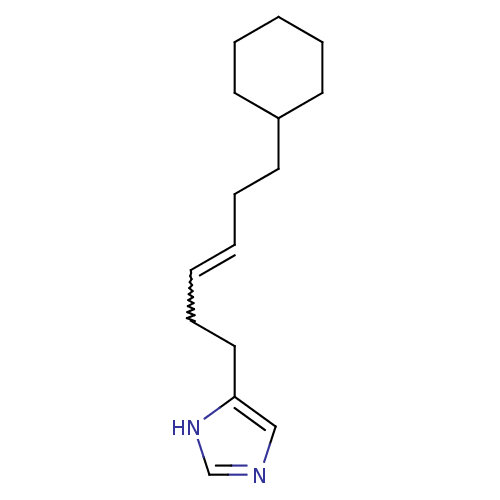 (GT 2227 | GT 2228) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM85412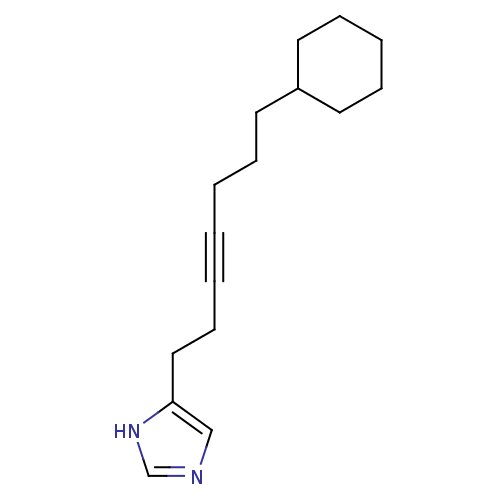 (GT 2287) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070216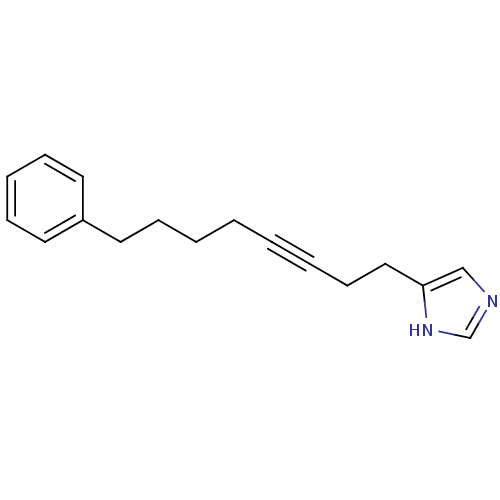 (4-(8-Phenyl-oct-3-ynyl)-1H-imidazole | CHEMBL14811) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070216 (4-(8-Phenyl-oct-3-ynyl)-1H-imidazole | CHEMBL14811) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070212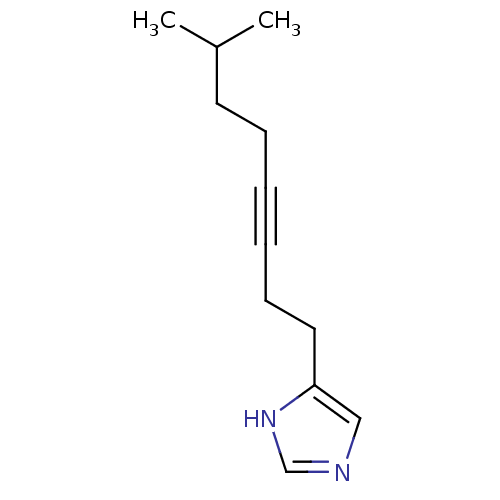 (4-(7-Methyl-oct-3-ynyl)-1H-imidazole | CHEMBL14497) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070212 (4-(7-Methyl-oct-3-ynyl)-1H-imidazole | CHEMBL14497) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055213 (1-(3,5-Dihydroxy-benzyl)-2,3-dioxo-2,3-dihydro-1H-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055232 (1-(6-Hydroxy-naphthalen-2-ylmethyl)-2,3-dioxo-2,3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055218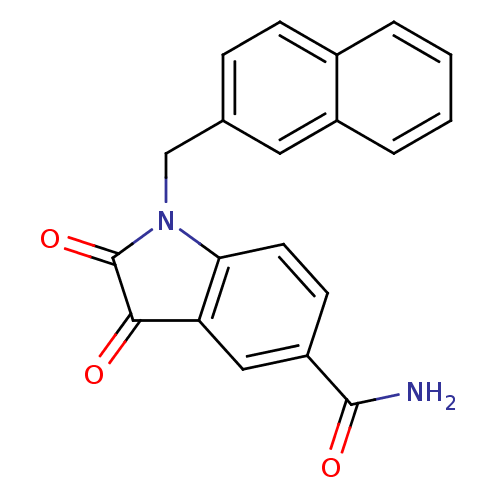 (1-(2-naphthlmethyl) isatin-5-carboxamide | 1-Napht...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM85405 (GT 2227 | GT 2228) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Antagonistic activity of the compound was evaluated against histamine H3 receptor using [3H]- N-alpha-methylhistamine as radioligand in experiment 1 | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... | Bioorg Med Chem Lett 7: 3017-3022 (1997) Article DOI: 10.1016/S0960-894X(97)10137-8 BindingDB Entry DOI: 10.7270/Q2280843 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065598 (CHEMBL419332 | {(S)-1-[(S)-1-((S)-3-Dimethylcarbam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1767 total ) | Next | Last >> |