

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50067796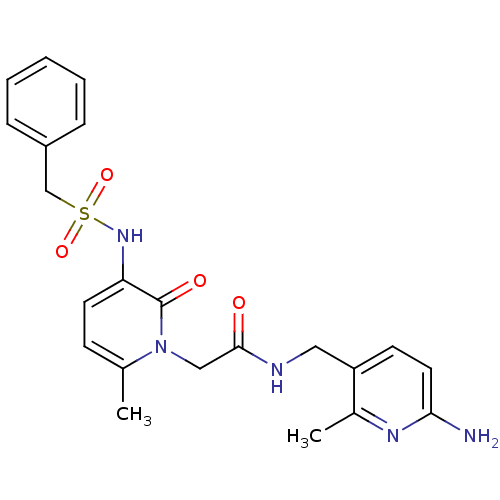 (CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067797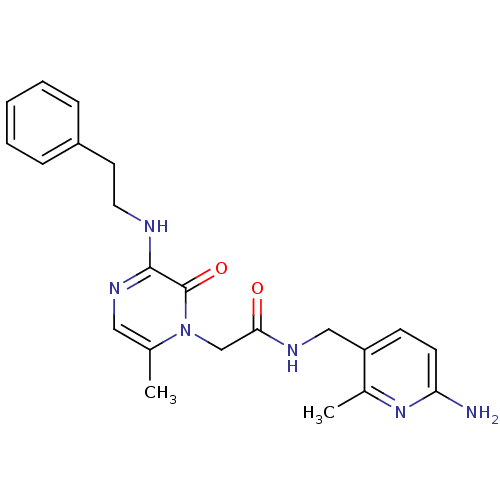 (CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215314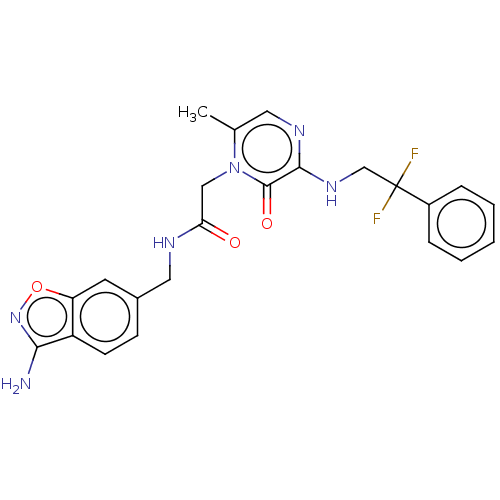 (CHEMBL100049) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215447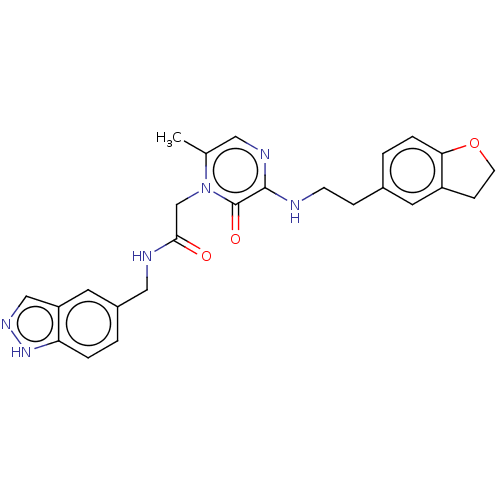 (CHEMBL101867) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221531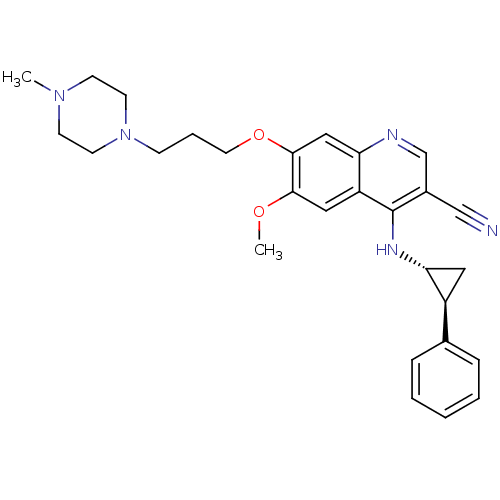 (6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50100973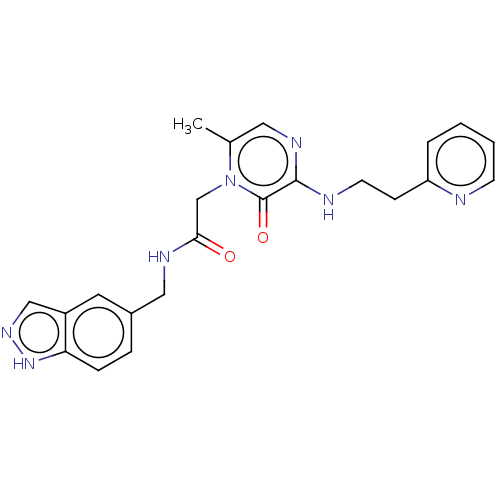 (CHEMBL101605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215308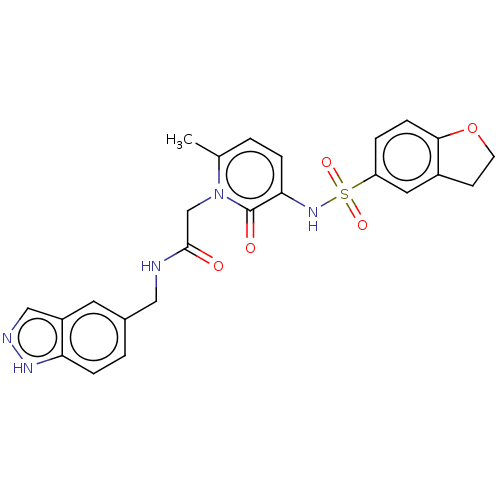 (CHEMBL99185) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50105480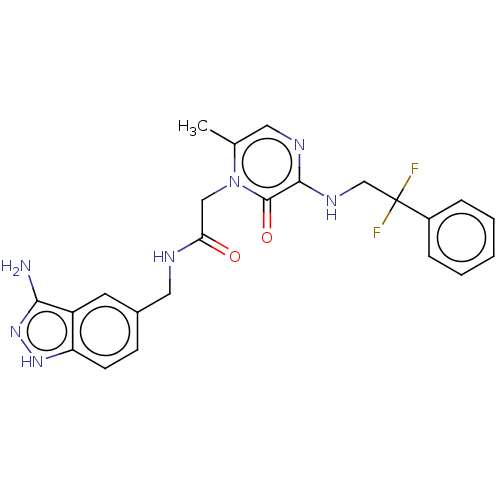 (CHEMBL98194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215441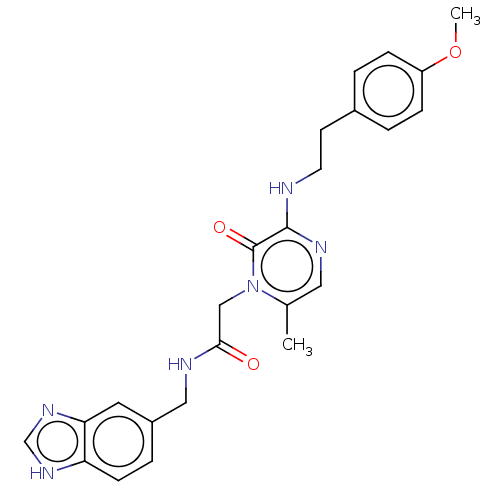 (CHEMBL101562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50100974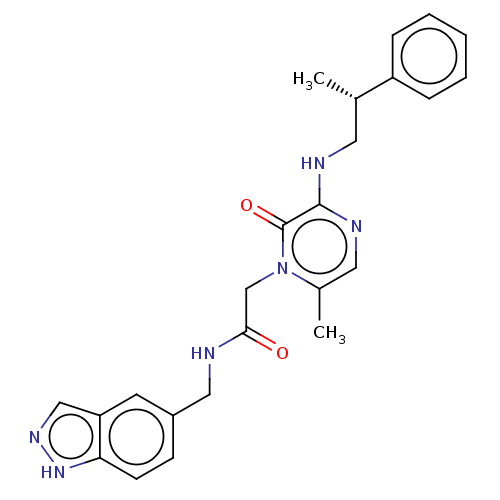 (CHEMBL317739) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215310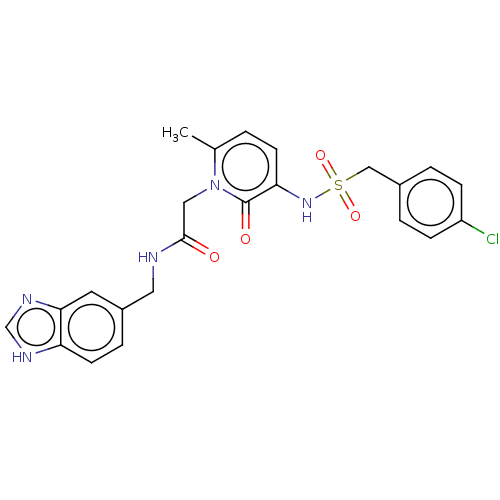 (CHEMBL97869) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221516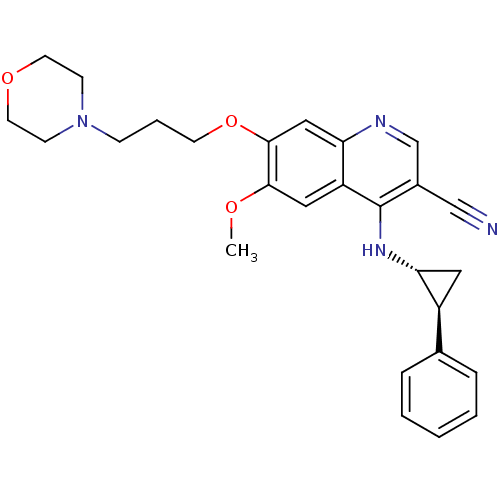 (6-methoxy-7-(3-morpholinopropoxy)-4-((1R,2S)-2-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215311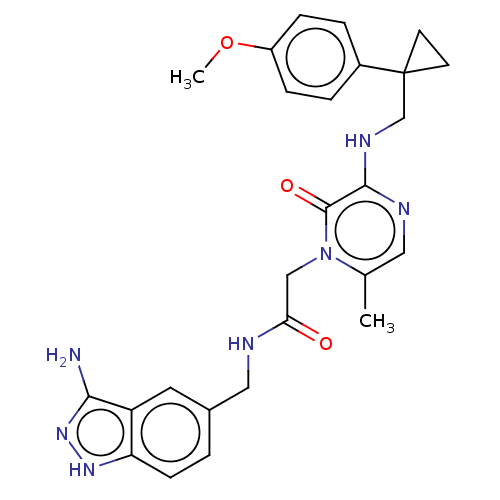 (CHEMBL317140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50100975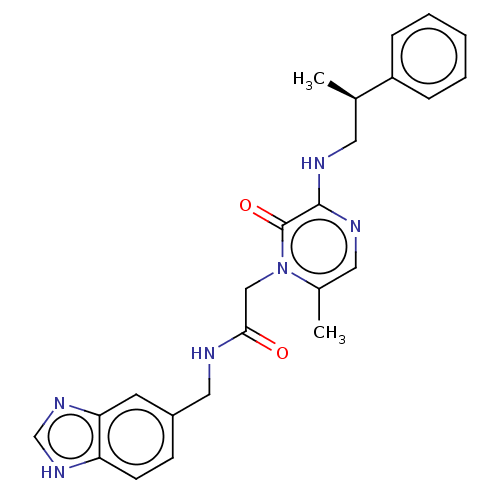 (CHEMBL100838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215302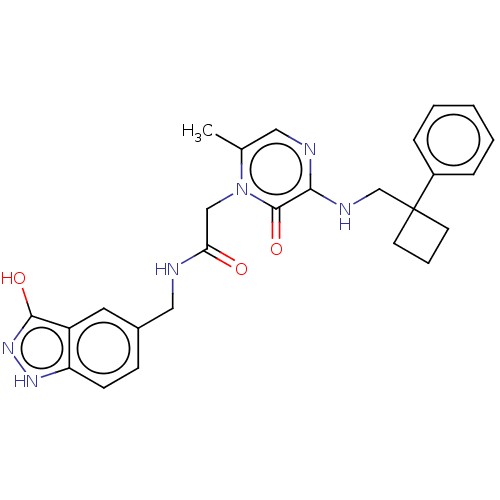 (CHEMBL98057) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50216995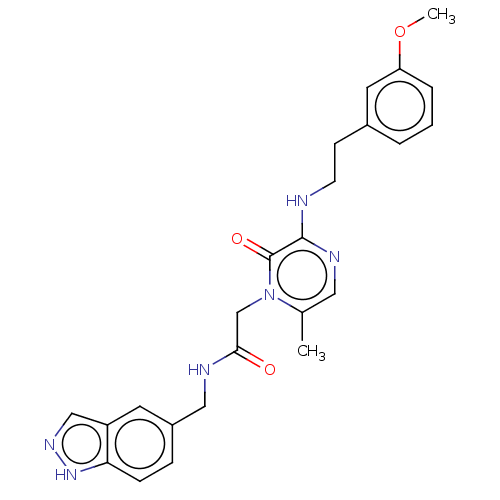 (CHEMBL319815) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215304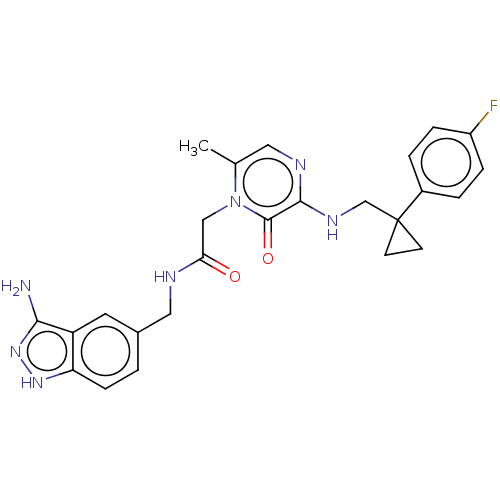 (CHEMBL98212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215317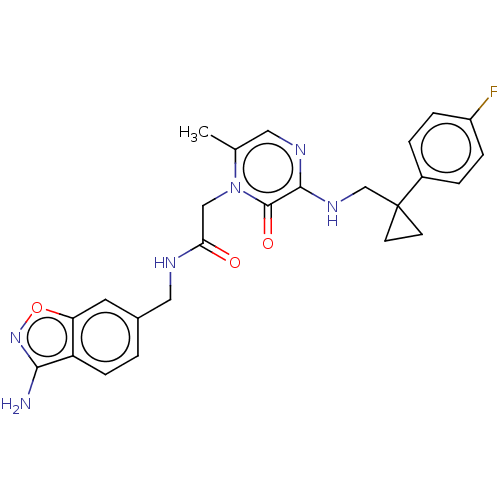 (CHEMBL97950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50124087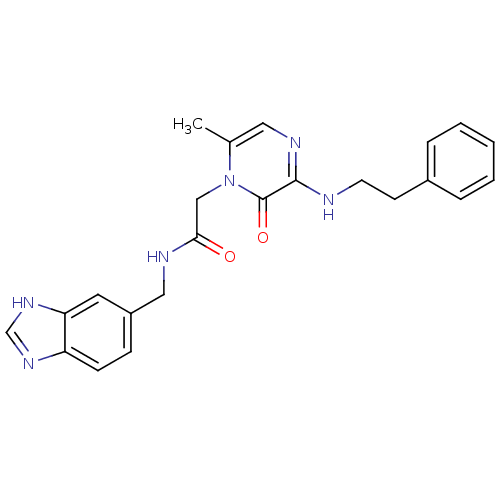 (CHEMBL101563 | N-(1H-Benzoimidazol-5-ylmethyl)-2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50216992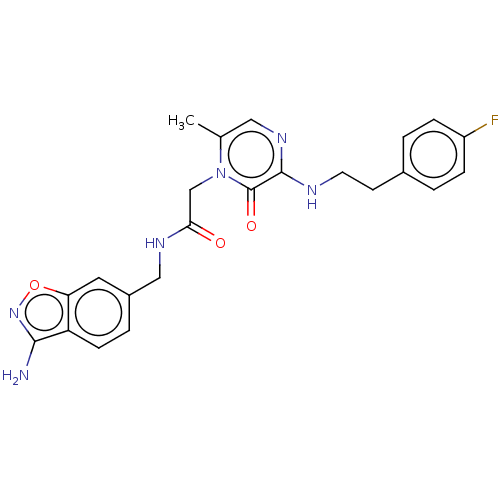 (CHEMBL98859) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215318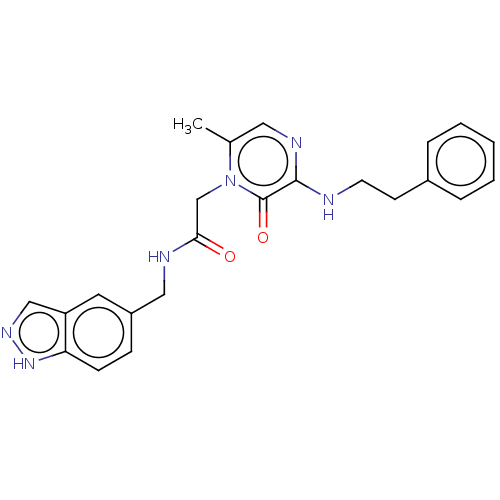 (CHEMBL101613) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215315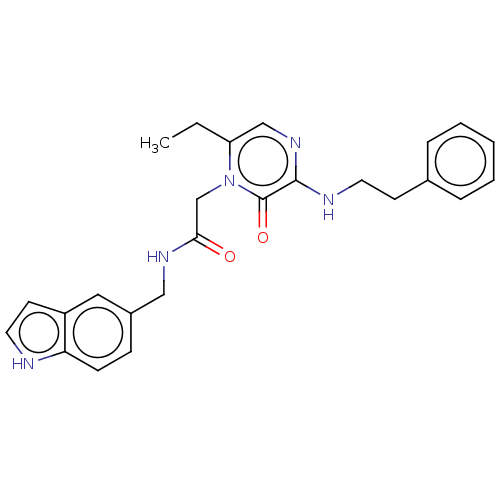 (CHEMBL100843) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221518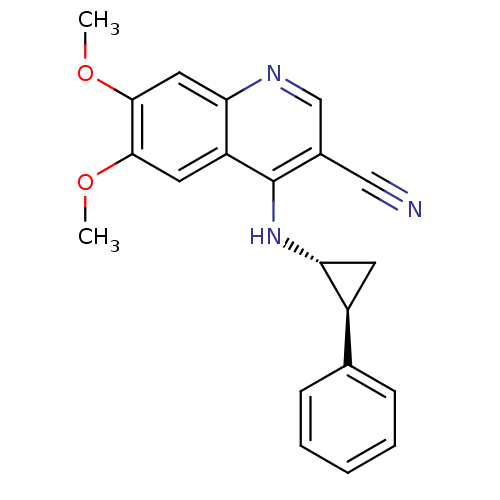 (6,7-dimethoxy-4-((1R,2S)-2-phenylcyclopropylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215305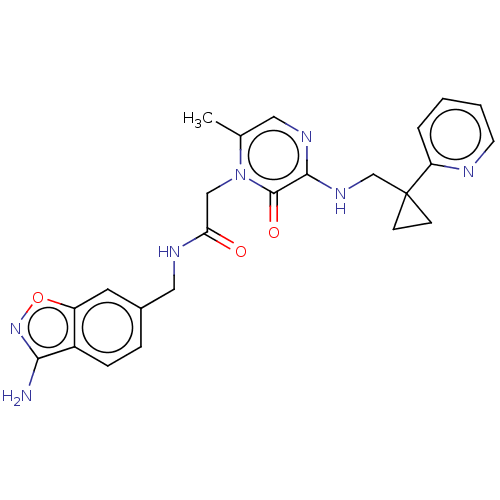 (CHEMBL100443) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50221531 (6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of SRC | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215309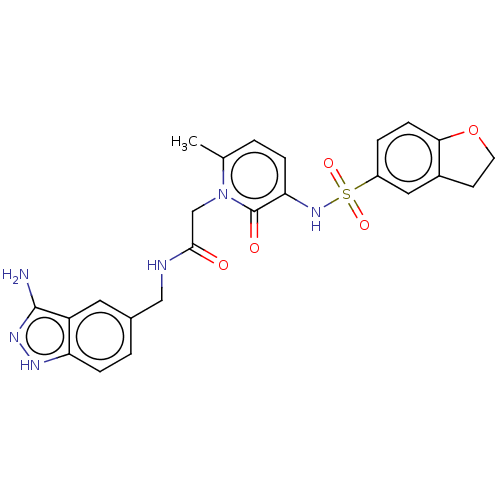 (CHEMBL102193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50100972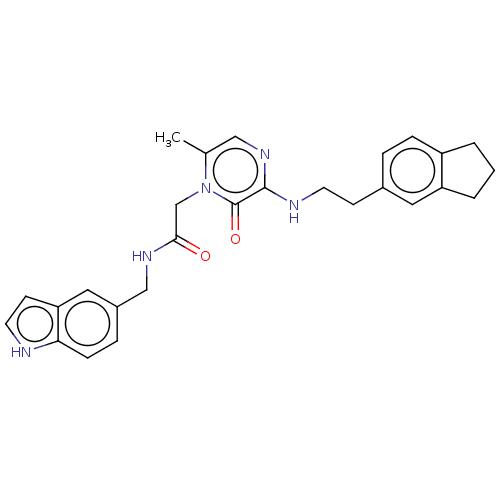 (CHEMBL98152) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215306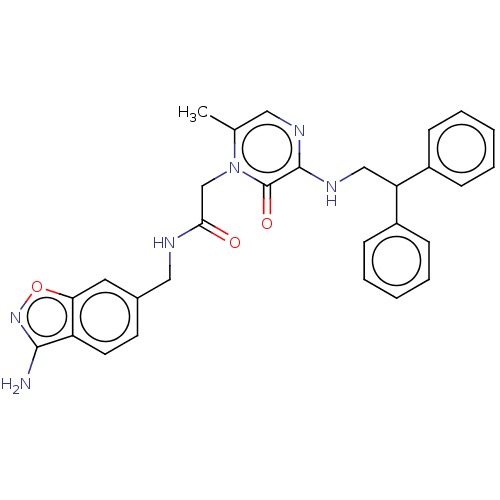 (CHEMBL100565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215313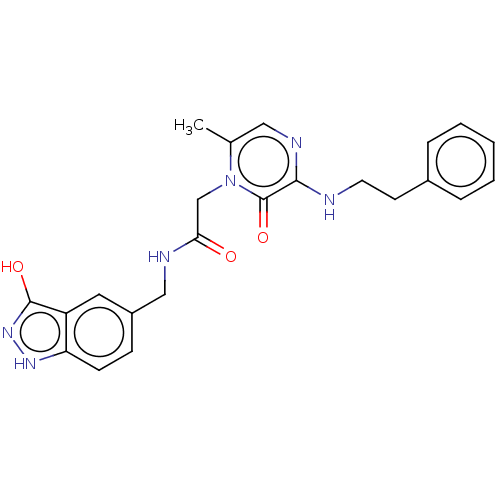 (CHEMBL101669) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221535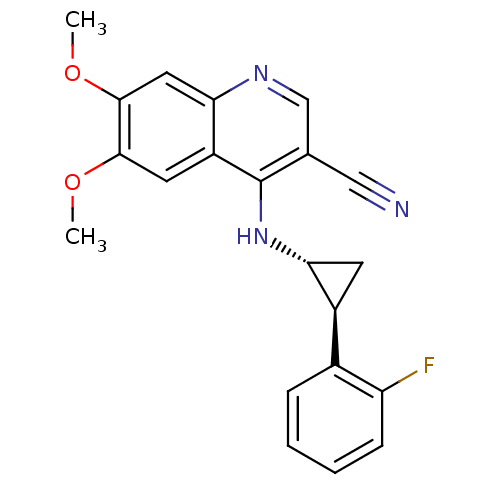 (4-((1R,2S)-2-(2-fluorophenyl)cyclopropylamino)-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221522 (6-(4-(aminomethyl)phenyl)-4-((1R,2S)-2-phenylcyclo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50221531 (6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of SYK | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50221531 (6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of ABL | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221530 (6,7-diethyl-2-ethynyl-1-(((1S,2R)-2-p-tolylcyclopr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50221516 (6-methoxy-7-(3-morpholinopropoxy)-4-((1R,2S)-2-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of SRC | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50221524 (6-methoxy-4-((1R,2S)-2-phenylcyclopropylamino)-7-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of SRC | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221533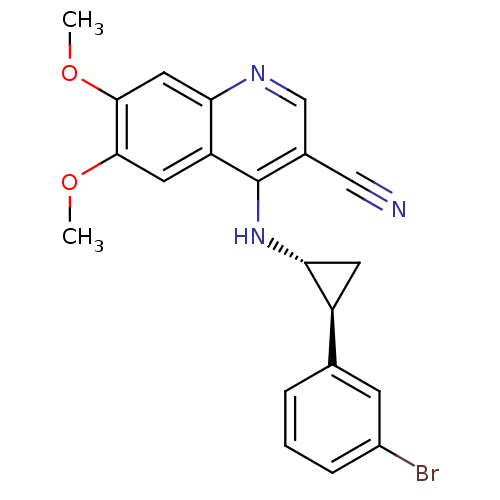 (4-((1R,2S)-2-(3-bromophenyl)cyclopropylamino)-6,7-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221527 (7-methoxy-6-(3-morpholinopropoxy)-4-((1R,2S)-2-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221517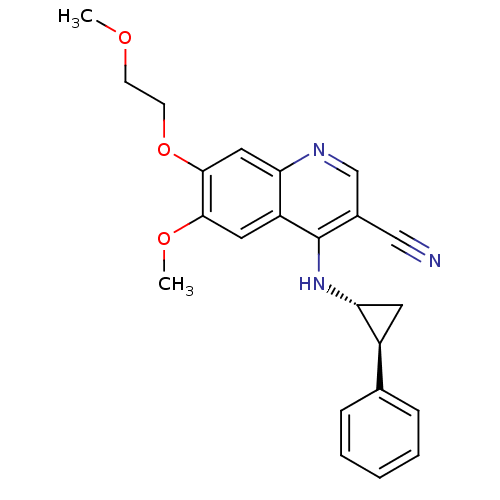 (6-methoxy-7-(2-methoxyethoxy)-4-((1R,2S)-2-phenylc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221521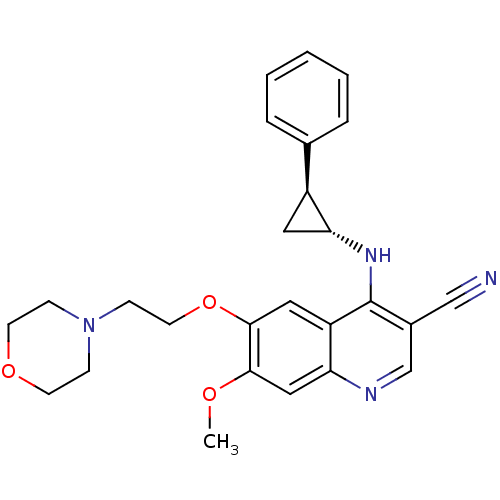 (7-methoxy-6-(2-morpholinoethoxy)-4-((1R,2S)-2-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221528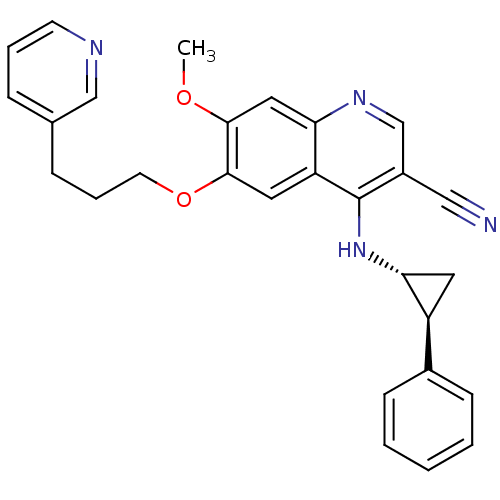 (7-methoxy-4-((1R,2S)-2-phenylcyclopropylamino)-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215442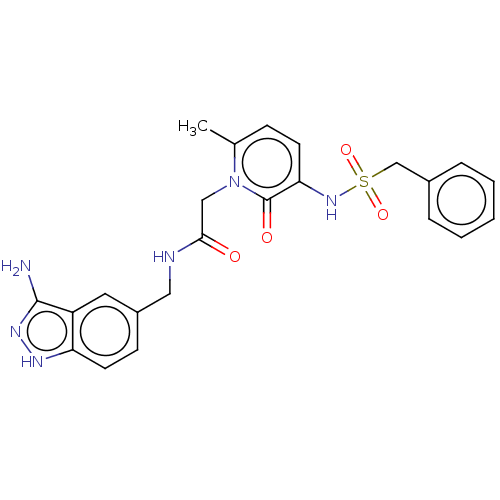 (CHEMBL99116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50216994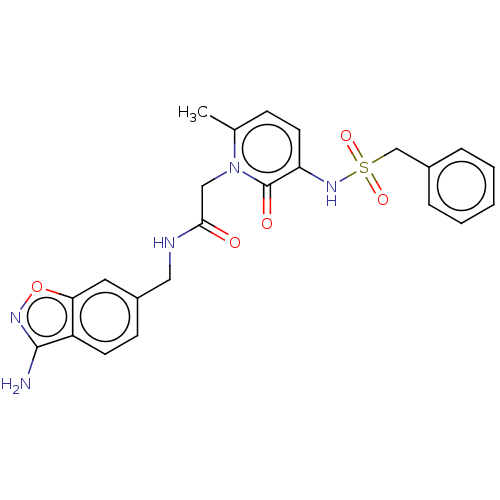 (CHEMBL99206) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221529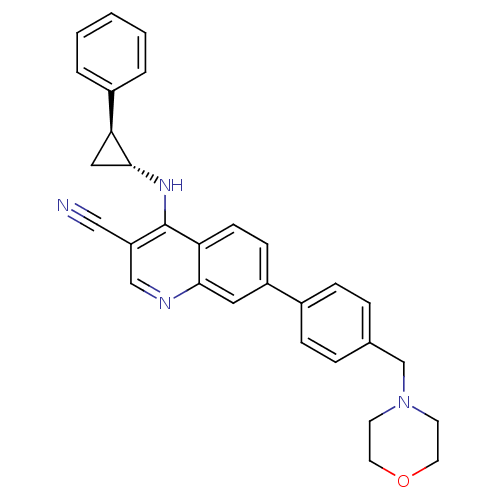 (7-(4-(morpholinomethyl)phenyl)-4-((1R,2S)-2-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50221532 (6,7-dimethoxy-4-((1R,2S)-2-(4-phenoxyphenyl)cyclop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by fluorescence polarization assay | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215316 (CHEMBL98281) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50221522 (6-(4-(aminomethyl)phenyl)-4-((1R,2S)-2-phenylcyclo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of SRC | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50216991 (CHEMBL318472) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50221524 (6-methoxy-4-((1R,2S)-2-phenylcyclopropylamino)-7-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of KDR | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50221517 (6-methoxy-7-(2-methoxyethoxy)-4-((1R,2S)-2-phenylc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA, Inc. Curated by ChEMBL | Assay Description Inhibition of SRC | Bioorg Med Chem Lett 17: 5978-82 (2007) Article DOI: 10.1016/j.bmcl.2007.07.071 BindingDB Entry DOI: 10.7270/Q2VM4C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 157 total ) | Next | Last >> |