

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258507 (CHEMBL4078345) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli (strain K12)) | BDBM36435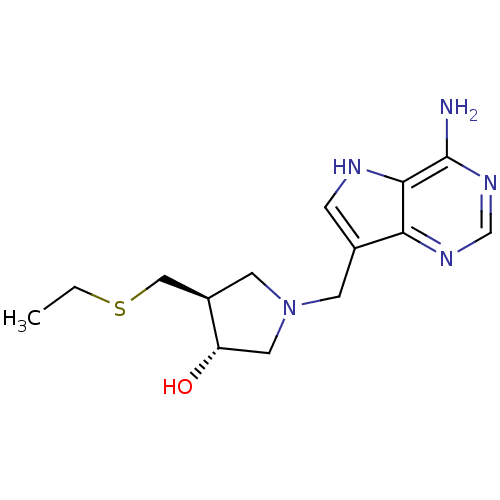 ((3R,4S)-1-[(9-Deaza-adenin-9-yl)methyl]-4-ethylthi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albert Einstein College of Medicine | Assay Description Purified MTAN activity in MTAN enzyme inhibition assay | Nat Chem Biol 5: 251-7 (2009) Article DOI: 10.1038/nchembio.153 BindingDB Entry DOI: 10.7270/Q28C9TM9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli (strain K12)) | BDBM22113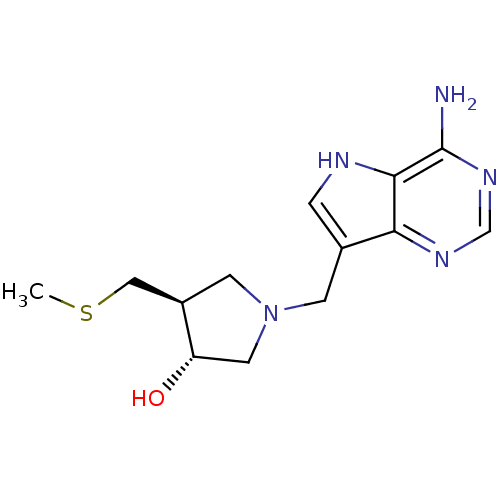 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0730 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albert Einstein College of Medicine | Assay Description Purified MTAN activity in MTAN enzyme inhibition assay | Nat Chem Biol 5: 251-7 (2009) Article DOI: 10.1038/nchembio.153 BindingDB Entry DOI: 10.7270/Q28C9TM9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258514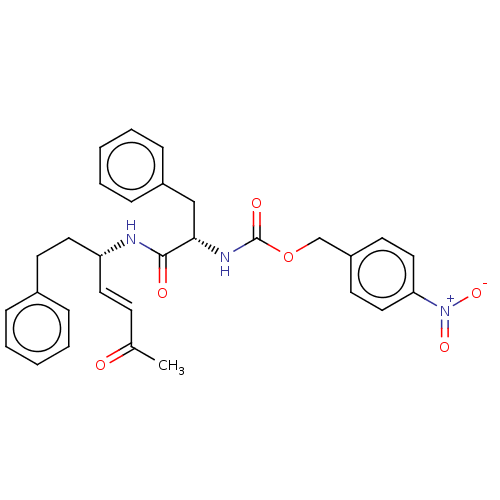 (CHEMBL4062015) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis | Bioorg Med Chem 20: 5181-7 (2012) Article DOI: 10.1016/j.bmc.2012.07.006 BindingDB Entry DOI: 10.7270/Q2XG9S6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli (strain K12)) | BDBM36436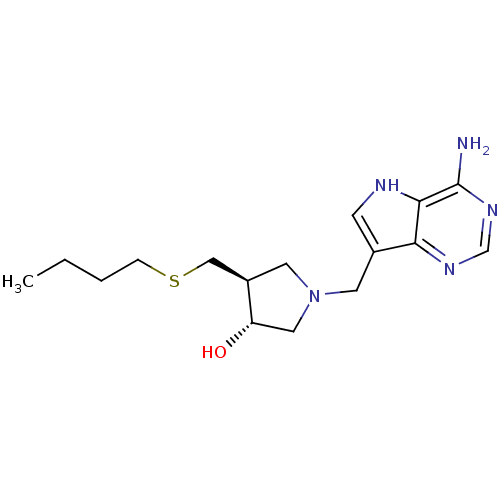 ((3R,4S)-1-[(9-Deaza-adenin-9-yl)methyl]-4-ethylthi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.208 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albert Einstein College of Medicine | Assay Description Purified MTAN activity in MTAN enzyme inhibition assay | Nat Chem Biol 5: 251-7 (2009) Article DOI: 10.1038/nchembio.153 BindingDB Entry DOI: 10.7270/Q28C9TM9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50000069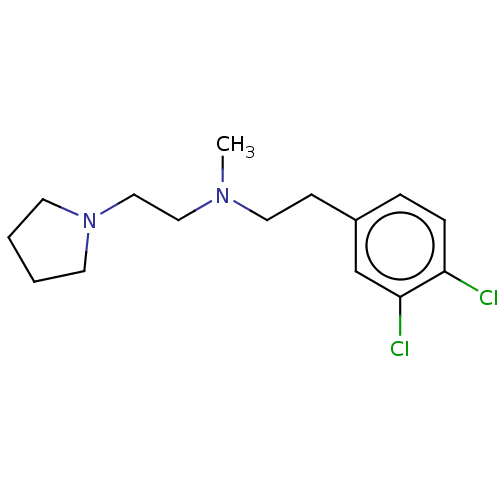 (CHEMBL20377 | [2-(3,4-Dichloro-phenyl)-ethyl]-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Tested for its binding affinity towards sigma-1 site in rat brain, using [3H](+)-3-PPP as radioligand | J Med Chem 37: 2285-91 (1994) BindingDB Entry DOI: 10.7270/Q29S1RPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50258507 (CHEMBL4078345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258506 (CHEMBL4072275) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chile Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from human alpha4beta2 nAChR overexpressed in human SHEP cells after 75 mins by liquid scintillation spectrometric analy... | Bioorg Med Chem 21: 2687-94 (2013) Article DOI: 10.1016/j.bmc.2013.03.024 BindingDB Entry DOI: 10.7270/Q2MW2JJQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50258515 (CHEMBL4083754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Grupo Ferrer Curated by ChEMBL | Assay Description Binding affinity measured at the 5-hydroxytryptamine 2A receptor by the inhibition of [3H]-ketanserin binding to rat cortex using unlabeled mianserin... | J Med Chem 41: 5402-9 (1999) Article DOI: 10.1021/jm9810396 BindingDB Entry DOI: 10.7270/Q2QC02N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326399 ((+/-)-trans-4-Butyl-1-[(9-deazaadenin-9-yl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326399 ((+/-)-trans-4-Butyl-1-[(9-deazaadenin-9-yl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258544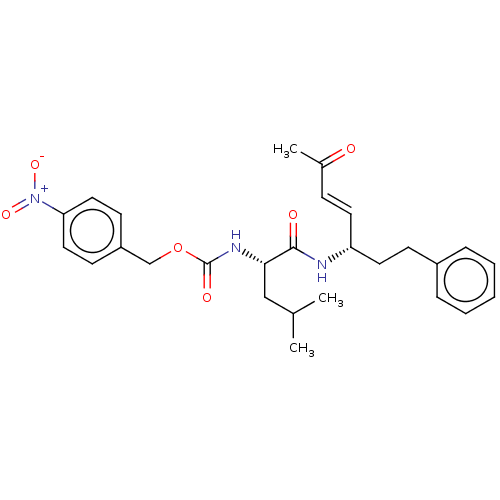 (CHEMBL4096388) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity towards muscarinic m2 receptor | J Med Chem 37: 2285-91 (1994) BindingDB Entry DOI: 10.7270/Q29S1RPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50390240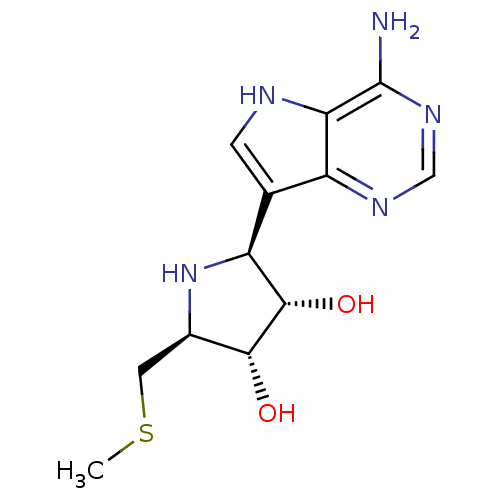 (CHEMBL1195586) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis | Bioorg Med Chem 20: 5181-7 (2012) Article DOI: 10.1016/j.bmc.2012.07.006 BindingDB Entry DOI: 10.7270/Q2XG9S6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50433566 (CHEMBL2381566) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chile Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from human alpha4beta2 nAChR overexpressed in human SHEP cells after 75 mins by liquid scintillation spectrometric analy... | Bioorg Med Chem 21: 2687-94 (2013) Article DOI: 10.1016/j.bmc.2013.03.024 BindingDB Entry DOI: 10.7270/Q2MW2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Grupo Ferrer Curated by ChEMBL | Assay Description Binding affinity measured at the sigma receptor by the inhibition of [3H]-3-PPP binding to guinea pig cerebellum using unlabeled 3-PPP for nonspecifi... | J Med Chem 41: 5402-9 (1999) Article DOI: 10.1021/jm9810396 BindingDB Entry DOI: 10.7270/Q2QC02N8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326400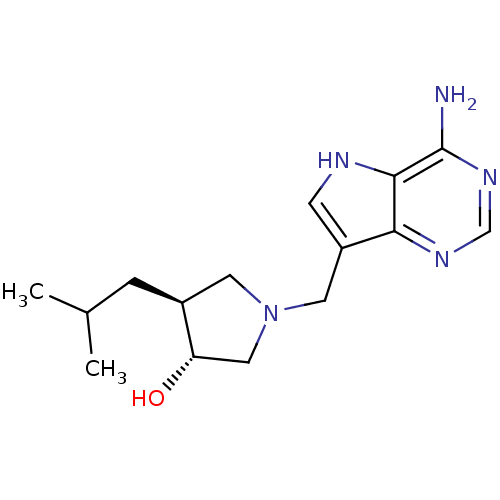 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50072822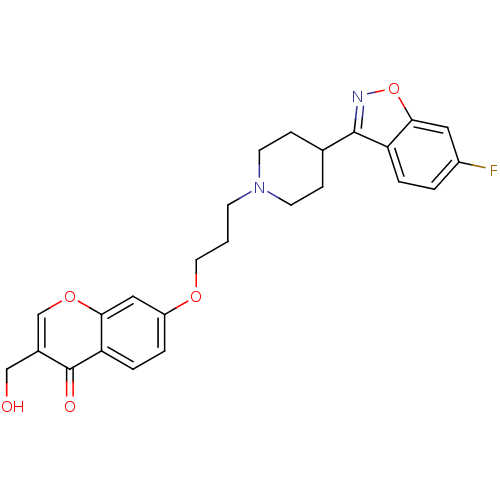 (7-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Grupo Ferrer Curated by ChEMBL | Assay Description Binding affinity measured at the 5-hydroxytryptamine 2A receptor by the inhibition of [3H]-ketanserin binding to rat cortex using unlabeled mianserin... | J Med Chem 41: 5402-9 (1999) Article DOI: 10.1021/jm9810396 BindingDB Entry DOI: 10.7270/Q2QC02N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity towards muscarinic m1 receptor | J Med Chem 37: 2285-91 (1994) BindingDB Entry DOI: 10.7270/Q29S1RPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258542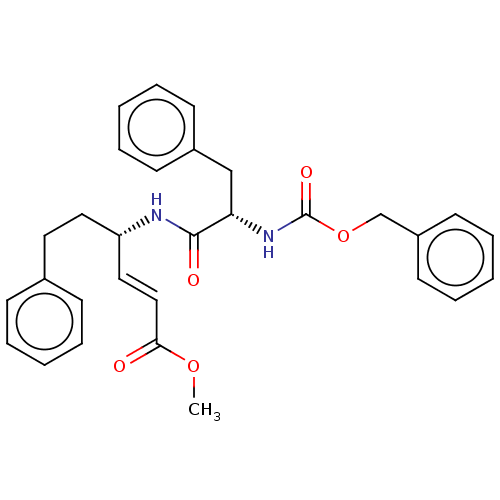 (CHEMBL4082758) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50039200 ((4-Phenyl-butyl)-(5-phenyl-pentyl)-amine | CHEMBL2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Tested for its binding affinity towards sigma-1 site in rat brain, using [3H]DTG as radioligand | J Med Chem 37: 2285-91 (1994) BindingDB Entry DOI: 10.7270/Q29S1RPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326402 ((+/-)-trans-4-Cyclopropyl-1-[(9-deazaadenin-9-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50258514 (CHEMBL4062015) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50072822 (7-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperidin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Grupo Ferrer Curated by ChEMBL | Assay Description Binding affinity measured at the Alpha-1A adrenergic receptor by the inhibition of [3H]-prazosin binding to rat cortex using unlabeled WB-4101 for no... | J Med Chem 41: 5402-9 (1999) Article DOI: 10.1021/jm9810396 BindingDB Entry DOI: 10.7270/Q2QC02N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326403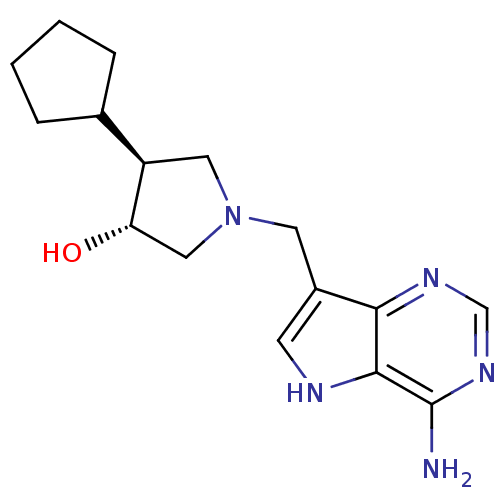 ((+/-)-trans-4-Cyclopentyl-1-[(9-deazaadenin-9-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326407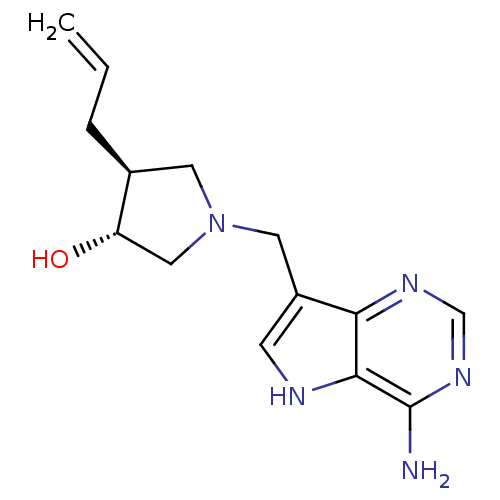 ((+/-)-trans-4-Allyl-1-[(9-deazaadenin-9-yl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326398 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326406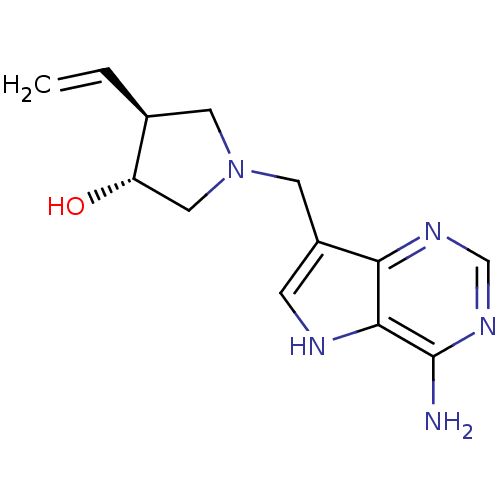 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326398 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50258544 (CHEMBL4096388) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258500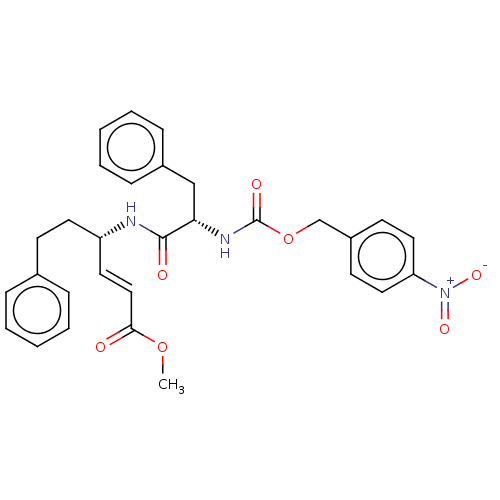 (CHEMBL4093034) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50304793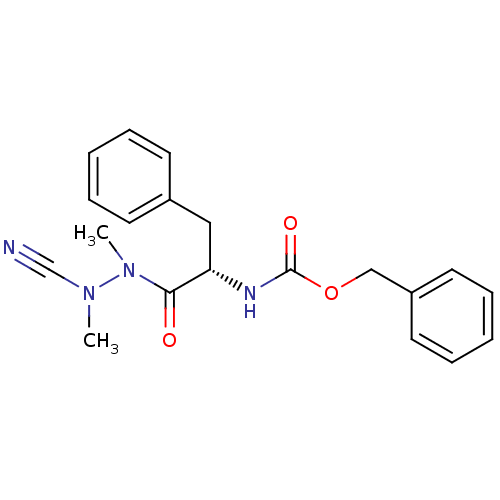 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by microtiter plate spectrofluorimetry | Bioorg Med Chem Lett 20: 252-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.122 BindingDB Entry DOI: 10.7270/Q24X57WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Grupo Ferrer Curated by ChEMBL | Assay Description Binding affinity measured at the Dopamine receptor D4 by the inhibition of [3H]-spiperone binding to human recombinant CHO cells using unlabeled halo... | J Med Chem 41: 5402-9 (1999) Article DOI: 10.1021/jm9810396 BindingDB Entry DOI: 10.7270/Q2QC02N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50390242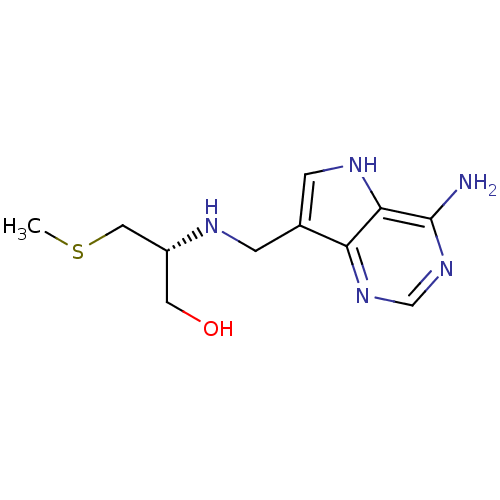 (CHEMBL2070308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis | Bioorg Med Chem 20: 5181-7 (2012) Article DOI: 10.1016/j.bmc.2012.07.006 BindingDB Entry DOI: 10.7270/Q2XG9S6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319585 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed noncompetitive inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258523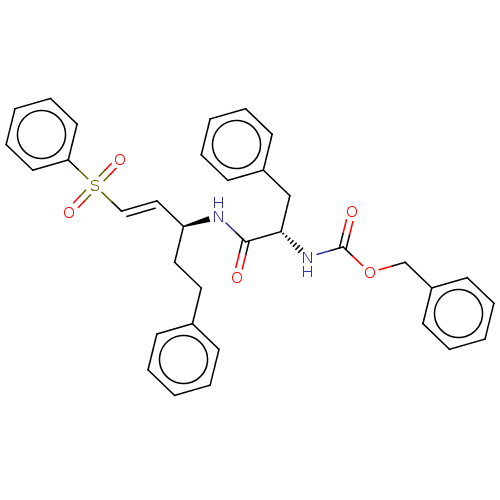 (CHEMBL2402204) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50390245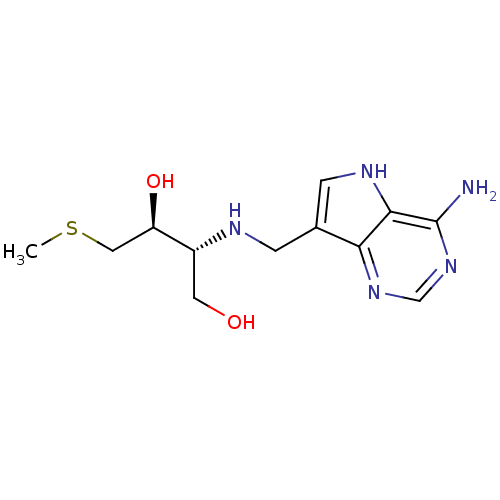 (CHEMBL2070311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis | Bioorg Med Chem 20: 5181-7 (2012) Article DOI: 10.1016/j.bmc.2012.07.006 BindingDB Entry DOI: 10.7270/Q2XG9S6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50072822 (7-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Grupo Ferrer Curated by ChEMBL | Assay Description Binding affinity measured at the Dopamine receptor D3 by the inhibition of [3H]-YM-09151-2 binding to human recombinant CCL 1.3 cells using unlabeled... | J Med Chem 41: 5402-9 (1999) Article DOI: 10.1021/jm9810396 BindingDB Entry DOI: 10.7270/Q2QC02N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Grupo Ferrer Curated by ChEMBL | Assay Description Binding affinity measured at the Dopamine receptor D4 by the inhibition of [3H]-spiperone binding to human recombinant CHO cells using unlabeled halo... | J Med Chem 41: 5402-9 (1999) Article DOI: 10.1021/jm9810396 BindingDB Entry DOI: 10.7270/Q2QC02N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50304796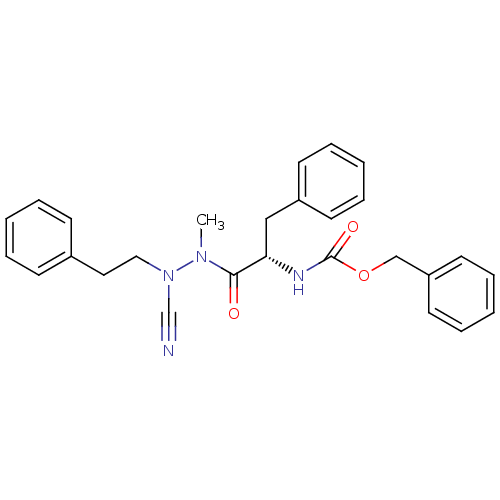 ((S)-benzyl 1-(2-cyano-1-methyl-2-phenethylhydrazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by microtiter plate spectrofluorimetry | Bioorg Med Chem Lett 20: 252-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.122 BindingDB Entry DOI: 10.7270/Q24X57WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258524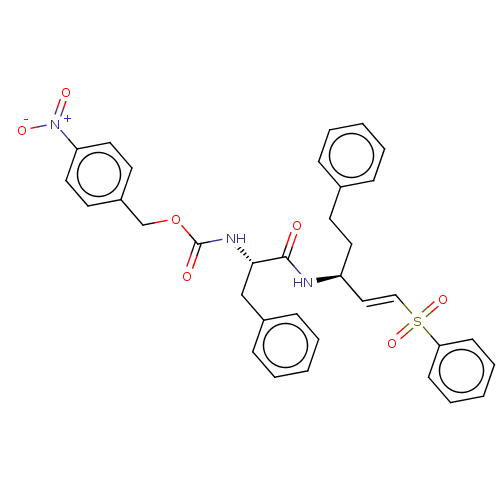 (CHEMBL4081250) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258498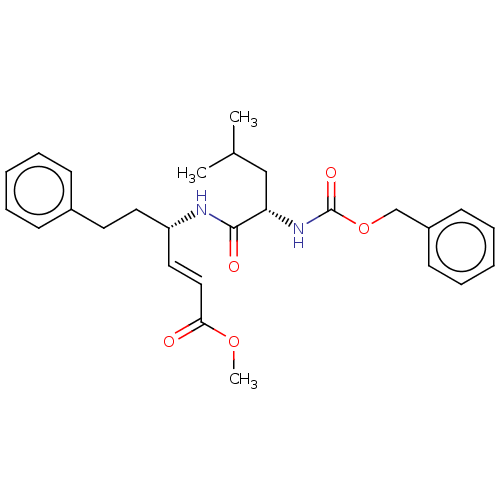 (CHEMBL4101714) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326405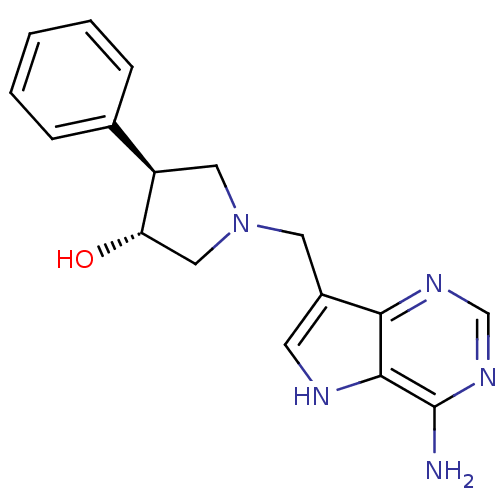 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50433573 (CHEMBL2381570) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chile Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from human alpha4beta2 nAChR overexpressed in human SHEP cells after 75 mins by liquid scintillation spectrometric analy... | Bioorg Med Chem 21: 2687-94 (2013) Article DOI: 10.1016/j.bmc.2013.03.024 BindingDB Entry DOI: 10.7270/Q2MW2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50304794 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-4-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by microtiter plate spectrofluorimetry | Bioorg Med Chem Lett 20: 252-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.122 BindingDB Entry DOI: 10.7270/Q24X57WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258515 (CHEMBL4083754) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Grupo Ferrer Curated by ChEMBL | Assay Description Binding affinity measured at the 5-hydroxytryptamine 2A receptor by the inhibition of [3H]-ketanserin binding to rat cortex using unlabeled mianserin... | J Med Chem 41: 5402-9 (1999) Article DOI: 10.1021/jm9810396 BindingDB Entry DOI: 10.7270/Q2QC02N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2843 total ) | Next | Last >> |