

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101823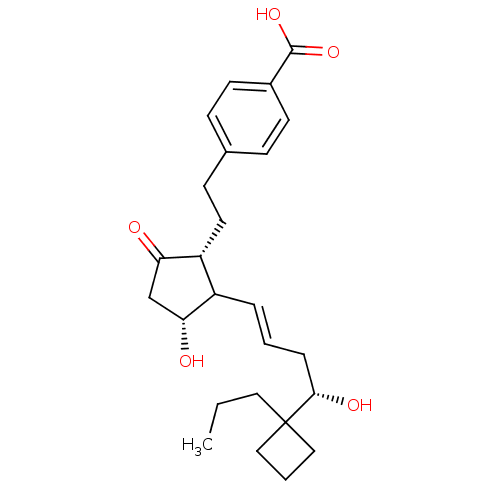 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101823 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101826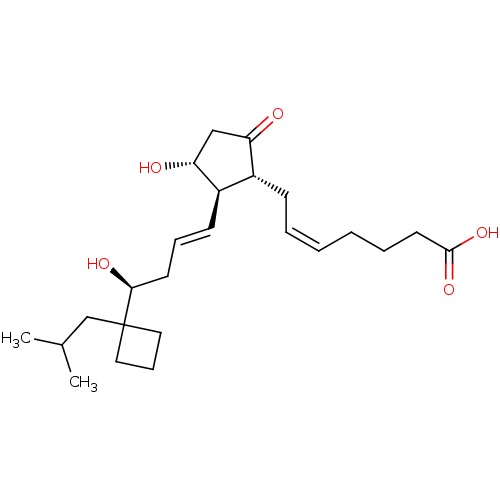 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101833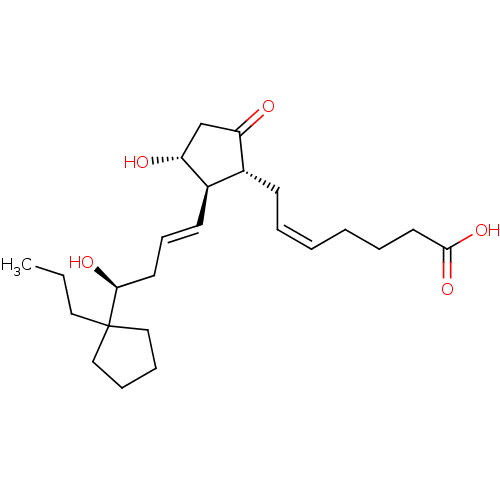 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101832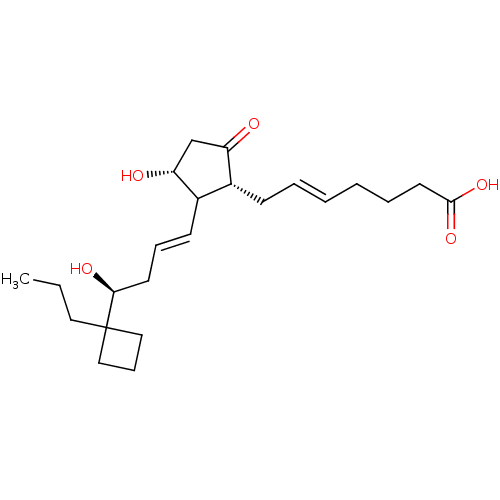 ((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101825 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101832 ((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101827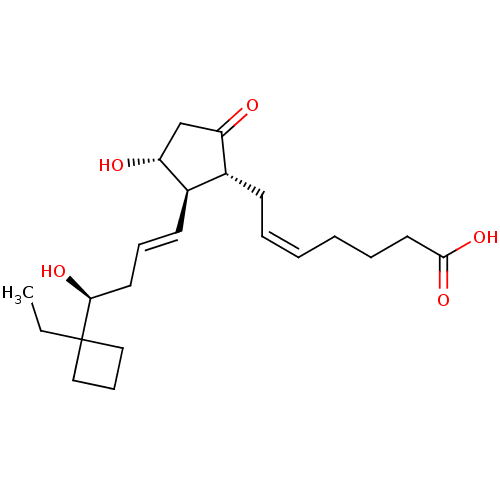 ((Z)-7-{(1R,2R,3R)-2-[(E)-(S)-4-(1-Ethyl-cyclobutyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101825 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126000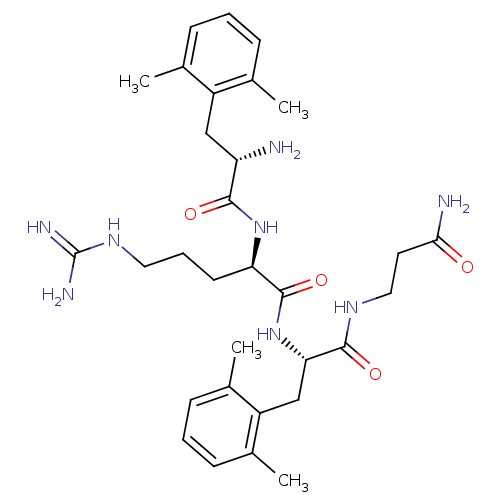 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126003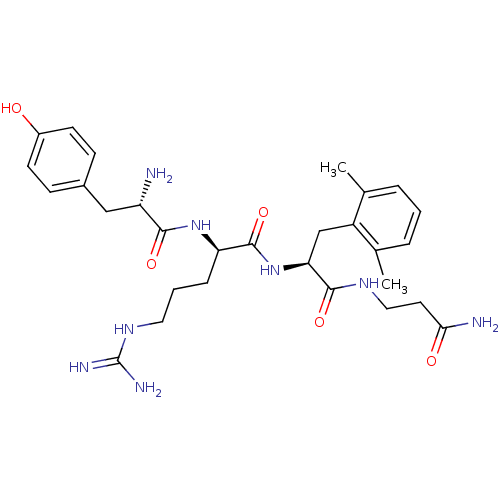 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125997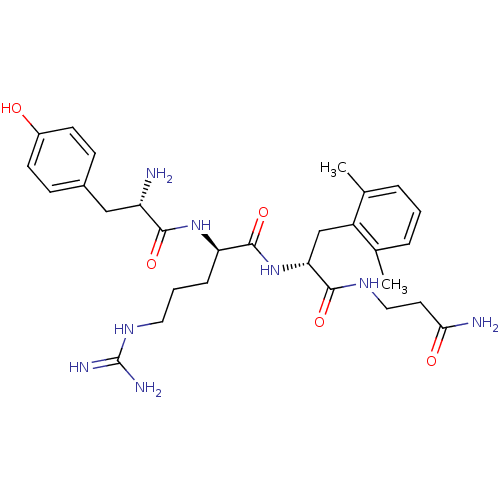 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0618 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125996 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0623 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50109635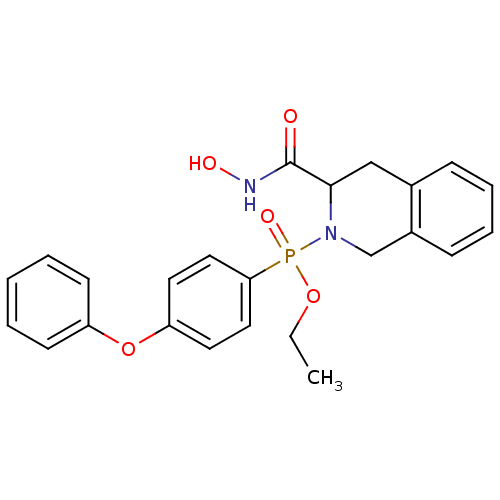 ((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50109621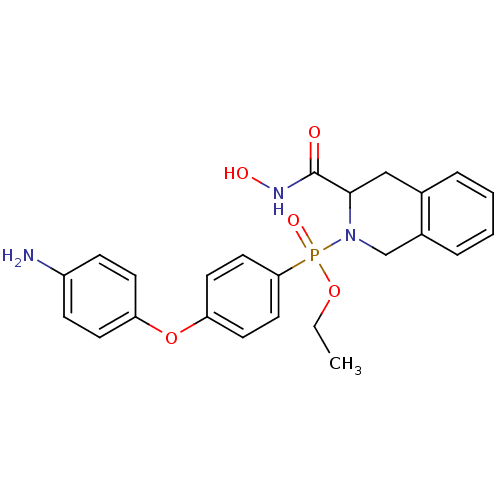 (CHEMBL425316 | [4-(4-Amino-phenoxy)-phenyl]-(3-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health Curated by PDSP Ki Database | Br J Pharmacol 117: 1558-64 (1996) Article DOI: 10.1111/j.1476-5381.1996.tb15321.x BindingDB Entry DOI: 10.7270/Q28W3BT3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125998 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50056419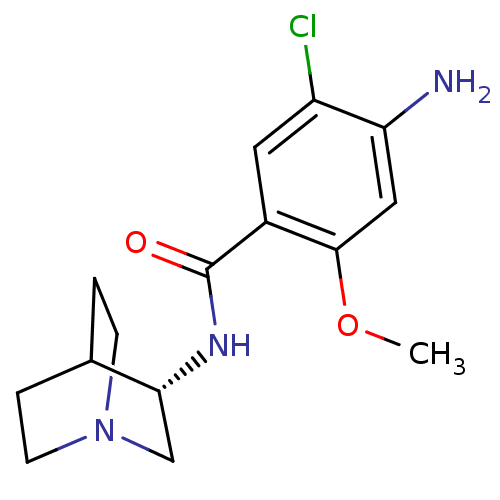 (4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to rat cortical membrane serotonin 5-hydroxytryptamine 3 receptor | J Med Chem 46: 702-15 (2003) Article DOI: 10.1021/jm020270n BindingDB Entry DOI: 10.7270/Q25H7H06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50109626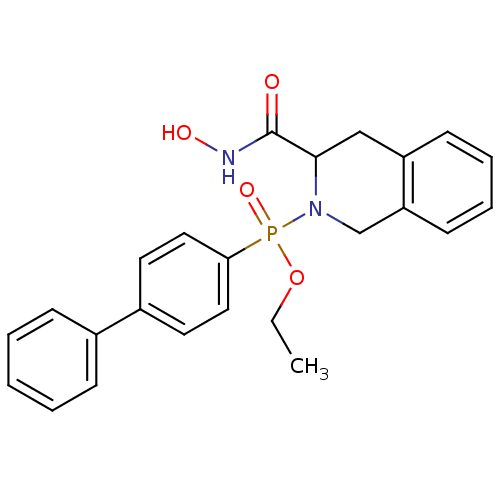 (Biphenyl-4-yl-(3-hydroxycarbamoyl-3,4-dihydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health Curated by PDSP Ki Database | Br J Pharmacol 117: 1558-64 (1996) Article DOI: 10.1111/j.1476-5381.1996.tb15321.x BindingDB Entry DOI: 10.7270/Q28W3BT3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50385105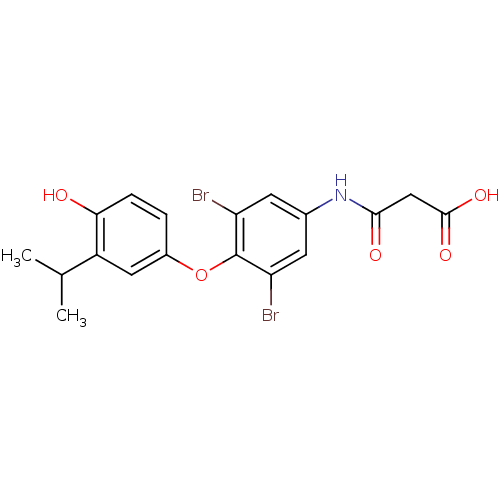 (CHEMBL2035874 | US10322118, Entry 8b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]T3 from human recombinant thyroid harmone receptor beta after 16 to 48 hrs by gamma-ray detection | Bioorg Med Chem 20: 3622-34 (2012) Article DOI: 10.1016/j.bmc.2012.03.056 BindingDB Entry DOI: 10.7270/Q2SF2X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50109621 (CHEMBL425316 | [4-(4-Amino-phenoxy)-phenyl]-(3-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50109635 ((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50109625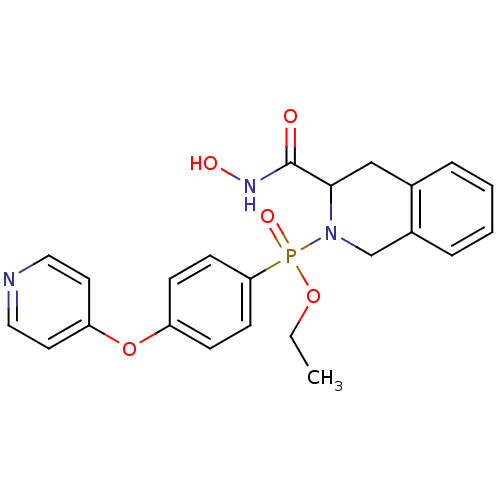 ((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50101830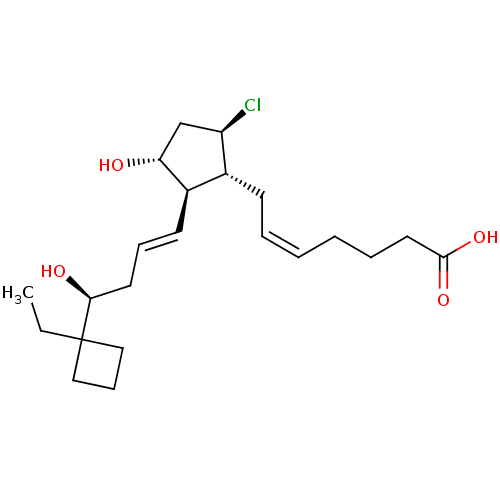 ((Z)-7-{(1R,2R,3R,5R)-5-Chloro-2-[(E)-(S)-4-(1-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its competitive binding affinity towards human Prostanoid EP2 receptor in CHO cells expressing prostanoid receptor | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM82519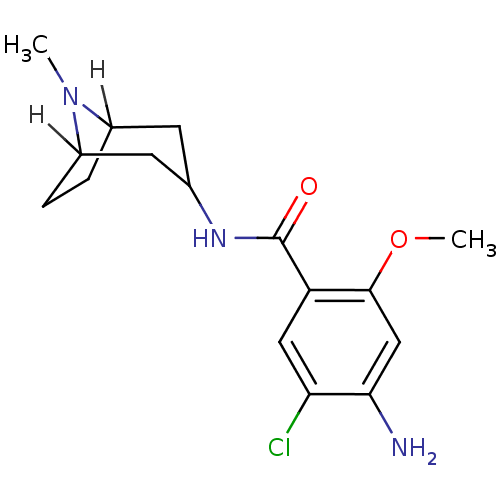 (4-amino-5-chloro-2-methoxy-N-(8-methyl-8-azabicycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to rat cortical membrane serotonin 5-hydroxytryptamine 3 receptor | J Med Chem 46: 702-15 (2003) Article DOI: 10.1021/jm020270n BindingDB Entry DOI: 10.7270/Q25H7H06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM82519 (4-amino-5-chloro-2-methoxy-N-(8-methyl-8-azabicycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to rat cortical membrane serotonin 5-hydroxytryptamine 3 receptor | J Med Chem 46: 702-15 (2003) Article DOI: 10.1021/jm020270n BindingDB Entry DOI: 10.7270/Q25H7H06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50109629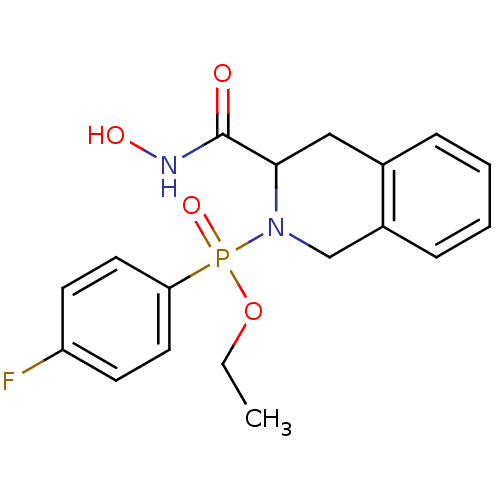 ((4-Fluoro-phenyl)-(3-hydroxycarbamoyl-3,4-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-1 (MMP-1)(recombinant human collagenase-1). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278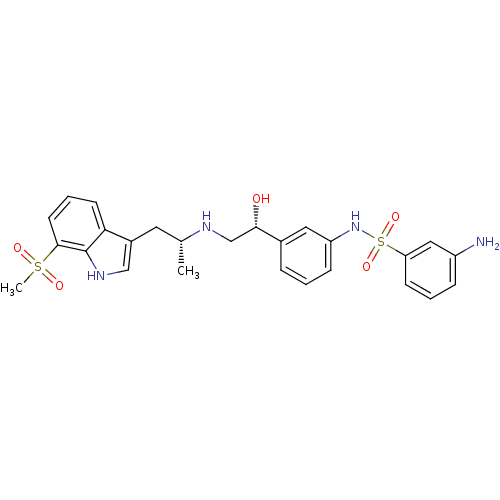 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50109633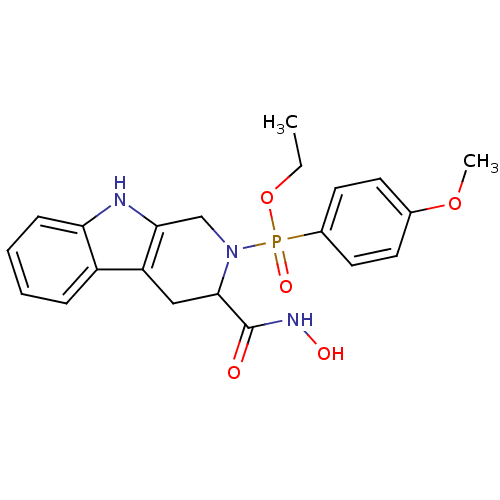 ((3-Hydroxycarbamoyl-1,3,4,9-tetrahydro-beta-carbol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50385109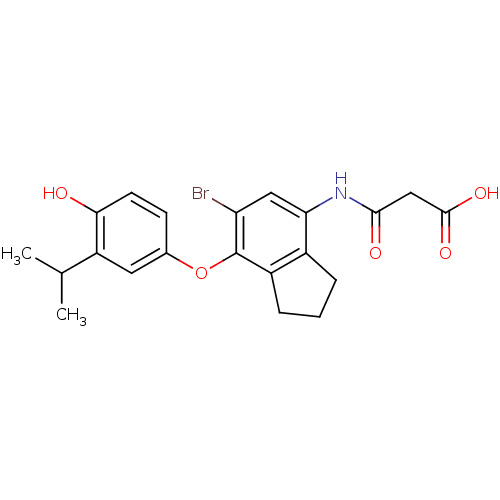 (CHEMBL2035879) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]T3 from human recombinant thyroid harmone receptor beta after 16 to 48 hrs by gamma-ray detection | Bioorg Med Chem 20: 3622-34 (2012) Article DOI: 10.1016/j.bmc.2012.03.056 BindingDB Entry DOI: 10.7270/Q2SF2X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM84958 (2-[[(R)-2-(1H-Indol-2-ylcarbonylamino)-3-(4-benzhy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health Curated by PDSP Ki Database | Br J Pharmacol 117: 1558-64 (1996) Article DOI: 10.1111/j.1476-5381.1996.tb15321.x BindingDB Entry DOI: 10.7270/Q28W3BT3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227831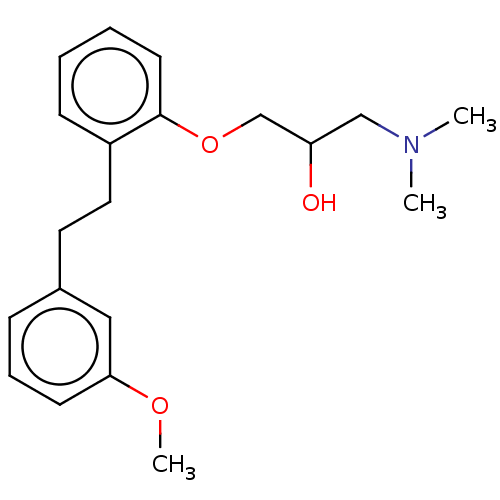 (CHEMBL52242) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50385101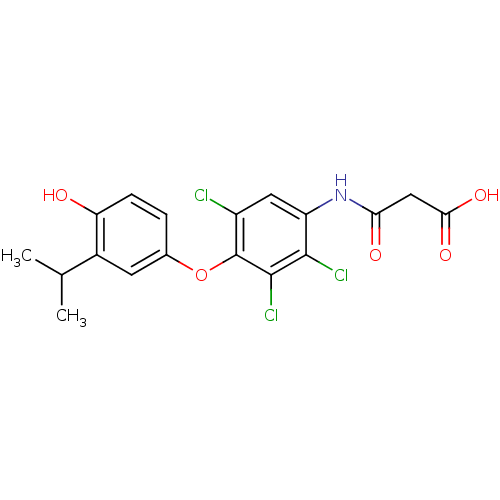 (CHEMBL2035876) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]T3 from human recombinant thyroid harmone receptor beta after 16 to 48 hrs by gamma-ray detection | Bioorg Med Chem 20: 3622-34 (2012) Article DOI: 10.1016/j.bmc.2012.03.056 BindingDB Entry DOI: 10.7270/Q2SF2X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273137 ((3-{[(3-Carbamimidoyl-phenyl)-({4-[1-(1-imino-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50109635 ((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-1 (MMP-1)(recombinant human collagenase-1). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50109633 ((3-Hydroxycarbamoyl-1,3,4,9-tetrahydro-beta-carbol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-1 (MMP-1)(recombinant human collagenase-1). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50109630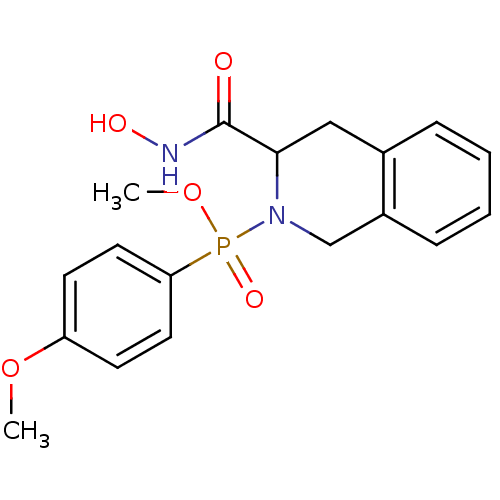 ((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50109633 ((3-Hydroxycarbamoyl-1,3,4,9-tetrahydro-beta-carbol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50109633 ((3-Hydroxycarbamoyl-1,3,4,9-tetrahydro-beta-carbol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against tumor necrosis factor alpha converting enzyme (TACE) from human acute monocytic leukemia cell line. | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101830 ((Z)-7-{(1R,2R,3R,5R)-5-Chloro-2-[(E)-(S)-4-(1-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP2 receptor expressed in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50036399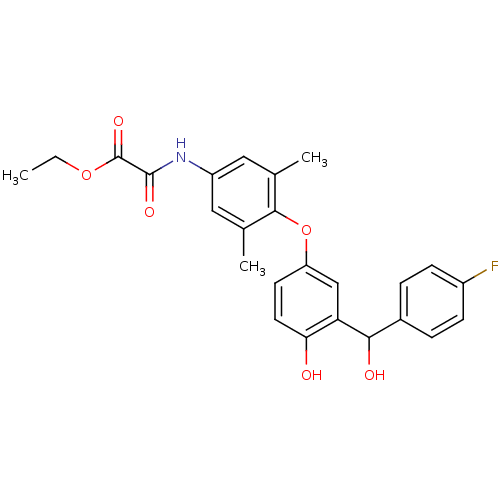 (Axitirome | CHEMBL159682 | N-(4-{3-[(4-Fluoro-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-T3 from human TRbeta expressed in insect cells after 16 to 48 hrs by gamma-counting | Bioorg Med Chem 21: 592-607 (2013) Article DOI: 10.1016/j.bmc.2012.12.002 BindingDB Entry DOI: 10.7270/Q2JS9RRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]T3 from human recombinant thyroid harmone receptor beta after 16 to 48 hrs by gamma-ray detection | Bioorg Med Chem 20: 3622-34 (2012) Article DOI: 10.1016/j.bmc.2012.03.056 BindingDB Entry DOI: 10.7270/Q2SF2X6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-T3 from human TRbeta expressed in insect cells after 16 to 48 hrs by gamma-counting | Bioorg Med Chem 21: 592-607 (2013) Article DOI: 10.1016/j.bmc.2012.12.002 BindingDB Entry DOI: 10.7270/Q2JS9RRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-T3 from human TRalpha expressed in insect cells after 16 to 48 hrs by gamma-counting | Bioorg Med Chem 21: 592-607 (2013) Article DOI: 10.1016/j.bmc.2012.12.002 BindingDB Entry DOI: 10.7270/Q2JS9RRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]T3 from human recombinant thyroid harmone receptor alpha after 16 to 48 hrs by gamma-ray detection | Bioorg Med Chem 20: 3622-34 (2012) Article DOI: 10.1016/j.bmc.2012.03.056 BindingDB Entry DOI: 10.7270/Q2SF2X6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50109622 ((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50385111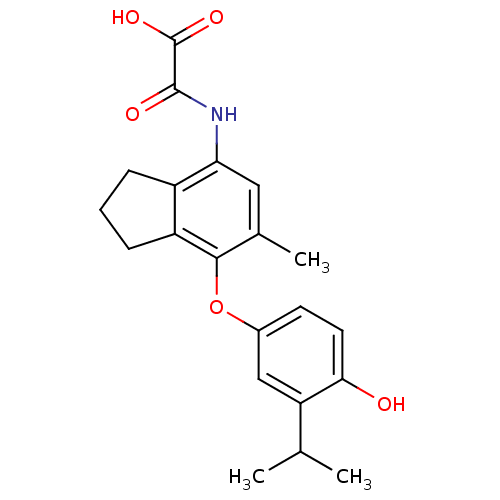 (CHEMBL2035881) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]T3 from human recombinant thyroid harmone receptor alpha after 16 to 48 hrs by gamma-ray detection | Bioorg Med Chem 20: 3622-34 (2012) Article DOI: 10.1016/j.bmc.2012.03.056 BindingDB Entry DOI: 10.7270/Q2SF2X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3638 total ) | Next | Last >> |