

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50273464 ((1S,9S,10S)-11-(2-phenylethyl)-8-oxa-11-azatetracy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50273935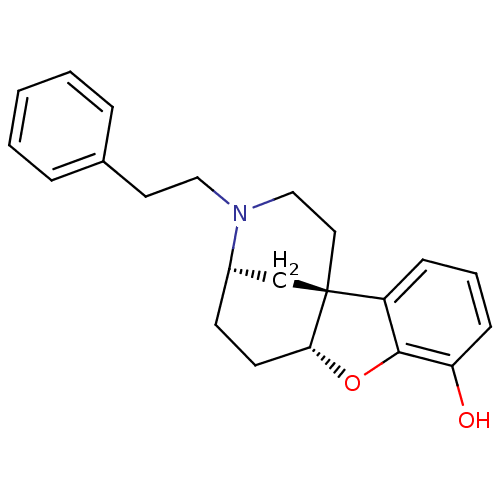 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-phenethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50273933 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-phenethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50273935 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-phenethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50273414 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-pheneth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50273414 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-pheneth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50273416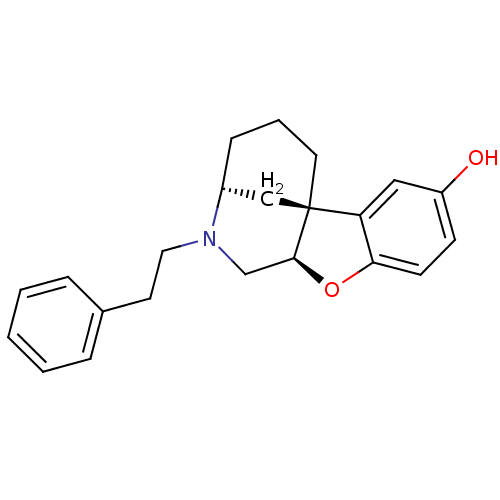 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-pheneth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50273415 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50273933 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-phenethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50273934 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50273416 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-pheneth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50273416 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-pheneth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant delta opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50273415 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant delta opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50273414 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-pheneth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant delta opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50273415 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-methyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50273934 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-methyl-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50273413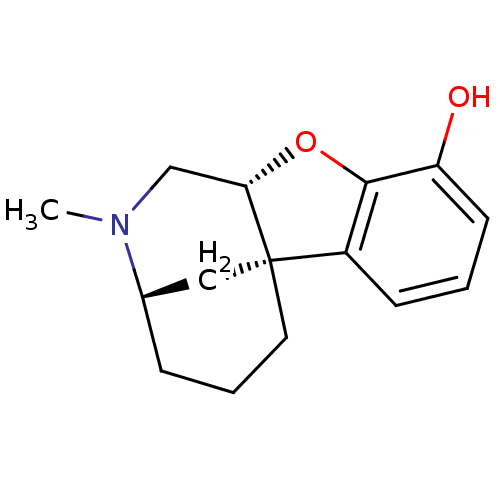 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant delta opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50273935 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-phenethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant delta opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50273933 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-phenethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant delta opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50273413 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50273413 ((3R*,6aS*,11aR*)-1,3,4,5,6,11a-Hexahydro-2-methyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50273932 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant delta opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50273934 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant delta opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50273932 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50273932 ((4R*,6aS*,11bR*)-2,3,4,5,6,6a-Hexahydro-3-methyl-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 51: 7866-81 (2008) Article DOI: 10.1021/jm800913d BindingDB Entry DOI: 10.7270/Q2639PM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50173539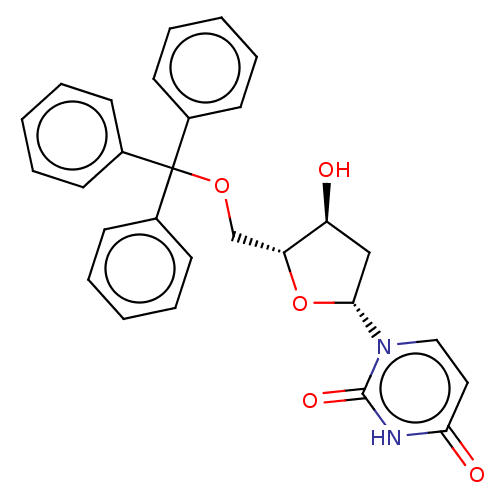 (1-(4-Hydroxy-5-trityloxymethyl-tetrahydro-furan-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis | J Med Chem 55: 2970-80 (2012) Article DOI: 10.1021/jm201628y BindingDB Entry DOI: 10.7270/Q2C82BB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM149712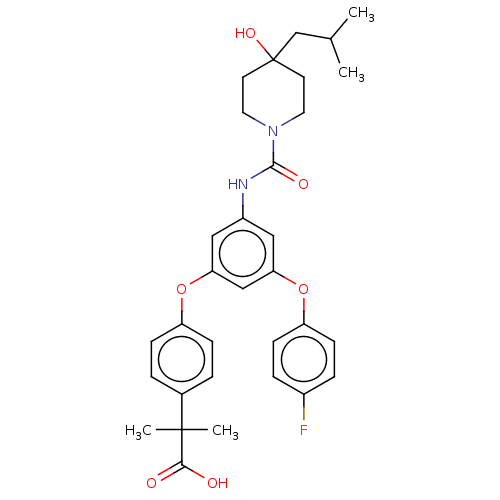 (US8975409, Example 9) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Rattus norvegicus (Rat)) | BDBM149705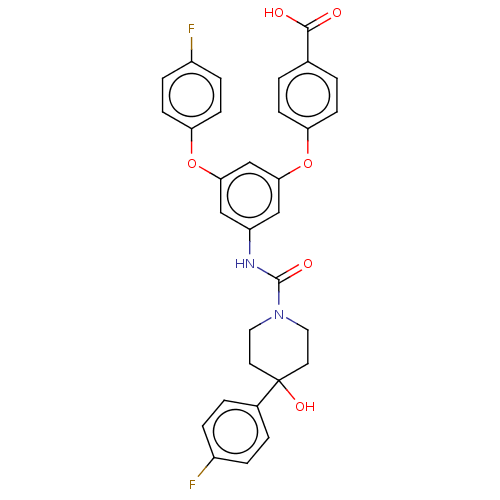 (US8975409, Example 9(3)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at rat S1P2 expressed in CHO cells assessed as Ca2+ level by FURA-2AM dye based fluorescence assay | Bioorg Med Chem Lett 25: 4387-92 (2015) Article DOI: 10.1016/j.bmcl.2015.09.022 BindingDB Entry DOI: 10.7270/Q2SX6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50143197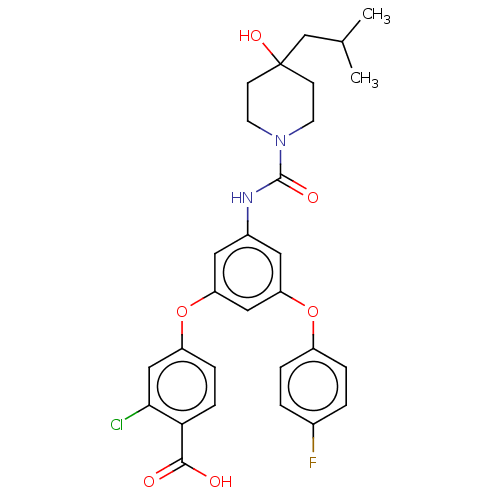 (CHEMBL3759037) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50065611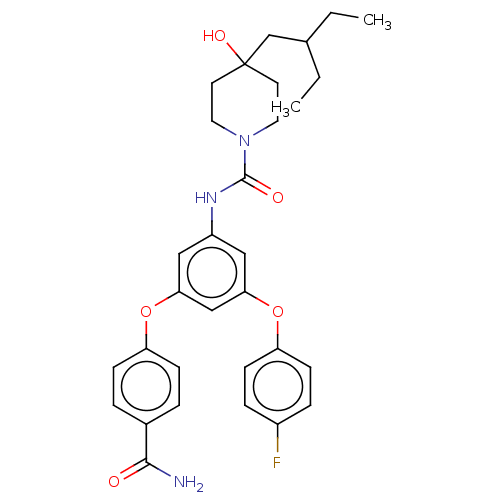 (CHEMBL3401383) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P2 expressed in CHO-K1 cells by scintillation counter | Bioorg Med Chem Lett 25: 4387-92 (2015) Article DOI: 10.1016/j.bmcl.2015.09.022 BindingDB Entry DOI: 10.7270/Q2SX6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50143192 (CHEMBL3758929) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Rattus norvegicus (Rat)) | BDBM50120582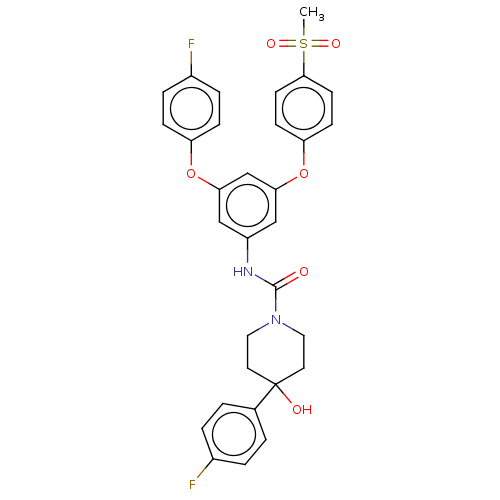 (CHEMBL3618199) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at rat S1P2 expressed in CHO cells assessed as Ca2+ level by FURA-2AM dye based fluorescence assay | Bioorg Med Chem Lett 25: 4387-92 (2015) Article DOI: 10.1016/j.bmcl.2015.09.022 BindingDB Entry DOI: 10.7270/Q2SX6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Rattus norvegicus (Rat)) | BDBM50065611 (CHEMBL3401383) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at rat S1P2 expressed in CHO cells assessed as Ca2+ level by FURA-2AM dye based fluorescence assay | Bioorg Med Chem Lett 25: 4387-92 (2015) Article DOI: 10.1016/j.bmcl.2015.09.022 BindingDB Entry DOI: 10.7270/Q2SX6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM149709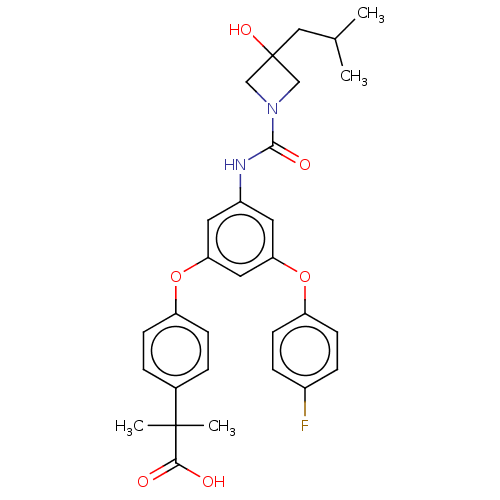 (US8975409, Example 19) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Rattus norvegicus (Rat)) | BDBM50120583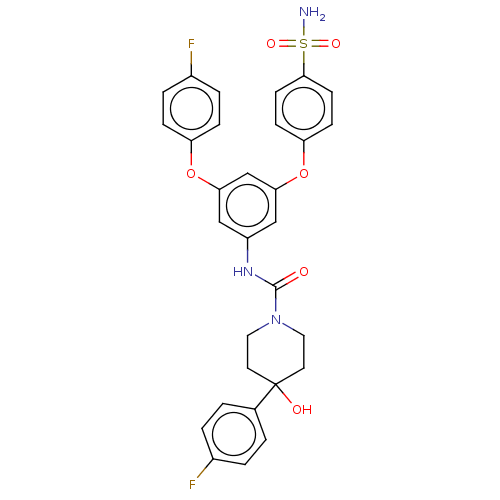 (CHEMBL3618200) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at rat S1P2 expressed in CHO cells assessed as Ca2+ level by FURA-2AM dye based fluorescence assay | Bioorg Med Chem Lett 25: 4387-92 (2015) Article DOI: 10.1016/j.bmcl.2015.09.022 BindingDB Entry DOI: 10.7270/Q2SX6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM149705 (US8975409, Example 9(3)) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM149705 (US8975409, Example 9(3)) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at human S1P2 expressed in CHO cells assessed as Ca2+ level by FURA-2AM dye based fluorescence assay | Bioorg Med Chem Lett 25: 4387-92 (2015) Article DOI: 10.1016/j.bmcl.2015.09.022 BindingDB Entry DOI: 10.7270/Q2SX6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50143201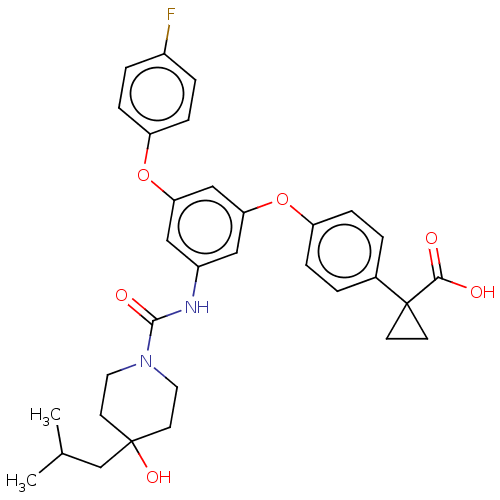 (CHEMBL3759864) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50143203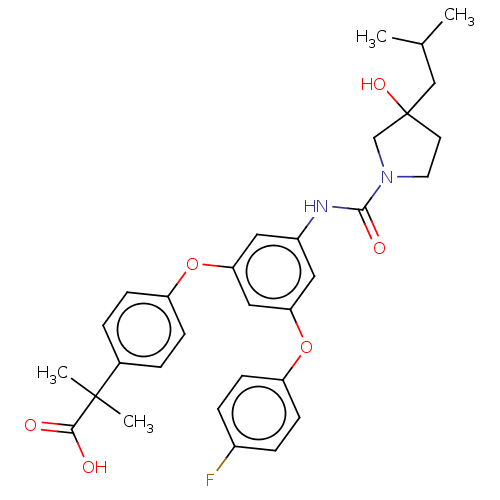 (CHEMBL3759434) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50131845 (CHEMBL3633535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Binding affinity against delta opioid receptor in mouse hot plate test | ACS Med Chem Lett 6: 1004-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00221 BindingDB Entry DOI: 10.7270/Q2WM1G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50143202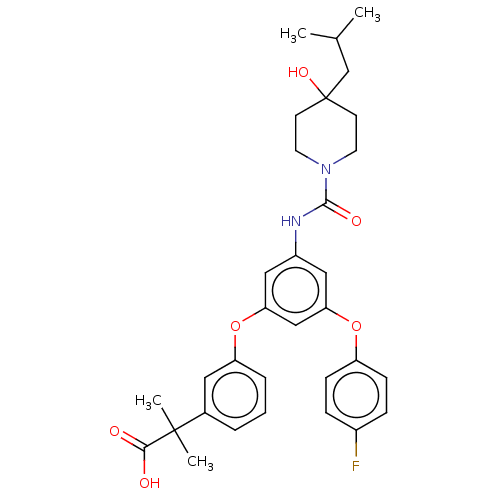 (CHEMBL3758872) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50143206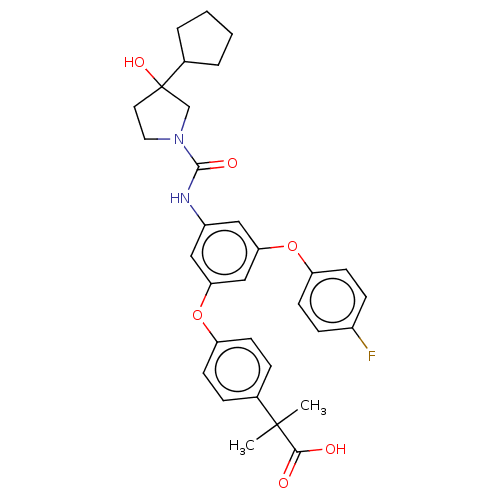 (CHEMBL3759532) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50143200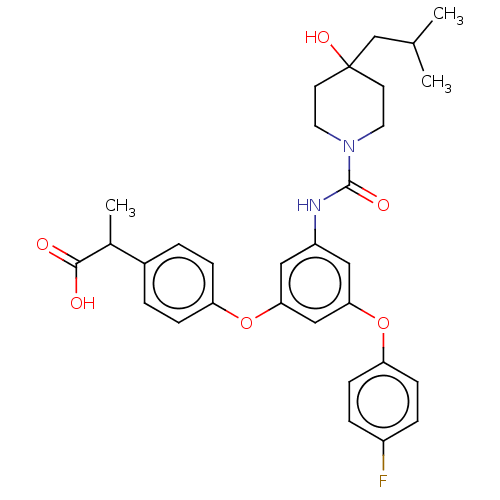 (CHEMBL3758775) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50131854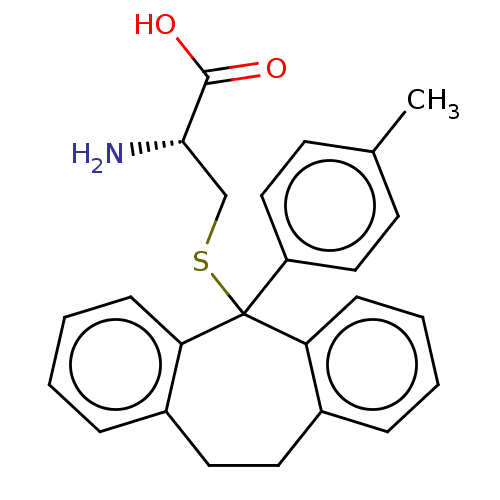 (CHEMBL3633526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Binding affinity against delta opioid receptor in mouse hot plate test | ACS Med Chem Lett 6: 1004-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00221 BindingDB Entry DOI: 10.7270/Q2WM1G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50131853 (CHEMBL3633527) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Binding affinity against delta opioid receptor in mouse hot plate test | ACS Med Chem Lett 6: 1004-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00221 BindingDB Entry DOI: 10.7270/Q2WM1G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50143198 (CHEMBL3758974) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50131859 (CHEMBL3633537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Binding affinity against delta opioid receptor in mouse hot plate test | ACS Med Chem Lett 6: 1004-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00221 BindingDB Entry DOI: 10.7270/Q2WM1G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50143191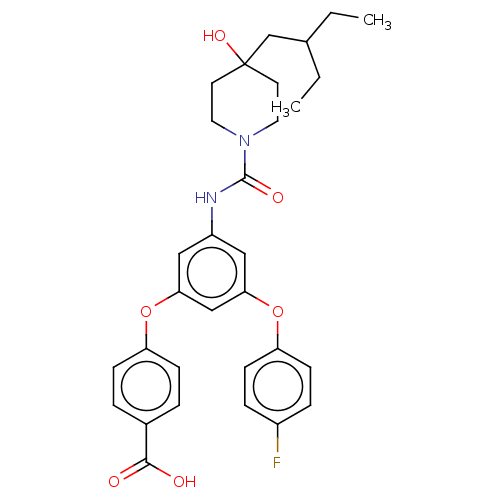 (CHEMBL3758214) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at S1P2 receptor (unknown origin) expressed in CHO cells assessed as inhibition of S1P-induced increase in intracellular calcium ... | Bioorg Med Chem Lett 26: 1209-13 (2016) Article DOI: 10.1016/j.bmcl.2016.01.031 BindingDB Entry DOI: 10.7270/Q2ST7RQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Rattus norvegicus (Rat)) | BDBM50120579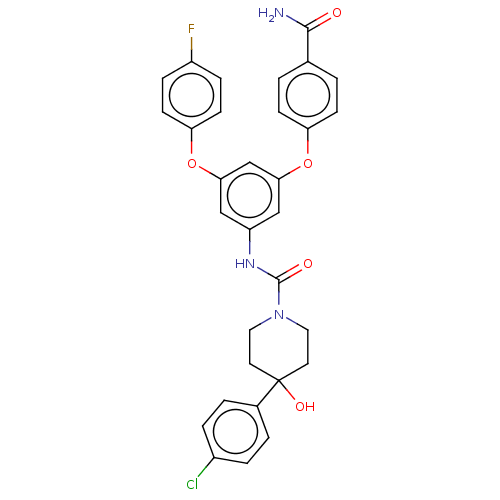 (CHEMBL3618196) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Curated by ChEMBL | Assay Description Antagonist activity at rat S1P2 expressed in CHO cells assessed as Ca2+ level by FURA-2AM dye based fluorescence assay | Bioorg Med Chem Lett 25: 4387-92 (2015) Article DOI: 10.1016/j.bmcl.2015.09.022 BindingDB Entry DOI: 10.7270/Q2SX6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50131855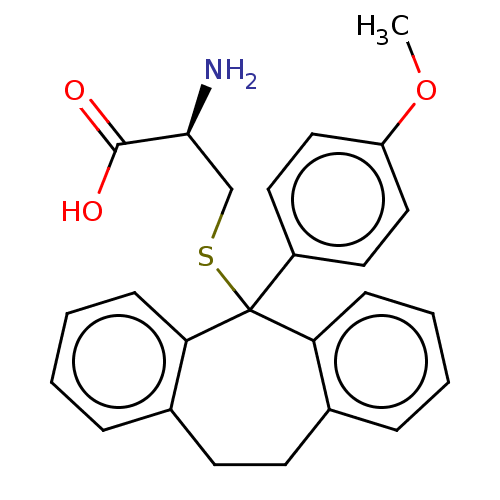 (CHEMBL3633366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Binding affinity against delta opioid receptor in mouse hot plate test | ACS Med Chem Lett 6: 1004-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00221 BindingDB Entry DOI: 10.7270/Q2WM1G76 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 4060 total ) | Next | Last >> |