

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50206243 (CHEMBL3918431) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]Tyr54-alpha-bungarotoxin from rat alpha7 nAChR expressed in HEK293 cell membranes co-expressing human RIC3 measured after 2 hrs... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50211195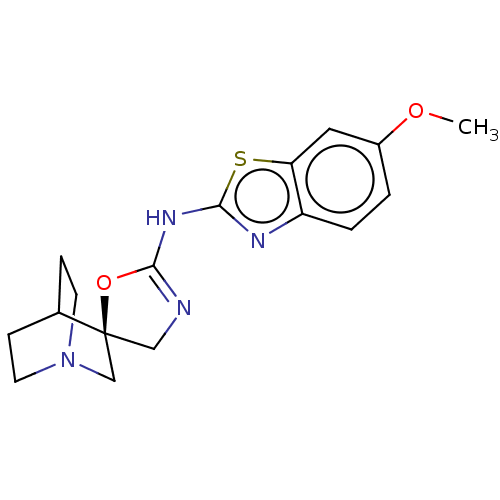 (CHEMBL3944506) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]Tyr54-alpha-bungarotoxin from rat alpha7 nAChR expressed in HEK293 cell membranes co-expressing human RIC3 measured after 2 hrs... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164613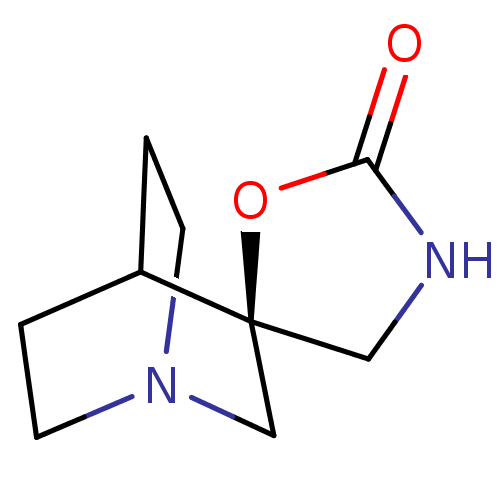 ((-)-Spiro[1-azabicyclo(2.2.2)octane-3,5'-oxazolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from rat hippocampal alpha7 nAChR measured after 2 hrs by TopCount scintillation counting method | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211207 (CHEMBL3950038) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211210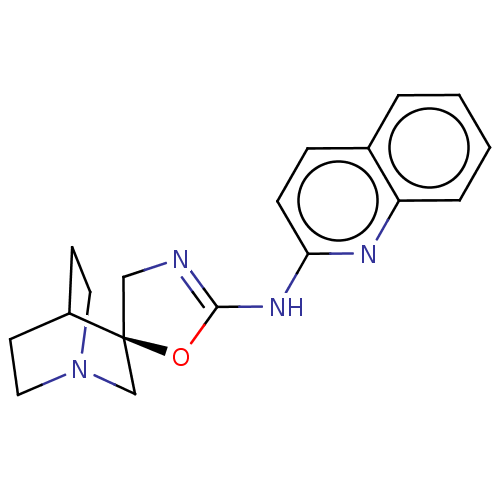 (CHEMBL3984925) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211200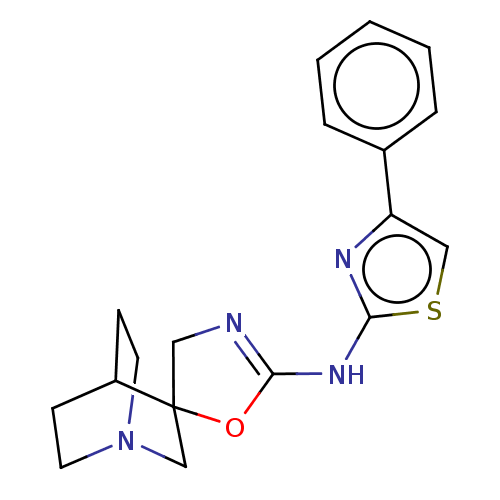 (CHEMBL3974854) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211198 (CHEMBL3961219) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211215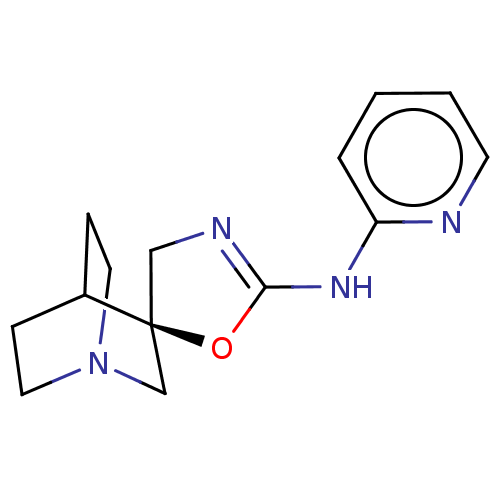 (CHEMBL3898922) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211212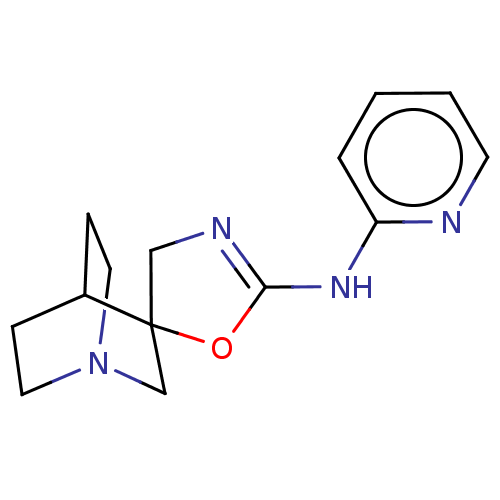 (CHEMBL3927589) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211202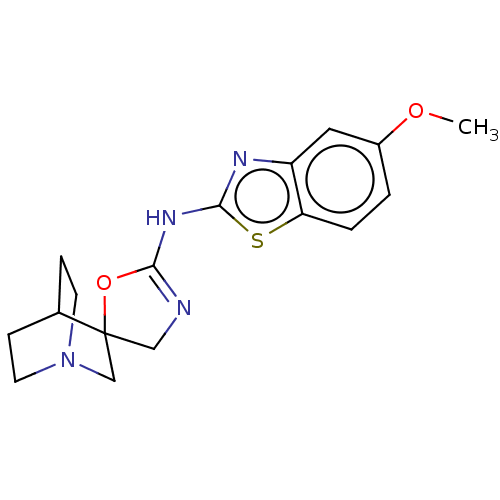 (CHEMBL3981300) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211220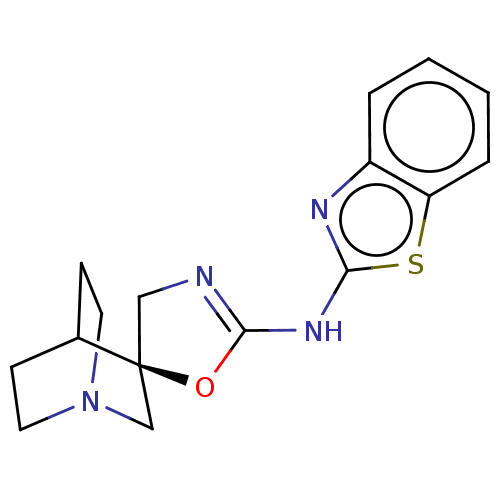 (CHEMBL3970846) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211196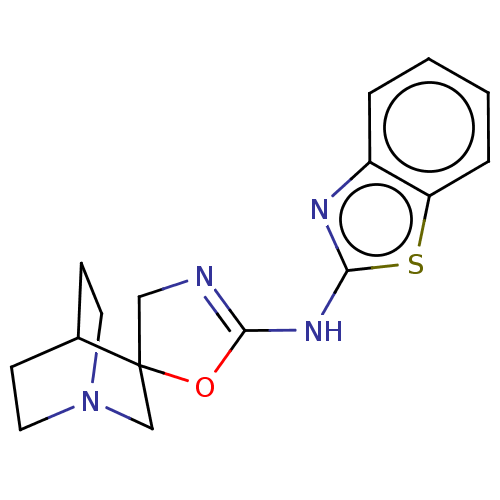 (CHEMBL3954179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211211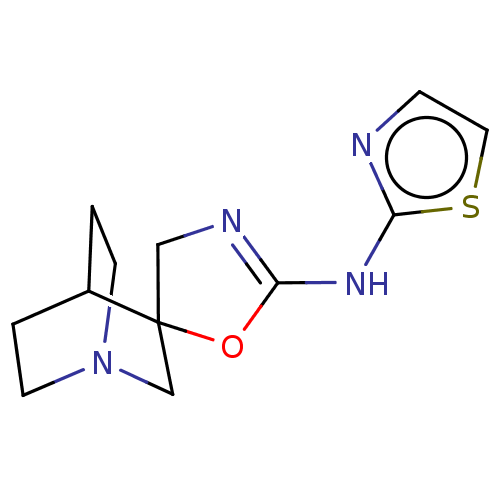 (CHEMBL3902912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259919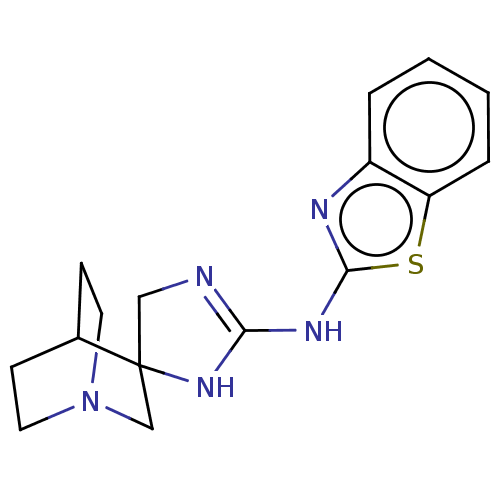 (CHEMBL4080813) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259938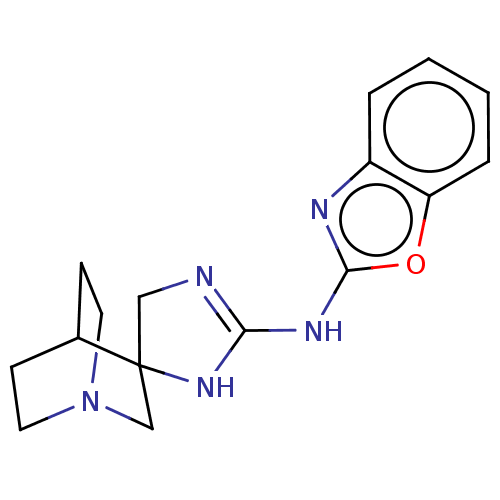 (CHEMBL4104136) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211206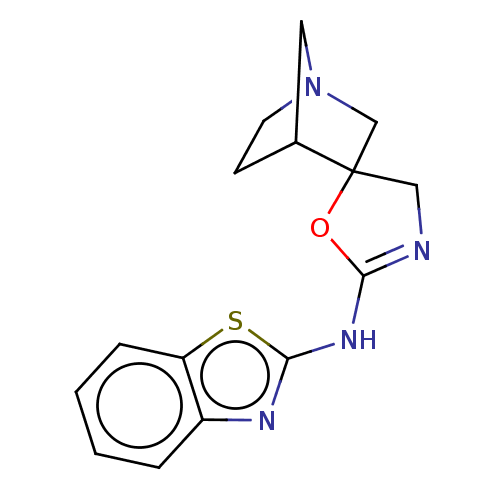 (CHEMBL3923346) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259915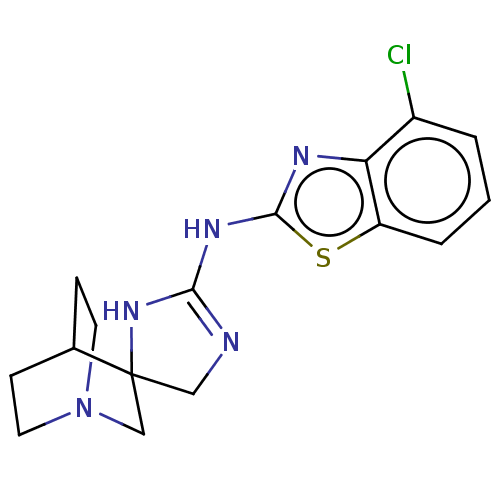 (CHEMBL4063687) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259914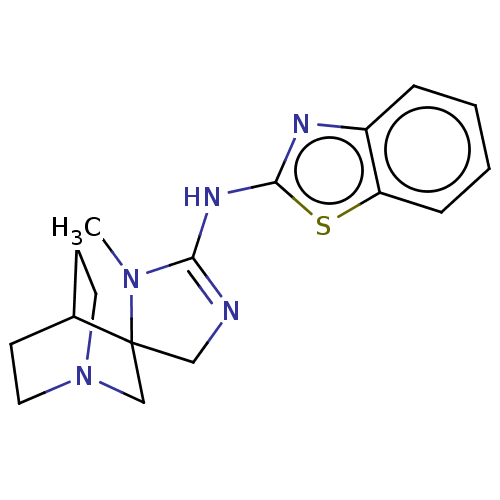 (CHEMBL4060728) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259947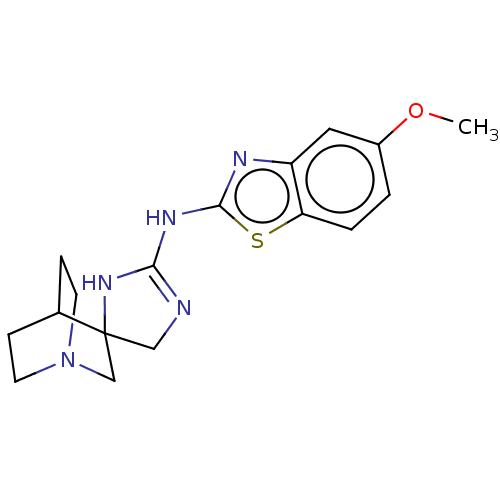 (CHEMBL4085299) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211208 (CHEMBL3926969) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211097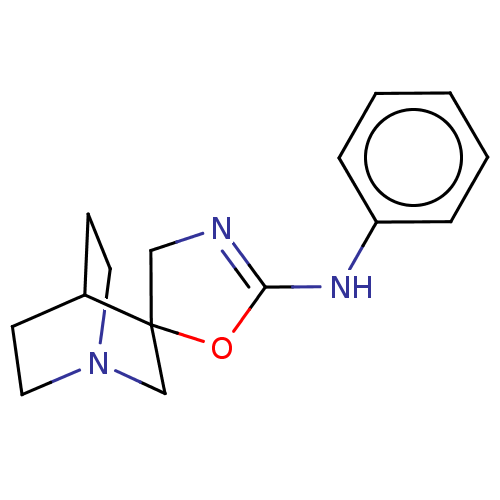 (CHEMBL3973872) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50206243 (CHEMBL3918431) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211197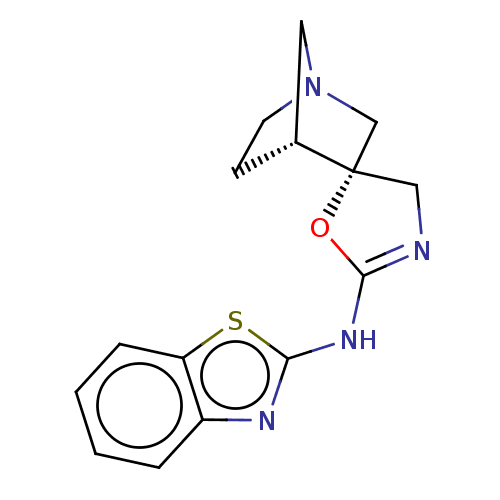 (CHEMBL3951064) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259936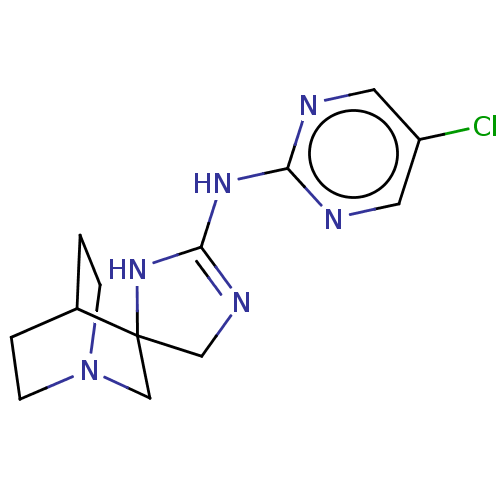 (CHEMBL4077622) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259937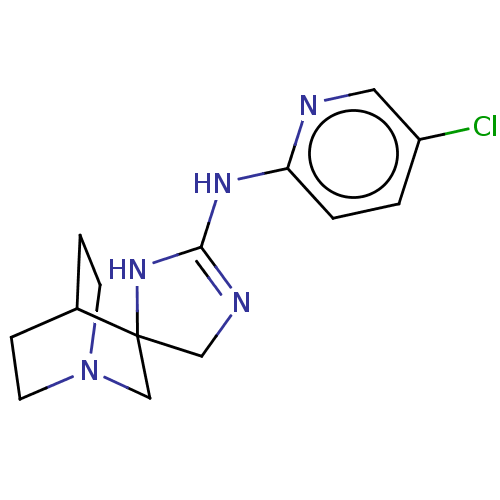 (CHEMBL4081582) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211214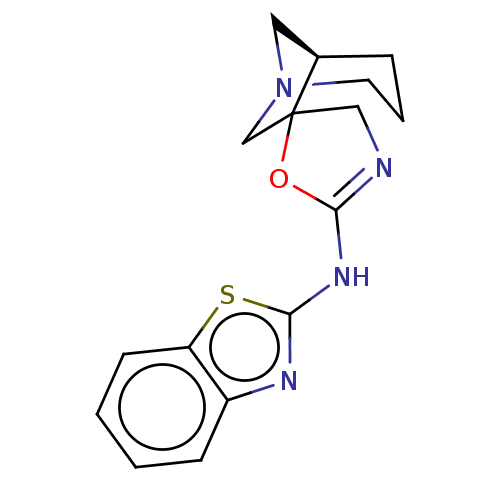 (CHEMBL3934387) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50211195 (CHEMBL3944506) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human ERG by patch clamp assay | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259944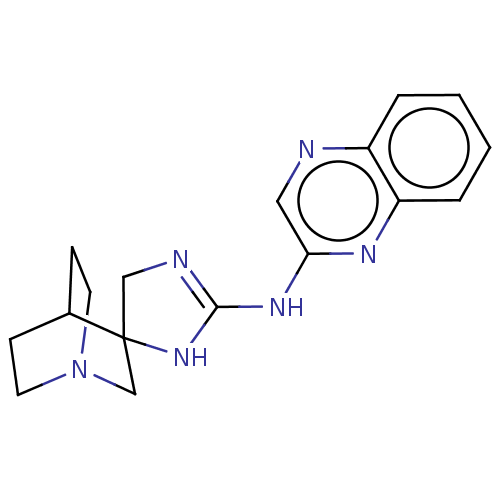 (CHEMBL4076554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50206265 (CHEMBL3909084) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211217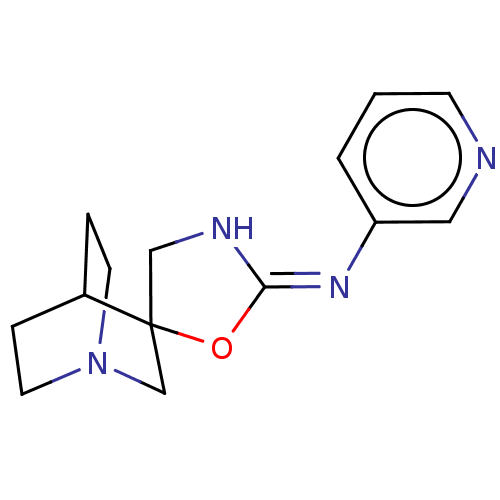 (CHEMBL3895986) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50206243 (CHEMBL3918431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human ERG by patch clamp assay | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211204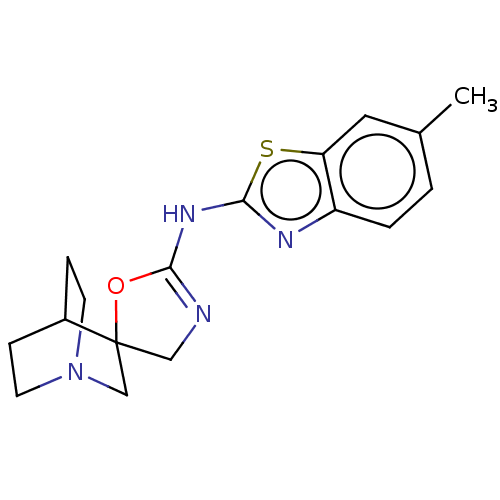 (CHEMBL3972330) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259945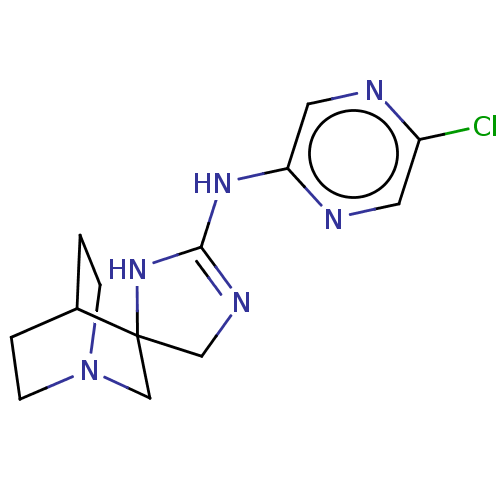 (CHEMBL4093984) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259925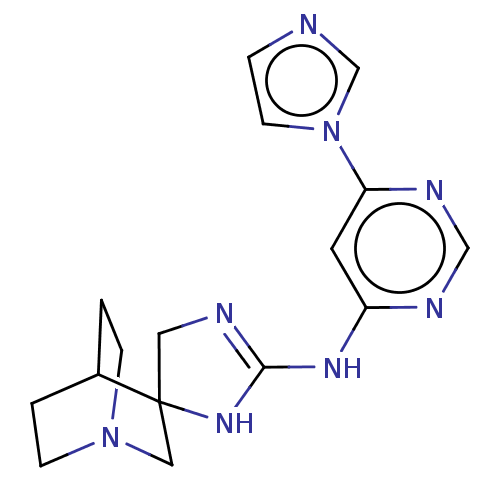 (CHEMBL4077127) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211096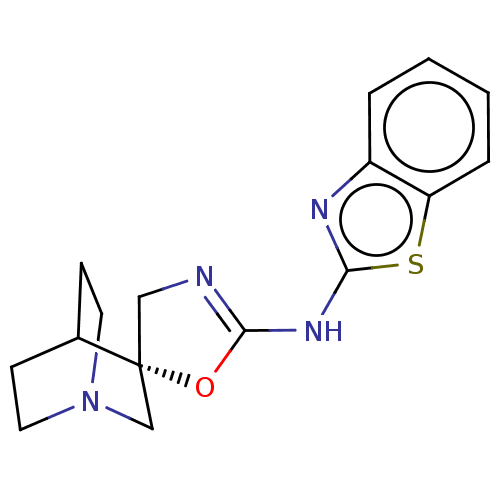 (CHEMBL3941279) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259921 (CHEMBL4086223) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211213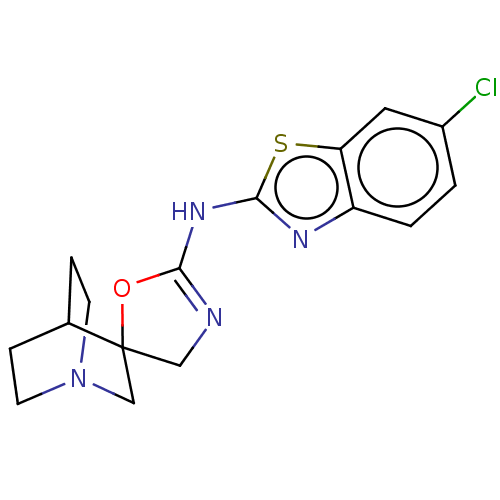 (CHEMBL3927478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211195 (CHEMBL3944506) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211199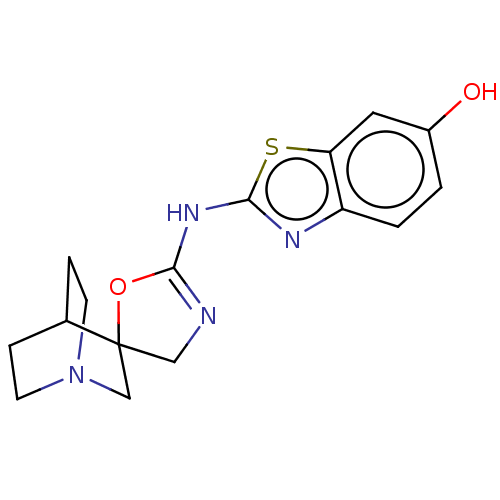 (CHEMBL3953577) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259946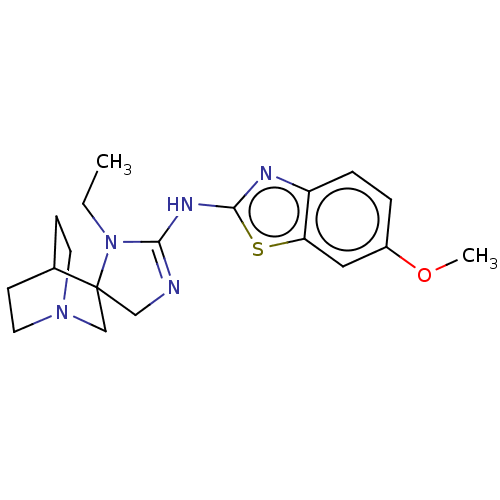 (CHEMBL4069408) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259913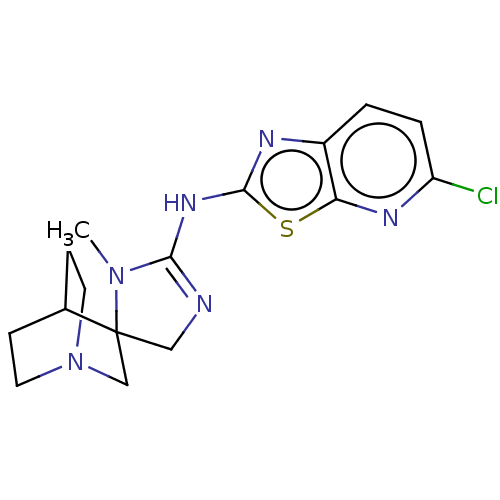 (CHEMBL4082605) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211203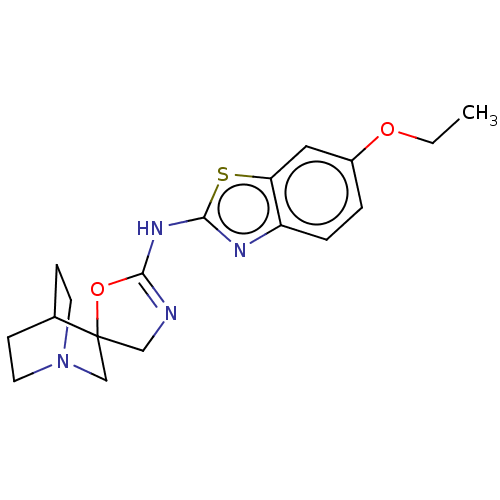 (CHEMBL3980382) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259924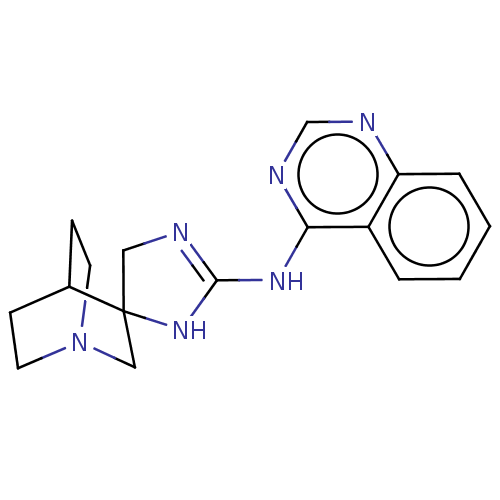 (CHEMBL4096397) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259918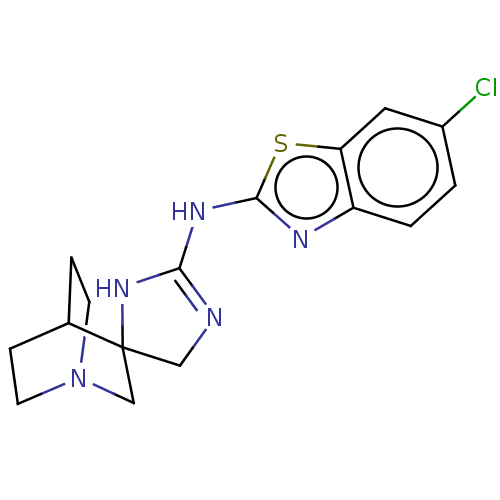 (CHEMBL4105038) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211194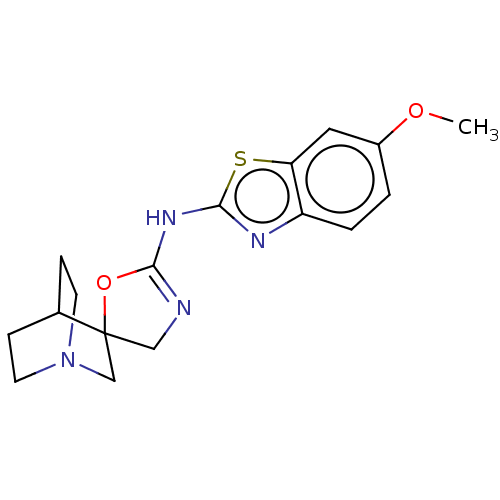 (CHEMBL3952532) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211201 (CHEMBL3945453) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211209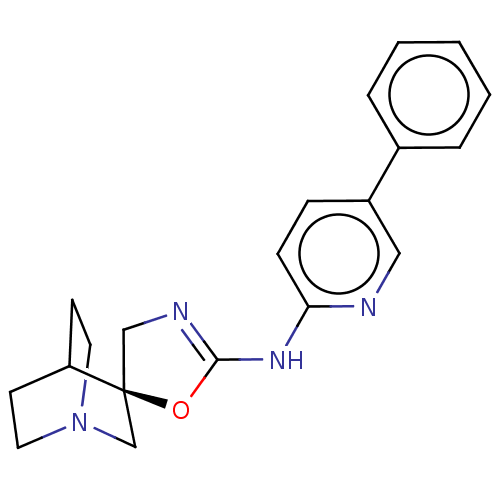 (CHEMBL3907949) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211190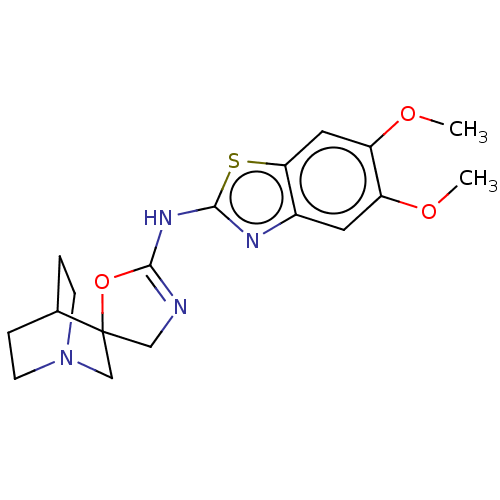 (CHEMBL3910268) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259916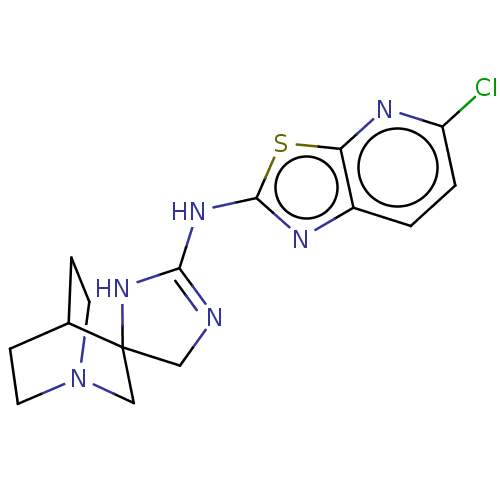 (CHEMBL4067446) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50259917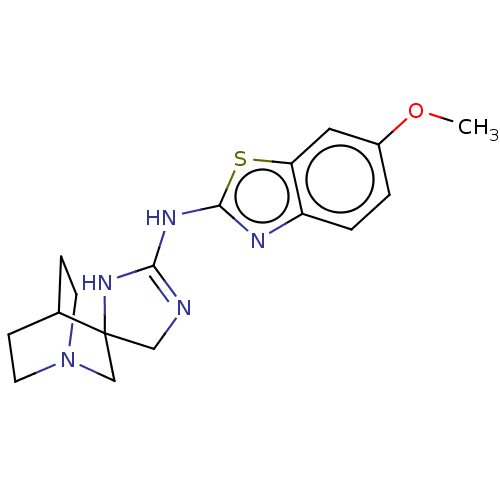 (CHEMBL4088682) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) assessed as decrease in calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 578-581 (2017) Article DOI: 10.1016/j.bmcl.2016.12.014 BindingDB Entry DOI: 10.7270/Q28P62ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 136 total ) | Next | Last >> |