

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581306 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581300 ((2S)-2-[(6-cyclopropoxy-5-{[(2,4- diamino-6-oxo-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27533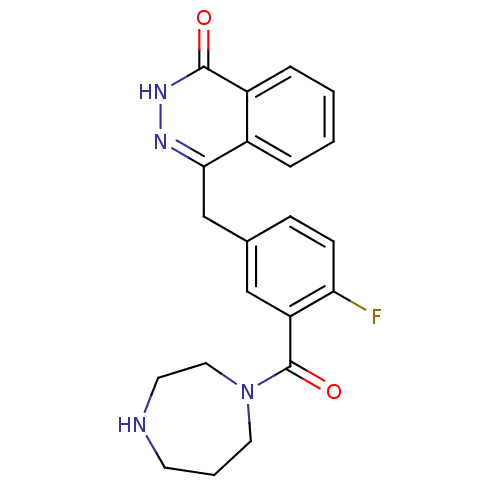 (4-(3-(1,4-diazepane-1-carbonyl)-4-fluorobenzyl)pht...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet Curated by ChEMBL | Assay Description Inhibition of human PARP1 | J Med Chem 52: 3108-11 (2009) Article DOI: 10.1021/jm900052j BindingDB Entry DOI: 10.7270/Q2B56JNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581317 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581313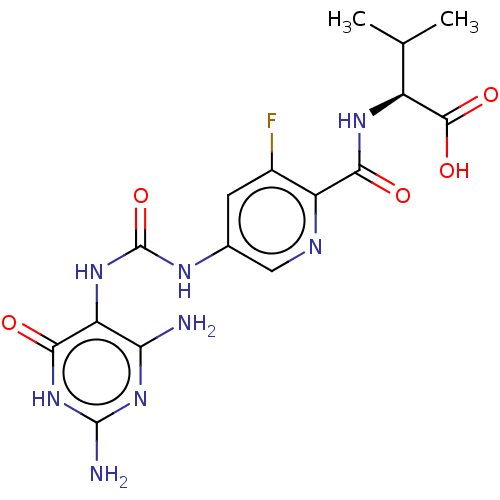 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581288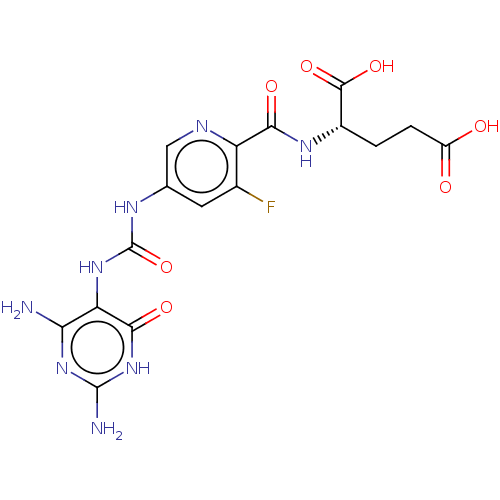 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581298 ((2S)-4- [(benzenesulfonyl)carbamoyl]-2- [(5-{[(2,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581286 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581314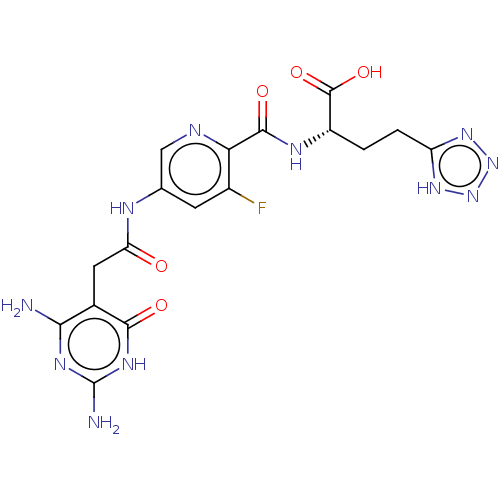 ((2S)-2-({5-[2-(2,4-diamino-6-oxo- 1,6-dihydropyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581308 ((2S)-2-({5-[2-(2,4-diamino-6-oxo- 1,6-dihydropyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581287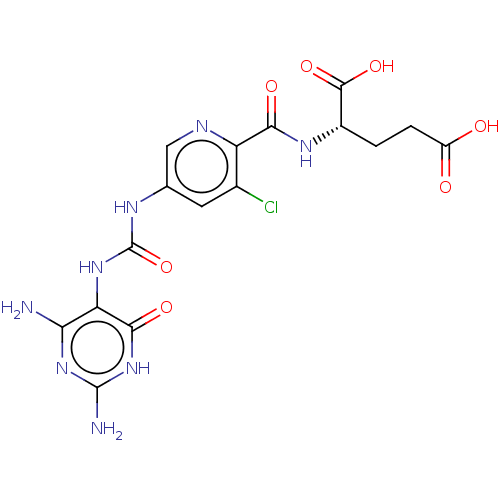 ((2S)-2-[(3-chloro-5-{[(2,4-diamino- 6-oxo-1,6-dihy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581294 ((2S)-2-cyclohexyl-2-[(5-{[(2,4- diamino-6-oxo-1,6-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50239529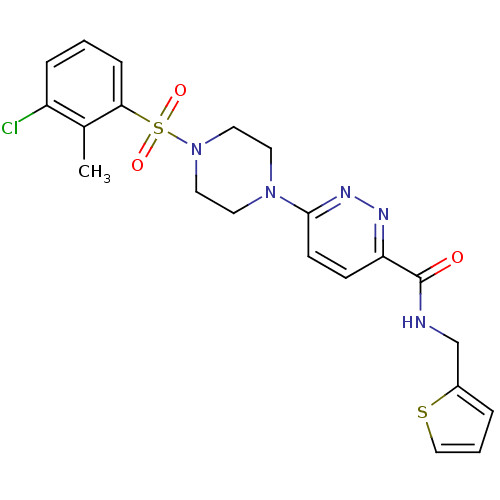 (CHEMBL4072033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged dCTPase expressed in Escherichia coli BL21(DE3) using dCTP as substrate by HTS-based malachite ... | J Med Chem 60: 4279-4292 (2017) Article DOI: 10.1021/acs.jmedchem.7b00182 BindingDB Entry DOI: 10.7270/Q22N54F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150038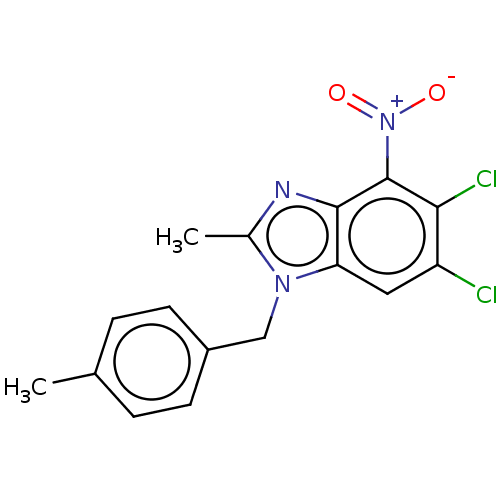 (CHEMBL3771346) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) Article DOI: 10.1021/acs.jmedchem.5b01741 BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581285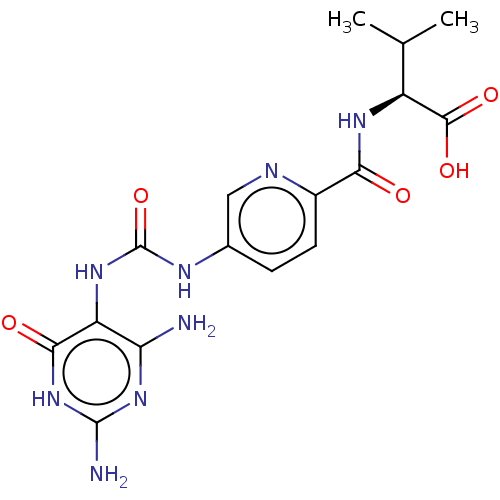 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM38321 (6-(3,4-dimethoxyphenyl)-3-(2-furanyl)-[1,2,4]triaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 receptor in rat cortical membrane using [3H]GR-65630 as radioligand | J Med Chem 60: 2148-2154 (2017) Article DOI: 10.1021/acs.jmedchem.6b01786 BindingDB Entry DOI: 10.7270/Q2BR8VGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50236118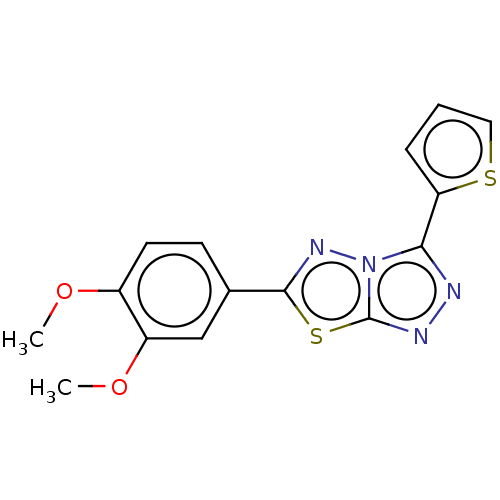 (CHEMBL4065181) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human dCTPase 1 | Bioorg Med Chem Lett 27: 3897-3904 (2017) Article DOI: 10.1016/j.bmcl.2017.06.038 BindingDB Entry DOI: 10.7270/Q23N25WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50236118 (CHEMBL4065181) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 receptor in rat cortical membrane using [3H]GR-65630 as radioligand | J Med Chem 60: 2148-2154 (2017) Article DOI: 10.1021/acs.jmedchem.6b01786 BindingDB Entry DOI: 10.7270/Q2BR8VGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150044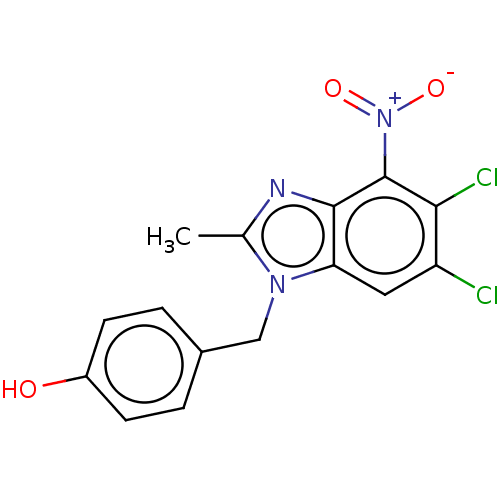 (CHEMBL3771039) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) Article DOI: 10.1021/acs.jmedchem.5b01741 BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581297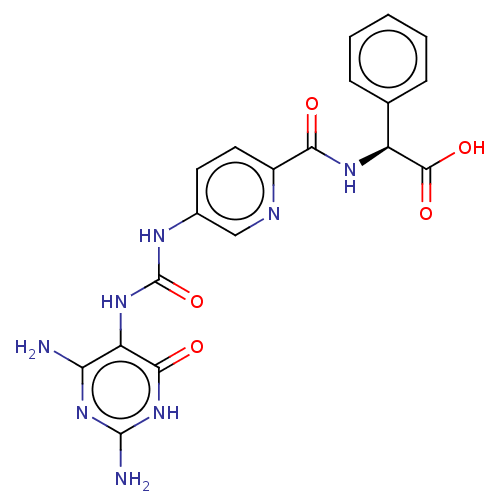 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581283 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50239517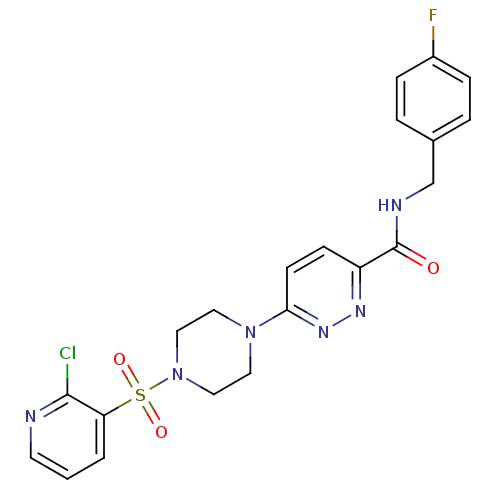 (CHEMBL4082508) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged dCTPase expressed in Escherichia coli BL21(DE3) using dCTP as substrate by HTS-based malachite ... | J Med Chem 60: 4279-4292 (2017) Article DOI: 10.1021/acs.jmedchem.7b00182 BindingDB Entry DOI: 10.7270/Q22N54F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150112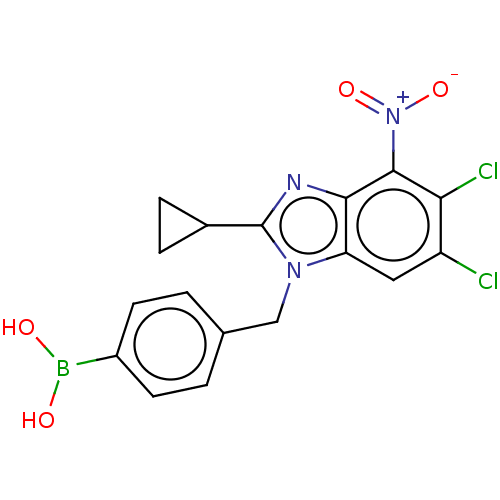 (CHEMBL3769835) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) Article DOI: 10.1021/acs.jmedchem.5b01741 BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581307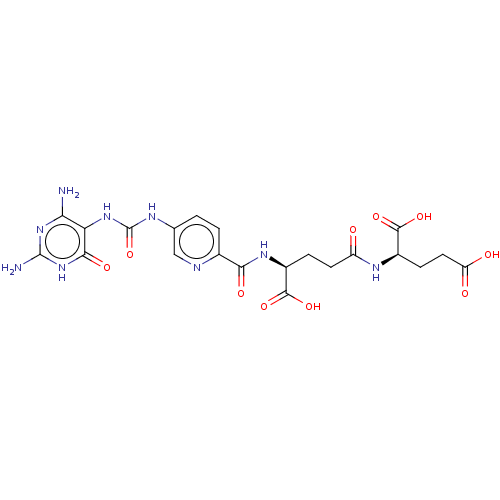 ((2R)-2-[(4S)-4-carboxy-4-[(5- {[(2,4-diamino-6-oxo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581299 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150043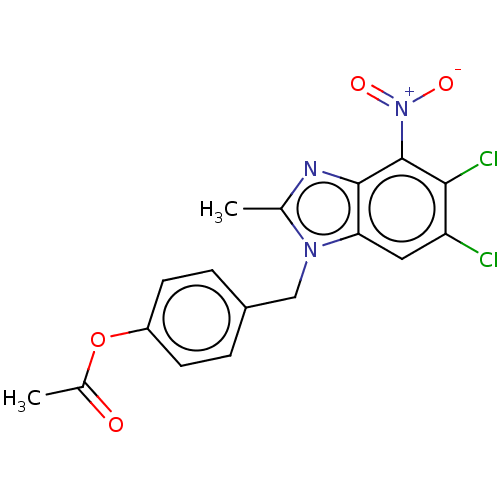 (CHEMBL3769727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) Article DOI: 10.1021/acs.jmedchem.5b01741 BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50239516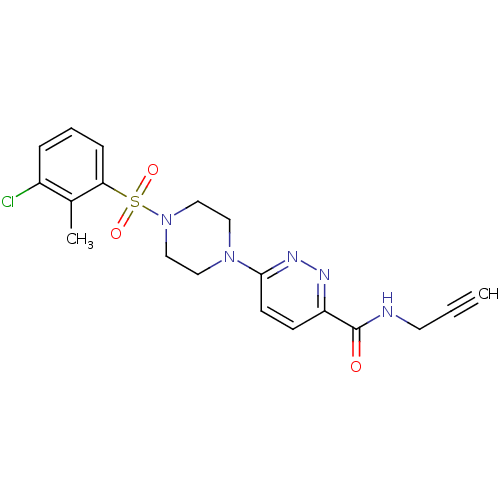 (CHEMBL4060634) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged dCTPase expressed in Escherichia coli BL21(DE3) using dCTP as substrate by HTS-based malachite ... | J Med Chem 60: 4279-4292 (2017) Article DOI: 10.1021/acs.jmedchem.7b00182 BindingDB Entry DOI: 10.7270/Q22N54F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50239518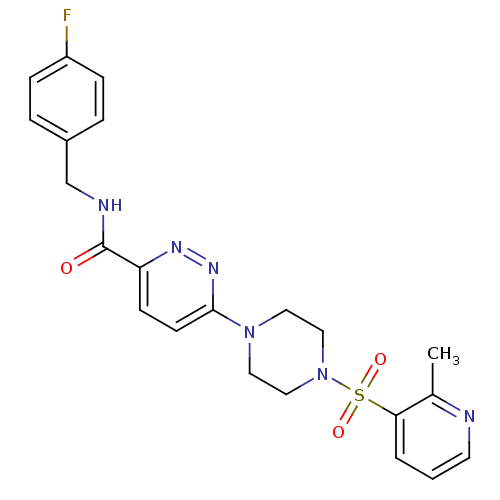 (CHEMBL4075978) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human dCTPase 1 | Bioorg Med Chem Lett 27: 3897-3904 (2017) Article DOI: 10.1016/j.bmcl.2017.06.038 BindingDB Entry DOI: 10.7270/Q23N25WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50239518 (CHEMBL4075978) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged dCTPase expressed in Escherichia coli BL21(DE3) using dCTP as substrate by HTS-based malachite ... | J Med Chem 60: 4279-4292 (2017) Article DOI: 10.1021/acs.jmedchem.7b00182 BindingDB Entry DOI: 10.7270/Q22N54F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581296 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50239530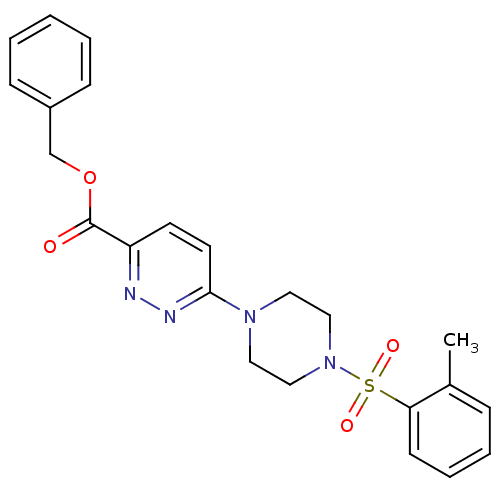 (CHEMBL4101470) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged dCTPase expressed in Escherichia coli BL21(DE3) using dCTP as substrate by HTS-based malachite ... | J Med Chem 60: 4279-4292 (2017) Article DOI: 10.1021/acs.jmedchem.7b00182 BindingDB Entry DOI: 10.7270/Q22N54F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50239519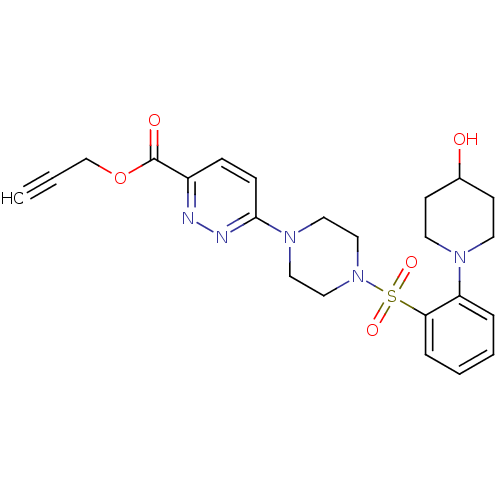 (CHEMBL4091797) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged dCTPase expressed in Escherichia coli BL21(DE3) using dCTP as substrate by HTS-based malachite ... | J Med Chem 60: 4279-4292 (2017) Article DOI: 10.1021/acs.jmedchem.7b00182 BindingDB Entry DOI: 10.7270/Q22N54F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150036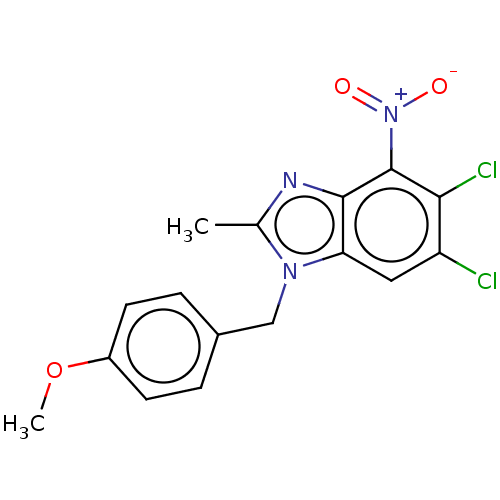 (CHEMBL3771227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) Article DOI: 10.1021/acs.jmedchem.5b01741 BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581305 ((2S)-2-[(4S)-4-carboxy-4-[(5- {[(2,4-diamino-6-oxo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581311 ((2S)-2-({5-[2-(2,4-diamino-6-oxo- 1,6-dihydropyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150040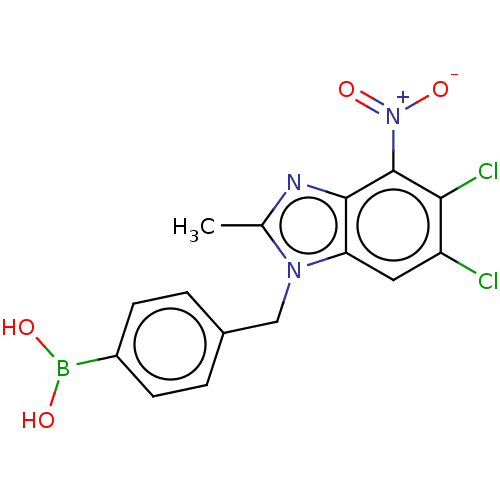 (CHEMBL3771237) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) Article DOI: 10.1021/acs.jmedchem.5b01741 BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150051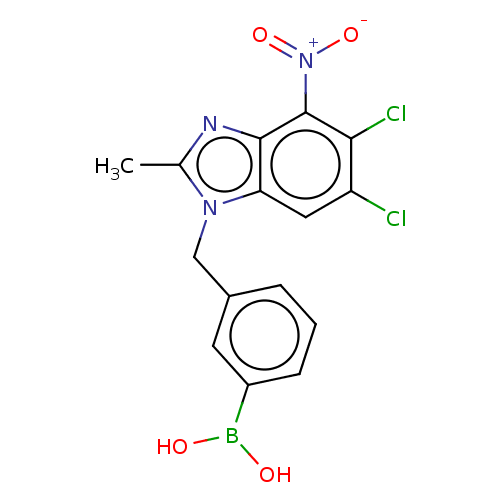 (CHEMBL3770446) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) Article DOI: 10.1021/acs.jmedchem.5b01741 BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581293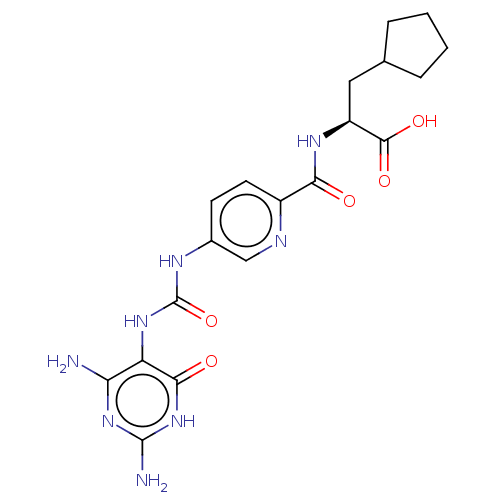 ((2S)-3-cyclopentyl-2-[(5-{[(2,4- diamino-6-oxo-1,6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150052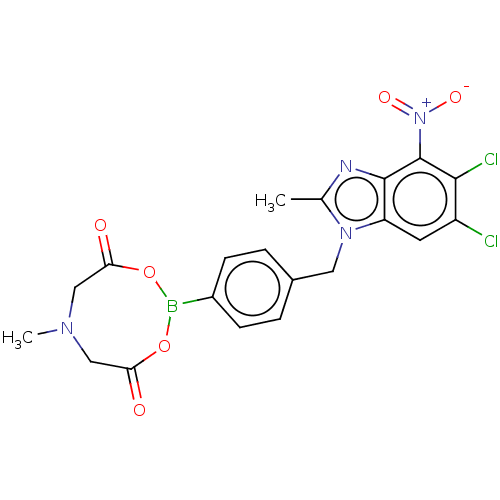 (CHEMBL3771105) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human dCTPase 1 | Bioorg Med Chem Lett 27: 3897-3904 (2017) Article DOI: 10.1016/j.bmcl.2017.06.038 BindingDB Entry DOI: 10.7270/Q23N25WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150052 (CHEMBL3771105) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) Article DOI: 10.1021/acs.jmedchem.5b01741 BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581292 ((2S)-2-({5-[2-(2,4-diamino-6-oxo- 1,6-dihydropyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150035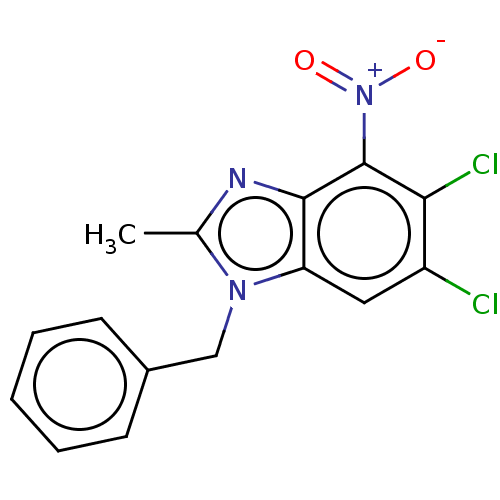 (CHEMBL3769687) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) Article DOI: 10.1021/acs.jmedchem.5b01741 BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50239514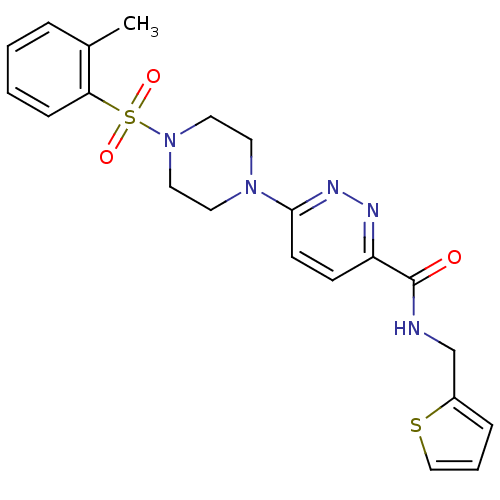 (CHEMBL4098065) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged dCTPase expressed in Escherichia coli BL21(DE3) using dCTP as substrate by HTS-based malachite ... | J Med Chem 60: 4279-4292 (2017) Article DOI: 10.1021/acs.jmedchem.7b00182 BindingDB Entry DOI: 10.7270/Q22N54F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581291 ((2S)-2-({5-[2-(2,4-diamino-6-oxo- 1,6-dihydropyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150026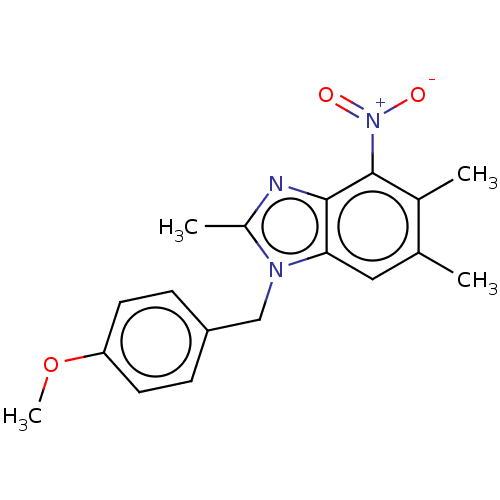 (CHEMBL3771138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) Article DOI: 10.1021/acs.jmedchem.5b01741 BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581316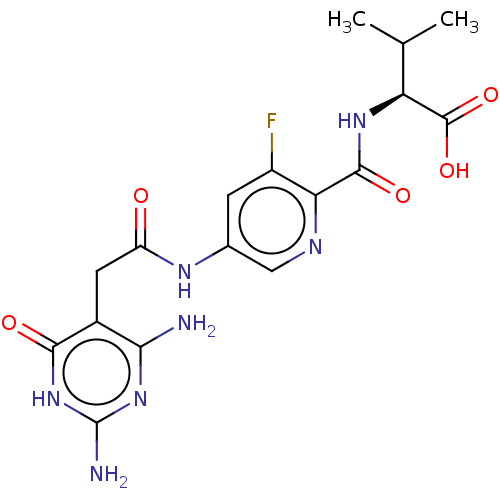 ((2S)-2-({5-[2-(2,4-diamino-6-oxo- 1,6-dihydropyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50239515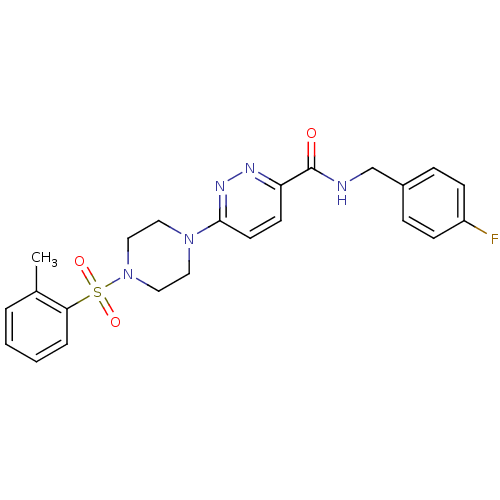 (CHEMBL4061657) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged dCTPase expressed in Escherichia coli BL21(DE3) using dCTP as substrate by HTS-based malachite ... | J Med Chem 60: 4279-4292 (2017) Article DOI: 10.1021/acs.jmedchem.7b00182 BindingDB Entry DOI: 10.7270/Q22N54F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581309 ((2S)-2-({5-[2-(2,4-diamino-6-oxo- 1,6-dihydropyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581284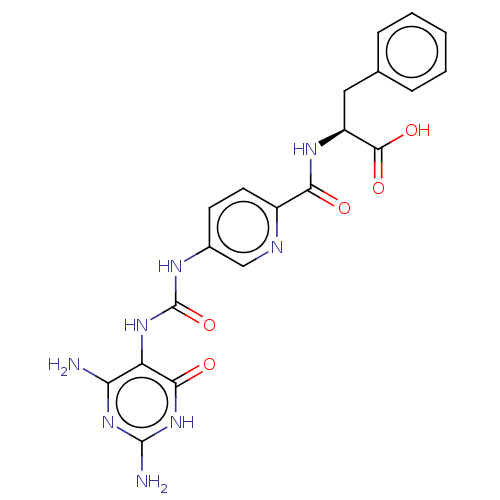 ((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM581301 ((2S)-2-({3-chloro-5-[2-(2,4- diamino-6-oxo-1,6- di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 263 total ) | Next | Last >> |