 Found 117 hits with Last Name = 'honda' and Initial = 'a'
Found 117 hits with Last Name = 'honda' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 3-kinase catalytic subunit type 3
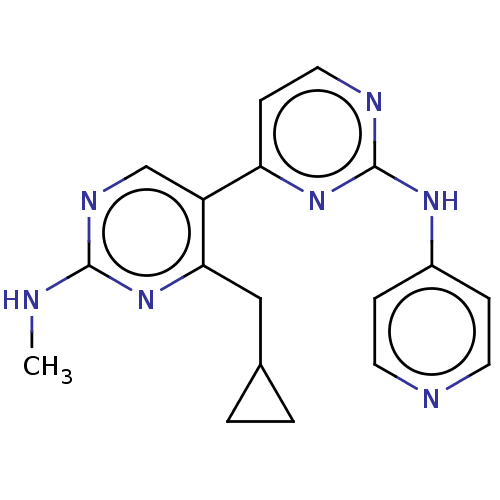
(Homo sapiens (Human)) | BDBM50156294

(CHEMBL3781415)Show InChI InChI=1S/C17H17N7/c18-16-21-10-13(15(23-16)9-11-1-2-11)14-5-8-20-17(24-14)22-12-3-6-19-7-4-12/h3-8,10-11H,1-2,9H2,(H2,18,21,23)(H,19,20,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156302
(CHEMBL3780029)Show InChI InChI=1S/C18H19N7/c1-19-17-22-11-14(16(25-17)10-12-2-3-12)15-6-9-21-18(24-15)23-13-4-7-20-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,22,25)(H,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156296

(CHEMBL3781466)Show SMILES CC(C)(O)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7O/c1-21(2,29)13-25-19-24-12-16(18(28-19)11-14-3-4-14)17-7-10-23-20(27-17)26-15-5-8-22-9-6-15/h5-10,12,14,29H,3-4,11,13H2,1-2H3,(H,24,25,28)(H,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375331
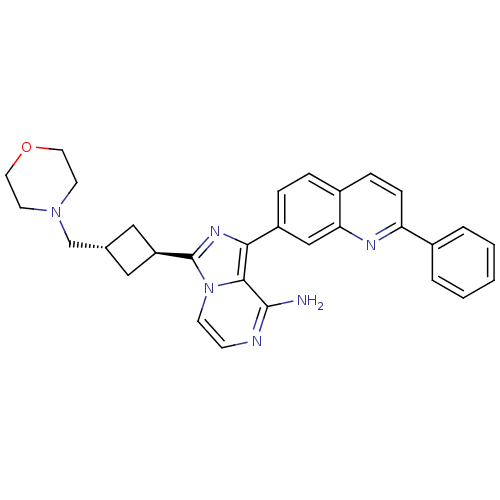
(CHEMBL261253)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@H]1C[C@H](CN2CCOCC2)C1 |wU:26.30,wD:28.33,(5.75,-42.52,;5.75,-44.06,;4.43,-44.83,;4.43,-46.37,;5.76,-47.14,;7.1,-46.37,;8.57,-46.85,;9.48,-45.59,;8.57,-44.34,;9.35,-43.01,;8.58,-41.68,;9.35,-40.35,;10.9,-40.36,;11.66,-39.02,;13.21,-39.04,;13.99,-40.37,;13.21,-41.71,;11.67,-41.7,;10.89,-43.02,;15.53,-40.38,;16.3,-41.71,;17.84,-41.71,;18.61,-40.38,;17.83,-39.04,;16.3,-39.05,;7.09,-44.82,;9.05,-48.31,;10.43,-49.01,;9.73,-50.38,;10.21,-51.85,;9.44,-53.18,;7.91,-53.17,;7.13,-54.5,;7.9,-55.83,;9.44,-55.84,;10.2,-54.51,;8.36,-49.68,)| Show InChI InChI=1S/C30H30N6O/c31-29-28-27(23-7-6-22-8-9-25(33-26(22)18-23)21-4-2-1-3-5-21)34-30(36(28)11-10-32-29)24-16-20(17-24)19-35-12-14-37-15-13-35/h1-11,18,20,24H,12-17,19H2,(H2,31,32)/t20-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156294

(CHEMBL3781415)Show InChI InChI=1S/C17H17N7/c18-16-21-10-13(15(23-16)9-11-1-2-11)14-5-8-20-17(24-14)22-12-3-6-19-7-4-12/h3-8,10-11H,1-2,9H2,(H2,18,21,23)(H,19,20,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375334

(CHEMBL411074)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@H](CN2CCOCC2)C1 |wU:26.30,28.33,(-9.18,-42.53,;-9.17,-44.07,;-10.49,-44.84,;-10.5,-46.39,;-9.16,-47.16,;-7.83,-46.39,;-6.35,-46.87,;-5.44,-45.61,;-6.35,-44.35,;-5.57,-43.03,;-6.34,-41.7,;-5.57,-40.37,;-4.02,-40.37,;-3.26,-39.04,;-1.71,-39.05,;-.93,-40.39,;-1.71,-41.72,;-3.25,-41.72,;-4.03,-43.04,;.61,-40.39,;1.38,-41.73,;2.92,-41.73,;3.69,-40.4,;2.91,-39.06,;1.37,-39.06,;-7.83,-44.83,;-5.87,-48.33,;-6.57,-49.7,;-5.19,-50.4,;-4.72,-51.86,;-5.5,-53.2,;-7.02,-53.19,;-7.8,-54.52,;-7.03,-55.85,;-5.49,-55.86,;-4.73,-54.53,;-4.49,-49.03,)| Show InChI InChI=1S/C30H30N6O/c31-29-28-27(23-7-6-22-8-9-25(33-26(22)18-23)21-4-2-1-3-5-21)34-30(36(28)11-10-32-29)24-16-20(17-24)19-35-12-14-37-15-13-35/h1-11,18,20,24H,12-17,19H2,(H2,31,32)/t20-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375339
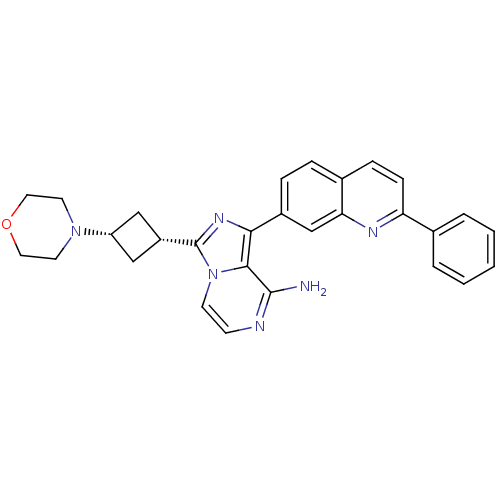
(CHEMBL258648)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCOCC1 |wU:26.30,28.35,(6.69,-13.61,;6.7,-15.15,;5.37,-15.92,;5.37,-17.47,;6.7,-18.24,;8.04,-17.47,;9.51,-17.95,;10.43,-16.69,;9.51,-15.44,;10.29,-14.11,;9.53,-12.78,;10.29,-11.45,;11.84,-11.45,;12.61,-10.12,;14.15,-10.13,;14.93,-11.47,;14.15,-12.8,;12.61,-12.8,;11.83,-14.12,;16.47,-11.47,;17.24,-12.81,;18.78,-12.81,;19.55,-11.48,;18.77,-10.14,;17.24,-10.14,;8.04,-15.92,;9.99,-19.41,;9.3,-20.78,;10.67,-21.48,;11.37,-20.11,;11.14,-22.94,;10.25,-24.19,;10.88,-25.58,;12.41,-25.73,;13.3,-24.48,;12.67,-23.08,)| Show InChI InChI=1S/C29H28N6O/c30-28-27-26(21-7-6-20-8-9-24(32-25(20)18-21)19-4-2-1-3-5-19)33-29(35(27)11-10-31-28)22-16-23(17-22)34-12-14-36-15-13-34/h1-11,18,22-23H,12-17H2,(H2,30,31)/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375337
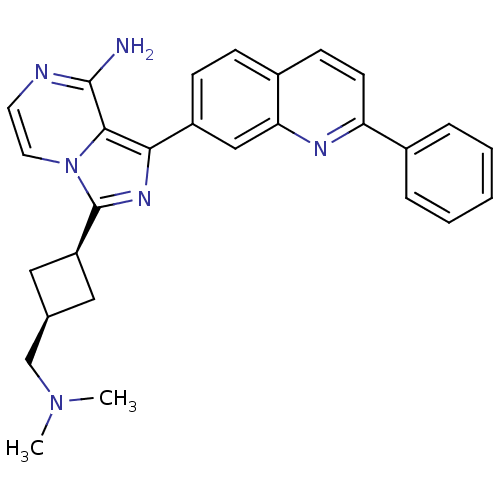
(CHEMBL413828)Show SMILES CN(C)C[C@H]1C[C@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |wU:6.8,4.3,(25.03,-21.69,;24.56,-20.23,;23.05,-19.91,;25.59,-19.08,;25.11,-17.62,;23.74,-16.92,;24.43,-15.55,;25.81,-16.24,;23.95,-14.09,;24.86,-12.83,;23.95,-11.57,;24.73,-10.25,;23.97,-8.92,;24.73,-7.59,;26.28,-7.59,;27.05,-6.26,;28.59,-6.27,;29.37,-7.61,;28.59,-8.94,;27.05,-8.94,;26.27,-10.26,;30.91,-7.61,;31.68,-8.95,;33.22,-8.95,;33.99,-7.62,;33.21,-6.28,;31.68,-6.29,;22.48,-12.05,;21.14,-11.29,;21.13,-9.75,;19.81,-12.06,;19.81,-13.61,;21.14,-14.38,;22.48,-13.61,)| Show InChI InChI=1S/C28H28N6/c1-33(2)17-18-14-22(15-18)28-32-25(26-27(29)30-12-13-34(26)28)21-9-8-20-10-11-23(31-24(20)16-21)19-6-4-3-5-7-19/h3-13,16,18,22H,14-15,17H2,1-2H3,(H2,29,30)/t18-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336316
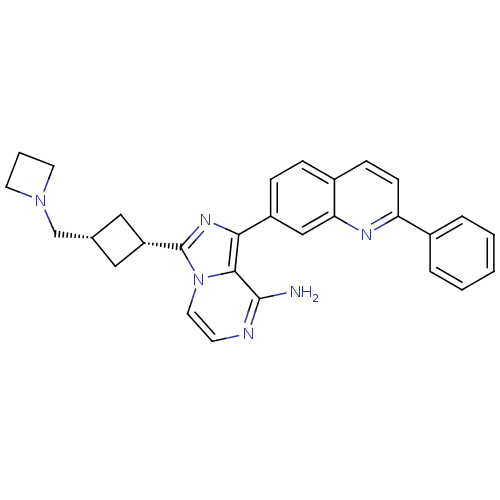
(CHEMBL410659 | cis-3-(3-(azetidin-1-ylmethyl)cyclo...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |wU:26.30,28.33,(7.04,-25.52,;7.05,-27.06,;5.72,-27.83,;5.72,-29.37,;7.05,-30.15,;8.39,-29.37,;9.86,-29.85,;10.77,-28.6,;9.86,-27.34,;10.64,-26.02,;9.88,-24.69,;10.64,-23.36,;12.19,-23.36,;12.96,-22.02,;14.5,-22.04,;15.28,-23.37,;14.5,-24.71,;12.96,-24.7,;12.18,-26.03,;16.82,-23.38,;17.59,-24.72,;19.13,-24.72,;19.9,-23.38,;19.12,-22.05,;17.59,-22.05,;8.39,-27.82,;10.34,-31.32,;9.65,-32.69,;11.02,-33.38,;11.5,-34.85,;10.47,-35.99,;8.93,-36.07,;9.01,-37.61,;10.55,-37.53,;11.72,-32.01,)| Show InChI InChI=1S/C29H28N6/c30-28-27-26(22-8-7-21-9-10-24(32-25(21)17-22)20-5-2-1-3-6-20)33-29(35(27)14-11-31-28)23-15-19(16-23)18-34-12-4-13-34/h1-3,5-11,14,17,19,23H,4,12-13,15-16,18H2,(H2,30,31)/t19-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375336
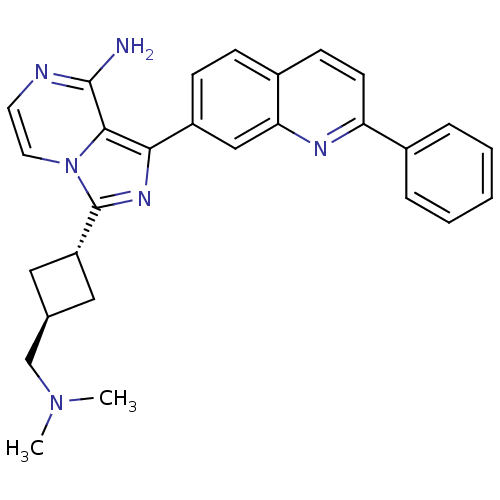
(CHEMBL411062)Show SMILES CN(C)C[C@H]1C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |wU:6.8,wD:4.3,(-5.34,-34.06,;-5.81,-32.6,;-7.32,-32.28,;-4.78,-31.45,;-5.26,-29.99,;-4.56,-28.62,;-5.94,-27.92,;-6.63,-29.29,;-6.42,-26.46,;-5.51,-25.2,;-6.42,-23.95,;-5.64,-22.62,;-6.41,-21.29,;-5.64,-19.96,;-4.09,-19.96,;-3.33,-18.63,;-1.78,-18.64,;-1,-19.98,;-1.78,-21.31,;-3.32,-21.31,;-4.1,-22.63,;.54,-19.98,;1.31,-21.32,;2.85,-21.32,;3.62,-19.99,;2.84,-18.65,;1.3,-18.66,;-7.89,-24.43,;-9.24,-23.66,;-9.24,-22.12,;-10.56,-24.43,;-10.56,-25.98,;-9.23,-26.75,;-7.89,-25.98,)| Show InChI InChI=1S/C28H28N6/c1-33(2)17-18-14-22(15-18)28-32-25(26-27(29)30-12-13-34(26)28)21-9-8-20-10-11-23(31-24(20)16-21)19-6-4-3-5-7-19/h3-13,16,18,22H,14-15,17H2,1-2H3,(H2,29,30)/t18-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156296

(CHEMBL3781466)Show SMILES CC(C)(O)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7O/c1-21(2,29)13-25-19-24-12-16(18(28-19)11-14-3-4-14)17-7-10-23-20(27-17)26-15-5-8-22-9-6-15/h5-10,12,14,29H,3-4,11,13H2,1-2H3,(H,24,25,28)(H,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156298

(CHEMBL3781926)Show SMILES CC(C)(C)Nc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7/c1-21(2,3)28-20-24-13-16(18(27-20)12-14-4-5-14)17-8-11-23-19(26-17)25-15-6-9-22-10-7-15/h6-11,13-14H,4-5,12H2,1-3H3,(H,24,27,28)(H,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336316

(CHEMBL410659 | cis-3-(3-(azetidin-1-ylmethyl)cyclo...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |wU:26.30,28.33,(7.04,-25.52,;7.05,-27.06,;5.72,-27.83,;5.72,-29.37,;7.05,-30.15,;8.39,-29.37,;9.86,-29.85,;10.77,-28.6,;9.86,-27.34,;10.64,-26.02,;9.88,-24.69,;10.64,-23.36,;12.19,-23.36,;12.96,-22.02,;14.5,-22.04,;15.28,-23.37,;14.5,-24.71,;12.96,-24.7,;12.18,-26.03,;16.82,-23.38,;17.59,-24.72,;19.13,-24.72,;19.9,-23.38,;19.12,-22.05,;17.59,-22.05,;8.39,-27.82,;10.34,-31.32,;9.65,-32.69,;11.02,-33.38,;11.5,-34.85,;10.47,-35.99,;8.93,-36.07,;9.01,-37.61,;10.55,-37.53,;11.72,-32.01,)| Show InChI InChI=1S/C29H28N6/c30-28-27-26(22-8-7-21-9-10-24(32-25(21)17-22)20-5-2-1-3-6-20)33-29(35(27)14-11-31-28)23-15-19(16-23)18-34-12-4-13-34/h1-3,5-11,14,17,19,23H,4,12-13,15-16,18H2,(H2,30,31)/t19-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156302

(CHEMBL3780029)Show InChI InChI=1S/C18H19N7/c1-19-17-22-11-14(16(25-17)10-12-2-3-12)15-6-9-21-18(24-15)23-13-4-7-20-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,22,25)(H,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375335
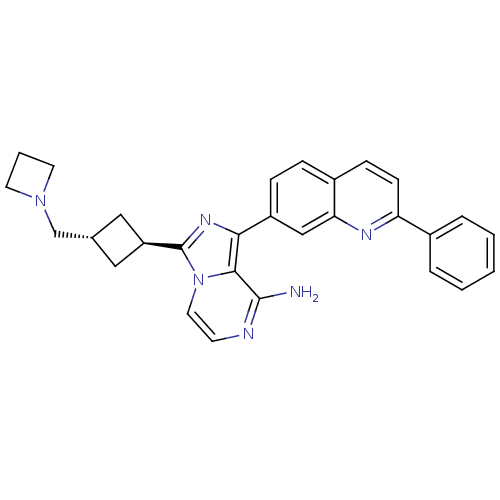
(CHEMBL260298)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@H]1C[C@H](CN2CCC2)C1 |wU:26.30,wD:28.33,(20.91,-25.07,;20.92,-26.61,;19.6,-27.38,;19.59,-28.92,;20.93,-29.69,;22.26,-28.92,;23.74,-29.4,;24.65,-28.14,;23.74,-26.89,;24.51,-25.56,;23.75,-24.23,;24.52,-22.9,;26.07,-22.91,;26.83,-21.57,;28.38,-21.59,;29.16,-22.92,;28.38,-24.26,;26.83,-24.25,;26.06,-25.57,;30.7,-22.93,;31.47,-24.26,;33,-24.27,;33.78,-22.93,;33,-21.59,;31.46,-21.6,;22.26,-27.37,;24.22,-30.86,;25.59,-31.56,;24.9,-32.93,;25.37,-34.4,;24.34,-35.54,;22.81,-35.62,;22.89,-37.16,;24.43,-37.07,;23.52,-32.23,)| Show InChI InChI=1S/C29H28N6/c30-28-27-26(22-8-7-21-9-10-24(32-25(21)17-22)20-5-2-1-3-6-20)33-29(35(27)14-11-31-28)23-15-19(16-23)18-34-12-4-13-34/h1-3,5-11,14,17,19,23H,4,12-13,15-16,18H2,(H2,30,31)/t19-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375330
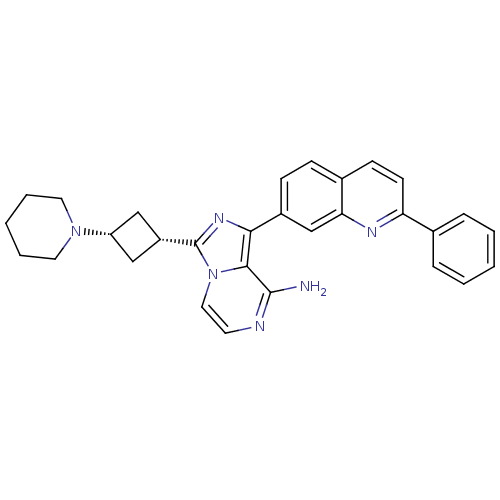
(CHEMBL408712)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCCCC1 |wU:26.30,28.35,(22.65,3.13,;22.66,1.59,;21.34,.82,;21.33,-.72,;22.66,-1.49,;24,-.72,;25.48,-1.2,;26.39,.05,;25.48,1.31,;26.25,2.63,;25.49,3.96,;26.26,5.29,;27.81,5.29,;28.57,6.63,;30.12,6.61,;30.9,5.28,;30.12,3.94,;28.57,3.95,;27.8,2.62,;32.44,5.27,;33.21,3.93,;34.75,3.93,;35.52,5.27,;34.74,6.61,;33.2,6.6,;24,.83,;25.96,-2.67,;25.26,-4.04,;26.64,-4.73,;27.33,-3.36,;27.11,-6.19,;26.22,-7.44,;26.85,-8.83,;28.38,-8.99,;29.27,-7.74,;28.64,-6.33,)| Show InChI InChI=1S/C30H30N6/c31-29-28-27(22-10-9-21-11-12-25(33-26(21)19-22)20-7-3-1-4-8-20)34-30(36(28)16-13-32-29)23-17-24(18-23)35-14-5-2-6-15-35/h1,3-4,7-13,16,19,23-24H,2,5-6,14-15,17-18H2,(H2,31,32)/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50336316

(CHEMBL410659 | cis-3-(3-(azetidin-1-ylmethyl)cyclo...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |wU:26.30,28.33,(7.04,-25.52,;7.05,-27.06,;5.72,-27.83,;5.72,-29.37,;7.05,-30.15,;8.39,-29.37,;9.86,-29.85,;10.77,-28.6,;9.86,-27.34,;10.64,-26.02,;9.88,-24.69,;10.64,-23.36,;12.19,-23.36,;12.96,-22.02,;14.5,-22.04,;15.28,-23.37,;14.5,-24.71,;12.96,-24.7,;12.18,-26.03,;16.82,-23.38,;17.59,-24.72,;19.13,-24.72,;19.9,-23.38,;19.12,-22.05,;17.59,-22.05,;8.39,-27.82,;10.34,-31.32,;9.65,-32.69,;11.02,-33.38,;11.5,-34.85,;10.47,-35.99,;8.93,-36.07,;9.01,-37.61,;10.55,-37.53,;11.72,-32.01,)| Show InChI InChI=1S/C29H28N6/c30-28-27-26(22-8-7-21-9-10-24(32-25(21)17-22)20-5-2-1-3-6-20)33-29(35(27)14-11-31-28)23-15-19(16-23)18-34-12-4-13-34/h1-3,5-11,14,17,19,23H,4,12-13,15-16,18H2,(H2,30,31)/t19-,23+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human purified insulin receptor expressed in HepG2 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156295
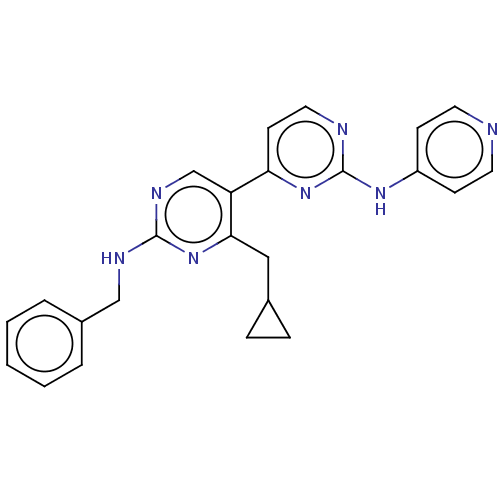
(CHEMBL3781615)Show SMILES C(Nc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1)c1ccccc1 Show InChI InChI=1S/C24H23N7/c1-2-4-18(5-3-1)15-27-23-28-16-20(22(31-23)14-17-6-7-17)21-10-13-26-24(30-21)29-19-8-11-25-12-9-19/h1-5,8-13,16-17H,6-7,14-15H2,(H,27,28,31)(H,25,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375341
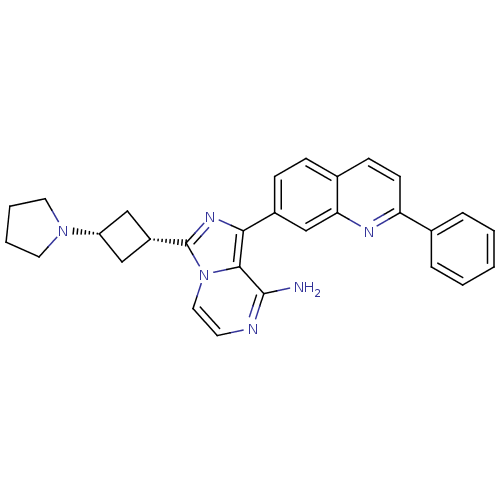
(CHEMBL261146)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCCC1 |wU:26.30,28.35,(6.76,3.51,;6.77,1.97,;5.45,1.2,;5.44,-.35,;6.78,-1.12,;8.11,-.35,;9.59,-.83,;10.5,.43,;9.59,1.69,;10.36,3.01,;9.6,4.34,;10.37,5.67,;11.92,5.67,;12.68,7,;14.23,6.99,;15.01,5.65,;14.23,4.32,;12.68,4.32,;11.91,3,;16.55,5.65,;17.32,4.31,;18.86,4.31,;19.63,5.64,;18.85,6.98,;17.31,6.98,;8.11,1.21,;10.07,-2.29,;9.37,-3.66,;10.75,-4.36,;11.44,-2.98,;11.22,-5.82,;10.33,-7.06,;11.23,-8.31,;12.7,-7.83,;12.7,-6.29,)| Show InChI InChI=1S/C29H28N6/c30-28-27-26(21-9-8-20-10-11-24(32-25(20)18-21)19-6-2-1-3-7-19)33-29(35(27)15-12-31-28)22-16-23(17-22)34-13-4-5-14-34/h1-3,6-12,15,18,22-23H,4-5,13-14,16-17H2,(H2,30,31)/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339059
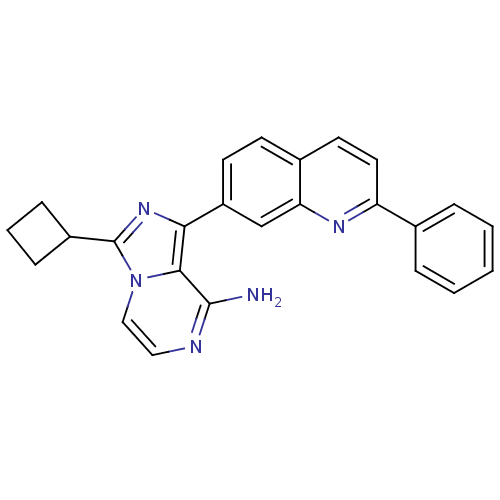
(3-cyclobutyl-1-(2-phenylquinolin-7-yl)-imidazo[1,5...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C25H21N5/c26-24-23-22(29-25(18-7-4-8-18)30(23)14-13-27-24)19-10-9-17-11-12-20(28-21(17)15-19)16-5-2-1-3-6-16/h1-3,5-6,9-15,18H,4,7-8H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1R by ELISA |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156297
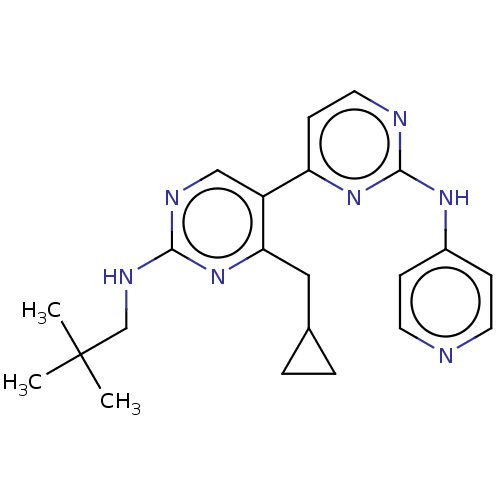
(CHEMBL3780339)Show SMILES CC(C)(C)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C22H27N7/c1-22(2,3)14-26-20-25-13-17(19(29-20)12-15-4-5-15)18-8-11-24-21(28-18)27-16-6-9-23-10-7-16/h6-11,13,15H,4-5,12,14H2,1-3H3,(H,25,26,29)(H,23,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339059

(3-cyclobutyl-1-(2-phenylquinolin-7-yl)-imidazo[1,5...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C25H21N5/c26-24-23-22(29-25(18-7-4-8-18)30(23)14-13-27-24)19-10-9-17-11-12-20(28-21(17)15-19)16-5-2-1-3-6-16/h1-3,5-6,9-15,18H,4,7-8H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156300

(CHEMBL3781634)Show InChI InChI=1S/C18H18N6O/c1-25-18-21-11-14(16(24-18)10-12-2-3-12)15-6-9-20-17(23-15)22-13-4-7-19-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156293
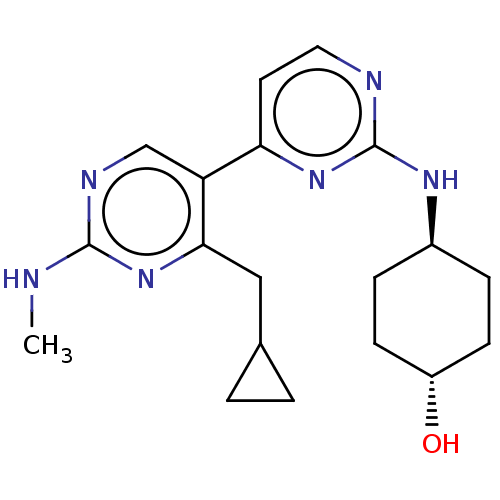
(CHEMBL3781916)Show SMILES CNc1ncc(c(CC2CC2)n1)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:21.23,wD:18.19,(-9.3,9.74,;-9.31,8.51,;-7.98,7.73,;-6.64,8.49,;-5.31,7.71,;-5.32,6.17,;-6.66,5.41,;-6.67,3.87,;-8.01,3.11,;-8.71,1.83,;-9.47,3.17,;-7.99,6.19,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.66,3.08,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;2.4,-1.39,;1.33,.77,;,1.54,;-4,3.86,)| Show InChI InChI=1S/C19H26N6O/c1-20-18-22-11-15(17(25-18)10-12-2-3-12)16-8-9-21-19(24-16)23-13-4-6-14(26)7-5-13/h8-9,11-14,26H,2-7,10H2,1H3,(H,20,22,25)(H,21,23,24)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
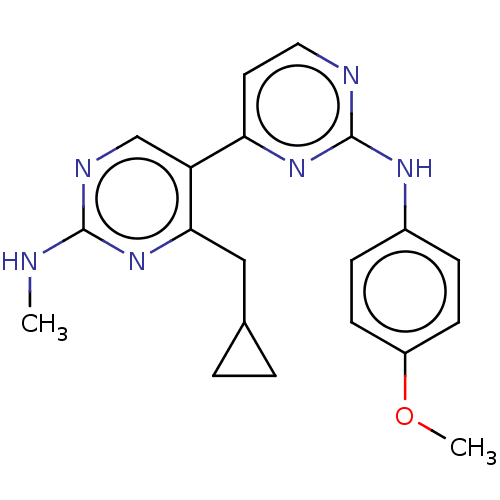
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156337
(CHEMBL3781236)Show SMILES CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccc(OC)cc2)n1 Show InChI InChI=1S/C20H22N6O/c1-21-19-23-12-16(18(26-19)11-13-3-4-13)17-9-10-22-20(25-17)24-14-5-7-15(27-2)8-6-14/h5-10,12-13H,3-4,11H2,1-2H3,(H,21,23,26)(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156338

(CHEMBL3782016)Show SMILES CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2cnc3ccccc3c2)n1 Show InChI InChI=1S/C22H21N7/c1-23-21-26-13-17(20(29-21)10-14-6-7-14)19-8-9-24-22(28-19)27-16-11-15-4-2-3-5-18(15)25-12-16/h2-5,8-9,11-14H,6-7,10H2,1H3,(H,23,26,29)(H,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375340
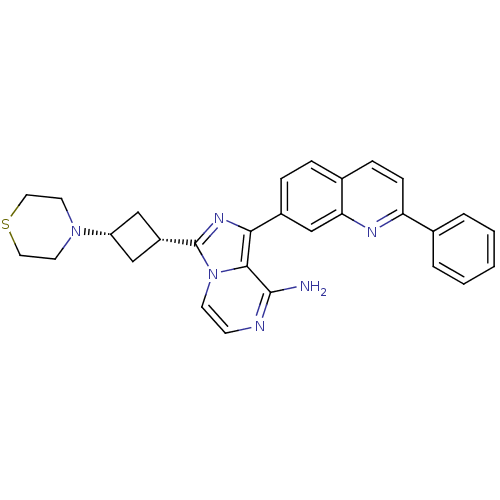
(CHEMBL258951)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCSCC1 |wU:26.30,28.35,(-8.5,-13.48,;-8.49,-15.02,;-9.82,-15.79,;-9.82,-17.34,;-8.49,-18.11,;-7.15,-17.34,;-5.67,-17.82,;-4.76,-16.56,;-5.68,-15.31,;-4.9,-13.98,;-5.66,-12.65,;-4.89,-11.32,;-3.34,-11.32,;-2.58,-9.99,;-1.03,-10,;-.25,-11.34,;-1.03,-12.67,;-2.58,-12.67,;-3.36,-13.99,;1.28,-11.35,;2.05,-12.68,;3.59,-12.68,;4.36,-11.35,;3.59,-10.01,;2.05,-10.02,;-7.15,-15.79,;-5.2,-19.28,;-5.89,-20.65,;-4.52,-21.35,;-3.82,-19.98,;-4.04,-22.81,;-4.94,-24.06,;-4.31,-25.45,;-2.78,-25.6,;-1.88,-24.35,;-2.52,-22.95,)| Show InChI InChI=1S/C29H28N6S/c30-28-27-26(21-7-6-20-8-9-24(32-25(20)18-21)19-4-2-1-3-5-19)33-29(35(27)11-10-31-28)22-16-23(17-22)34-12-14-36-15-13-34/h1-11,18,22-23H,12-17H2,(H2,30,31)/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375332
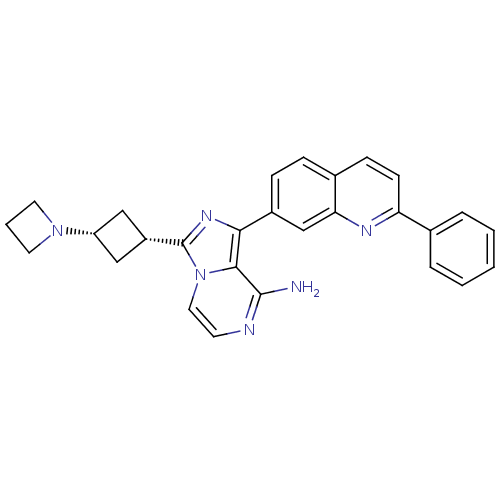
(CHEMBL411082)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCC1 |wU:26.30,28.35,(-8.61,2.61,;-8.6,1.07,;-9.93,.3,;-9.93,-1.25,;-8.6,-2.02,;-7.26,-1.25,;-5.78,-1.72,;-4.87,-.47,;-5.79,.79,;-5.01,2.11,;-5.77,3.44,;-5,4.77,;-3.46,4.77,;-2.69,6.1,;-1.14,6.09,;-.37,4.76,;-1.15,3.42,;-2.69,3.43,;-3.47,2.1,;1.17,4.75,;1.94,3.41,;3.48,3.41,;4.25,4.75,;3.47,6.08,;1.94,6.08,;-7.26,.31,;-5.31,-3.19,;-6,-4.56,;-4.63,-5.26,;-3.93,-3.88,;-4.15,-6.72,;-4.85,-8.09,;-3.47,-8.79,;-2.78,-7.41,)| Show InChI InChI=1S/C28H26N6/c29-27-26-25(32-28(34(26)14-11-30-27)21-15-22(16-21)33-12-4-13-33)20-8-7-19-9-10-23(31-24(19)17-20)18-5-2-1-3-6-18/h1-3,5-11,14,17,21-22H,4,12-13,15-16H2,(H2,29,30)/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375333
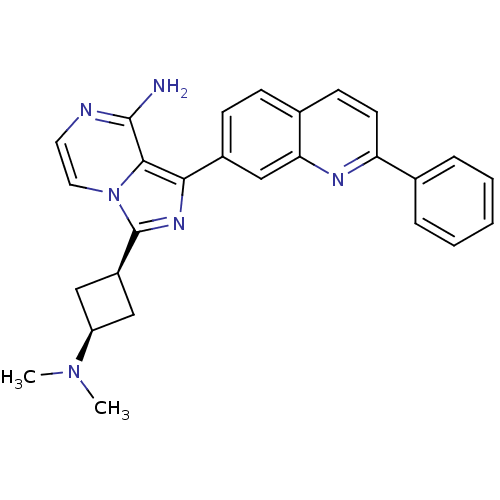
(CHEMBL430051)Show SMILES CN(C)[C@H]1C[C@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |wU:5.7,3.2,(24.37,-53.29,;25.4,-52.15,;26.9,-52.47,;24.92,-50.68,;23.55,-49.98,;24.24,-48.62,;25.62,-49.31,;23.77,-47.15,;24.68,-45.9,;23.76,-44.64,;24.54,-43.32,;23.78,-41.99,;24.55,-40.66,;26.09,-40.66,;26.86,-39.32,;28.4,-39.34,;29.18,-40.67,;28.4,-42.01,;26.86,-42,;26.08,-43.33,;30.72,-40.68,;31.49,-42.02,;33.03,-42.02,;33.8,-40.68,;33.02,-39.34,;31.49,-39.35,;22.29,-45.12,;20.95,-44.36,;20.94,-42.82,;19.62,-45.13,;19.62,-46.67,;20.95,-47.44,;22.29,-46.67,)| Show InChI InChI=1S/C27H26N6/c1-32(2)21-14-20(15-21)27-31-24(25-26(28)29-12-13-33(25)27)19-9-8-18-10-11-22(30-23(18)16-19)17-6-4-3-5-7-17/h3-13,16,20-21H,14-15H2,1-2H3,(H2,28,29)/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50340954
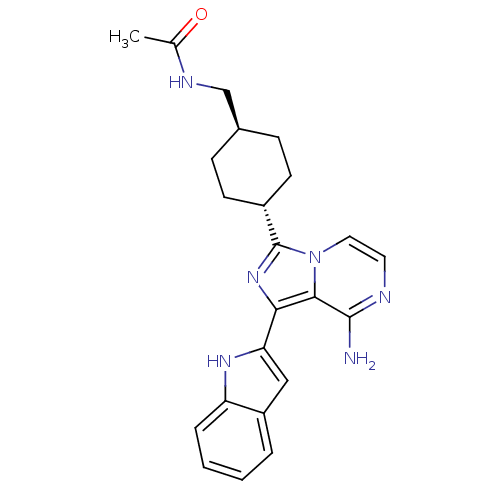
(CHEMBL1762110 | Trans-N-((-4-(8-amino-1-(1H-indol-...)Show SMILES CC(=O)NC[C@H]1CC[C@@H](CC1)c1nc(-c2cc3ccccc3[nH]2)c2c(N)nccn12 |r,wU:8.11,wD:5.4,(30.4,-52.78,;31.44,-51.63,;32.94,-51.96,;30.97,-50.17,;32,-49.03,;31.53,-47.56,;32.56,-46.41,;32.09,-44.94,;30.58,-44.63,;29.54,-45.77,;30.01,-47.24,;30.1,-43.16,;31.01,-41.9,;30.1,-40.65,;30.58,-39.19,;29.67,-37.94,;30.58,-36.7,;30.27,-35.2,;31.41,-34.17,;32.87,-34.65,;33.19,-36.15,;32.04,-37.18,;32.04,-38.72,;28.62,-41.13,;27.29,-40.37,;27.29,-38.83,;25.96,-41.14,;25.96,-42.68,;27.29,-43.45,;28.63,-42.68,)| Show InChI InChI=1S/C23H26N6O/c1-14(30)26-13-15-6-8-16(9-7-15)23-28-20(21-22(24)25-10-11-29(21)23)19-12-17-4-2-3-5-18(17)27-19/h2-5,10-12,15-16,27H,6-9,13H2,1H3,(H2,24,25)(H,26,30)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of cRaf |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156301
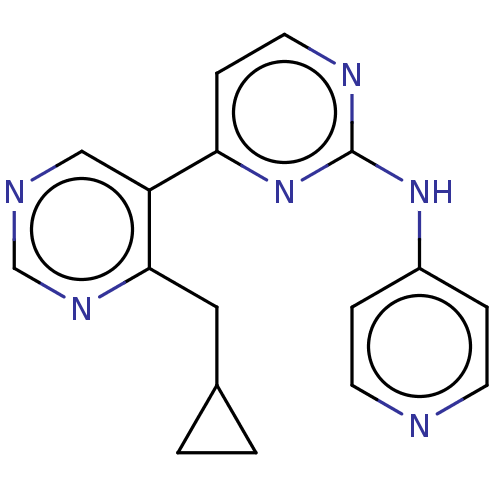
(CHEMBL3781020)Show InChI InChI=1S/C17H16N6/c1-2-12(1)9-16-14(10-19-11-21-16)15-5-8-20-17(23-15)22-13-3-6-18-7-4-13/h3-8,10-12H,1-2,9H2,(H,18,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156299
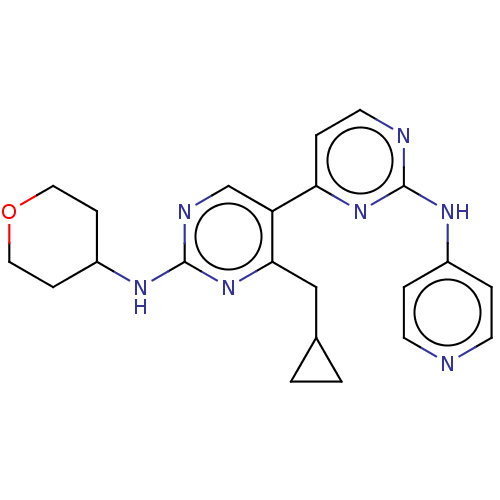
(CHEMBL3780762)Show SMILES C(C1CC1)c1nc(NC2CCOCC2)ncc1-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C22H25N7O/c1-2-15(1)13-20-18(14-25-22(29-20)27-17-6-11-30-12-7-17)19-5-10-24-21(28-19)26-16-3-8-23-9-4-16/h3-5,8-10,14-15,17H,1-2,6-7,11-13H2,(H,25,27,29)(H,23,24,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156299

(CHEMBL3780762)Show SMILES C(C1CC1)c1nc(NC2CCOCC2)ncc1-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C22H25N7O/c1-2-15(1)13-20-18(14-25-22(29-20)27-17-6-11-30-12-7-17)19-5-10-24-21(28-19)26-16-3-8-23-9-4-16/h3-5,8-10,14-15,17H,1-2,6-7,11-13H2,(H,25,27,29)(H,23,24,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156336
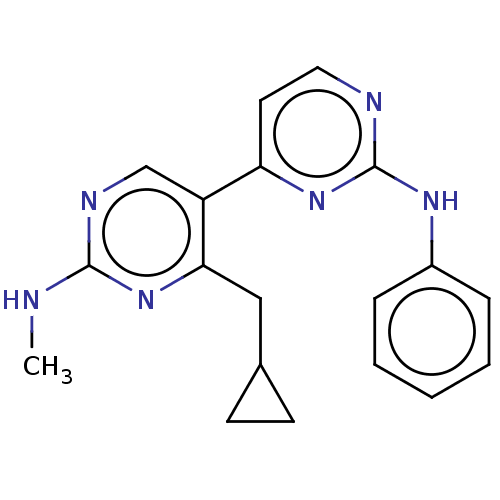
(CHEMBL3780896)Show InChI InChI=1S/C19H20N6/c1-20-18-22-12-15(17(25-18)11-13-7-8-13)16-9-10-21-19(24-16)23-14-5-3-2-4-6-14/h2-6,9-10,12-13H,7-8,11H2,1H3,(H,20,22,25)(H,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156301

(CHEMBL3781020)Show InChI InChI=1S/C17H16N6/c1-2-12(1)9-16-14(10-19-11-21-16)15-5-8-20-17(23-15)22-13-3-6-18-7-4-13/h3-8,10-12H,1-2,9H2,(H,18,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156333

(CHEMBL3781964)Show InChI InChI=1S/C18H24N6O/c1-19-17-21-11-14(16(24-17)10-12-2-3-12)15-4-7-20-18(23-15)22-13-5-8-25-9-6-13/h4,7,11-13H,2-3,5-6,8-10H2,1H3,(H,19,21,24)(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156332

(CHEMBL3780864)Show SMILES CNc1ncc(c(CC2CC2)n1)-c1ccnc(N[C@H]2CC[C@H](N)CC2)n1 |r,wU:21.23,wD:18.19,(-1.08,8.31,;-.01,7.7,;-0,6.16,;-1.34,5.39,;-1.33,3.85,;,3.08,;1.33,3.85,;2.67,3.09,;4,3.86,;5.46,3.82,;4.68,5.15,;1.33,5.39,;,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;2.67,-1.54,;2.66,-3.08,;1.33,-3.85,;1.32,-5.39,;2.65,-6.16,;2.65,-7.4,;3.99,-5.4,;4,-3.86,;1.33,.77,)| Show InChI InChI=1S/C19H27N7/c1-21-18-23-11-15(17(26-18)10-12-2-3-12)16-8-9-22-19(25-16)24-14-6-4-13(20)5-7-14/h8-9,11-14H,2-7,10,20H2,1H3,(H,21,23,26)(H,22,24,25)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50156297

(CHEMBL3780339)Show SMILES CC(C)(C)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C22H27N7/c1-22(2,3)14-26-20-25-13-17(19(29-20)12-15-4-5-15)18-8-11-24-21(28-18)27-16-6-9-23-10-7-16/h6-11,13,15H,4-5,12,14H2,1-3H3,(H,25,26,29)(H,23,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199683

(3-(8-amino-3-cyclobutylimidazo[1,5-a]pyrazin-1-yl)...)Show InChI InChI=1S/C16H16N4O/c17-15-14-13(11-5-2-6-12(21)9-11)19-16(10-3-1-4-10)20(14)8-7-18-15/h2,5-10,21H,1,3-4H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1R by ELISA |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50156298

(CHEMBL3781926)Show SMILES CC(C)(C)Nc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7/c1-21(2,3)28-20-24-13-16(18(27-20)12-14-4-5-14)17-8-11-23-19(26-17)25-15-6-9-22-10-7-15/h6-11,13-14H,4-5,12H2,1-3H3,(H,24,27,28)(H,22,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50340952
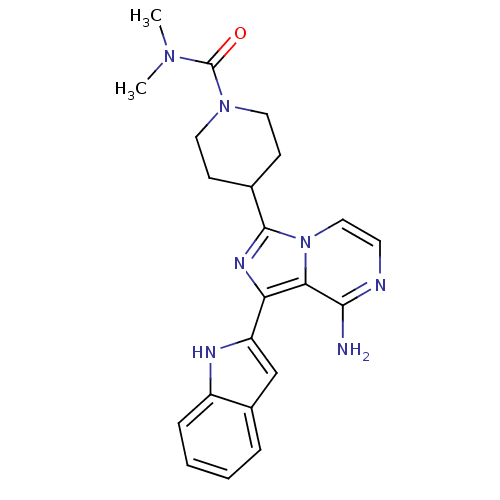
(4-(8-amino-1-(1H-indol-2-yl)imidazo[1,5-a]pyrazin-...)Show SMILES CN(C)C(=O)N1CCC(CC1)c1nc(-c2cc3ccccc3[nH]2)c2c(N)nccn12 Show InChI InChI=1S/C22H25N7O/c1-27(2)22(30)28-10-7-14(8-11-28)21-26-18(19-20(23)24-9-12-29(19)21)17-13-15-5-3-4-6-16(15)25-17/h3-6,9,12-14,25H,7-8,10-11H2,1-2H3,(H2,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Src |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50156296

(CHEMBL3781466)Show SMILES CC(C)(O)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7O/c1-21(2,29)13-25-19-24-12-16(18(28-19)11-14-3-4-14)17-7-10-23-20(27-17)26-15-5-8-22-9-6-15/h5-10,12,14,29H,3-4,11,13H2,1-2H3,(H,24,25,28)(H,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50340954

(CHEMBL1762110 | Trans-N-((-4-(8-amino-1-(1H-indol-...)Show SMILES CC(=O)NC[C@H]1CC[C@@H](CC1)c1nc(-c2cc3ccccc3[nH]2)c2c(N)nccn12 |r,wU:8.11,wD:5.4,(30.4,-52.78,;31.44,-51.63,;32.94,-51.96,;30.97,-50.17,;32,-49.03,;31.53,-47.56,;32.56,-46.41,;32.09,-44.94,;30.58,-44.63,;29.54,-45.77,;30.01,-47.24,;30.1,-43.16,;31.01,-41.9,;30.1,-40.65,;30.58,-39.19,;29.67,-37.94,;30.58,-36.7,;30.27,-35.2,;31.41,-34.17,;32.87,-34.65,;33.19,-36.15,;32.04,-37.18,;32.04,-38.72,;28.62,-41.13,;27.29,-40.37,;27.29,-38.83,;25.96,-41.14,;25.96,-42.68,;27.29,-43.45,;28.63,-42.68,)| Show InChI InChI=1S/C23H26N6O/c1-14(30)26-13-15-6-8-16(9-7-15)23-28-20(21-22(24)25-10-11-29(21)23)19-12-17-4-2-3-5-18(17)27-19/h2-5,10-12,15-16,27H,6-9,13H2,1H3,(H2,24,25)(H,26,30)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50375338
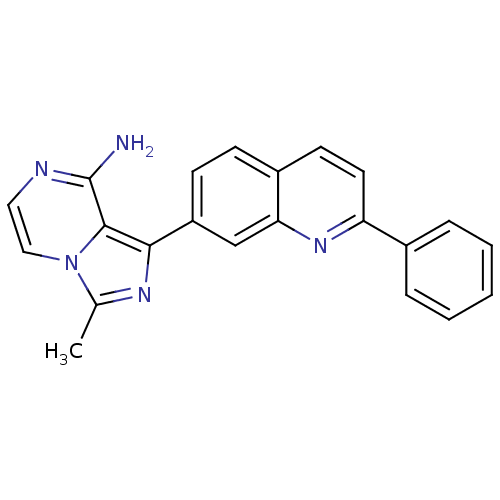
(CHEMBL260888)Show SMILES Cc1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 Show InChI InChI=1S/C22H17N5/c1-14-25-20(21-22(23)24-11-12-27(14)21)17-8-7-16-9-10-18(26-19(16)13-17)15-5-3-2-4-6-15/h2-13H,1H3,(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM1779
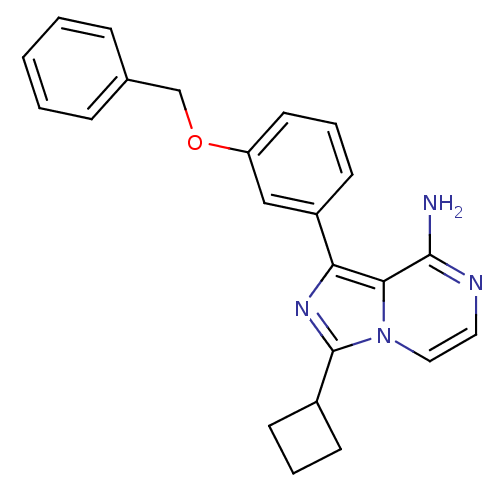
(CHEMBL392106 | US8481733, Comparator 2)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)C1CCC1 Show InChI InChI=1S/C23H22N4O/c24-22-21-20(26-23(17-8-4-9-17)27(21)13-12-25-22)18-10-5-11-19(14-18)28-15-16-6-2-1-3-7-16/h1-3,5-7,10-14,17H,4,8-9,15H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length IGF1R expressed in mouse 3T3 cells |
Bioorg Med Chem 16: 1359-75 (2008)
Article DOI: 10.1016/j.bmc.2007.10.061
BindingDB Entry DOI: 10.7270/Q2WH2QVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50156294

(CHEMBL3781415)Show InChI InChI=1S/C17H17N7/c18-16-21-10-13(15(23-16)9-11-1-2-11)14-5-8-20-17(24-14)22-12-3-6-19-7-4-12/h3-8,10-11H,1-2,9H2,(H2,18,21,23)(H,19,20,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50340952

(4-(8-amino-1-(1H-indol-2-yl)imidazo[1,5-a]pyrazin-...)Show SMILES CN(C)C(=O)N1CCC(CC1)c1nc(-c2cc3ccccc3[nH]2)c2c(N)nccn12 Show InChI InChI=1S/C22H25N7O/c1-27(2)22(30)28-10-7-14(8-11-28)21-26-18(19-20(23)24-9-12-29(19)21)17-13-15-5-3-4-6-16(15)25-17/h3-6,9,12-14,25H,7-8,10-11H2,1-2H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of cRaf |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50156302

(CHEMBL3780029)Show InChI InChI=1S/C18H19N7/c1-19-17-22-11-14(16(25-17)10-12-2-3-12)15-6-9-21-18(24-15)23-13-4-7-20-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,22,25)(H,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50340954

(CHEMBL1762110 | Trans-N-((-4-(8-amino-1-(1H-indol-...)Show SMILES CC(=O)NC[C@H]1CC[C@@H](CC1)c1nc(-c2cc3ccccc3[nH]2)c2c(N)nccn12 |r,wU:8.11,wD:5.4,(30.4,-52.78,;31.44,-51.63,;32.94,-51.96,;30.97,-50.17,;32,-49.03,;31.53,-47.56,;32.56,-46.41,;32.09,-44.94,;30.58,-44.63,;29.54,-45.77,;30.01,-47.24,;30.1,-43.16,;31.01,-41.9,;30.1,-40.65,;30.58,-39.19,;29.67,-37.94,;30.58,-36.7,;30.27,-35.2,;31.41,-34.17,;32.87,-34.65,;33.19,-36.15,;32.04,-37.18,;32.04,-38.72,;28.62,-41.13,;27.29,-40.37,;27.29,-38.83,;25.96,-41.14,;25.96,-42.68,;27.29,-43.45,;28.63,-42.68,)| Show InChI InChI=1S/C23H26N6O/c1-14(30)26-13-15-6-8-16(9-7-15)23-28-20(21-22(24)25-10-11-29(21)23)19-12-17-4-2-3-5-18(17)27-19/h2-5,10-12,15-16,27H,6-9,13H2,1H3,(H2,24,25)(H,26,30)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50340954

(CHEMBL1762110 | Trans-N-((-4-(8-amino-1-(1H-indol-...)Show SMILES CC(=O)NC[C@H]1CC[C@@H](CC1)c1nc(-c2cc3ccccc3[nH]2)c2c(N)nccn12 |r,wU:8.11,wD:5.4,(30.4,-52.78,;31.44,-51.63,;32.94,-51.96,;30.97,-50.17,;32,-49.03,;31.53,-47.56,;32.56,-46.41,;32.09,-44.94,;30.58,-44.63,;29.54,-45.77,;30.01,-47.24,;30.1,-43.16,;31.01,-41.9,;30.1,-40.65,;30.58,-39.19,;29.67,-37.94,;30.58,-36.7,;30.27,-35.2,;31.41,-34.17,;32.87,-34.65,;33.19,-36.15,;32.04,-37.18,;32.04,-38.72,;28.62,-41.13,;27.29,-40.37,;27.29,-38.83,;25.96,-41.14,;25.96,-42.68,;27.29,-43.45,;28.63,-42.68,)| Show InChI InChI=1S/C23H26N6O/c1-14(30)26-13-15-6-8-16(9-7-15)23-28-20(21-22(24)25-10-11-29(21)23)19-12-17-4-2-3-5-18(17)27-19/h2-5,10-12,15-16,27H,6-9,13H2,1H3,(H2,24,25)(H,26,30)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1R by ELISA |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data