 Found 10517 hits with Last Name = 'hua' and Initial = 'j'
Found 10517 hits with Last Name = 'hua' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016326
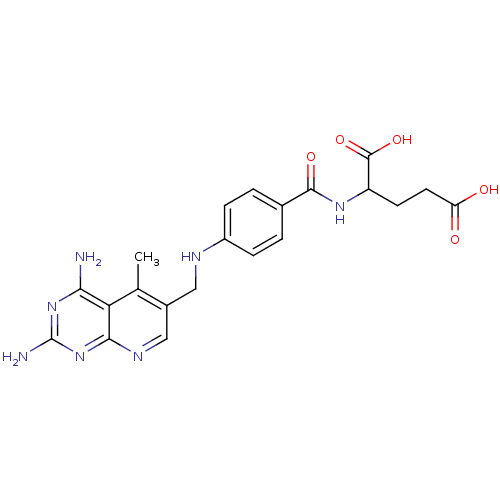
(2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C21H23N7O5/c1-10-12(9-25-18-16(10)17(22)27-21(23)28-18)8-24-13-4-2-11(3-5-13)19(31)26-14(20(32)33)6-7-15(29)30/h2-5,9,14,24H,6-8H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023681

(2-{4-[(2,4-Diamino-5,7-dimethyl-pyrido[2,3-d]pyrim...)Show SMILES Cc1nc2nc(N)nc(N)c2c(C)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-10-14(11(2)26-19-17(10)18(23)28-22(24)29-19)9-25-13-5-3-12(4-6-13)20(32)27-15(21(33)34)7-8-16(30)31/h3-6,15,25H,7-9H2,1-2H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023680

(2-{4-[(2,4-Diamino-7-phenyl-pyrido[2,3-d]pyrimidin...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c(nc2n1)-c1ccccc1 Show InChI InChI=1S/C26H25N7O5/c27-22-18-12-16(21(14-4-2-1-3-5-14)31-23(18)33-26(28)32-22)13-29-17-8-6-15(7-9-17)24(36)30-19(25(37)38)10-11-20(34)35/h1-9,12,19,29H,10-11,13H2,(H,30,36)(H,34,35)(H,37,38)(H4,27,28,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023682

(2-{4-[(2,4-Diamino-5-methyl-7-phenyl-pyrido[2,3-d]...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)c(nc2nc(N)nc(N)c12)-c1ccccc1 Show InChI InChI=1S/C27H27N7O5/c1-14-18(22(15-5-3-2-4-6-15)32-24-21(14)23(28)33-27(29)34-24)13-30-17-9-7-16(8-10-17)25(37)31-19(26(38)39)11-12-20(35)36/h2-10,19,30H,11-13H2,1H3,(H,31,37)(H,35,36)(H,38,39)(H4,28,29,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023683
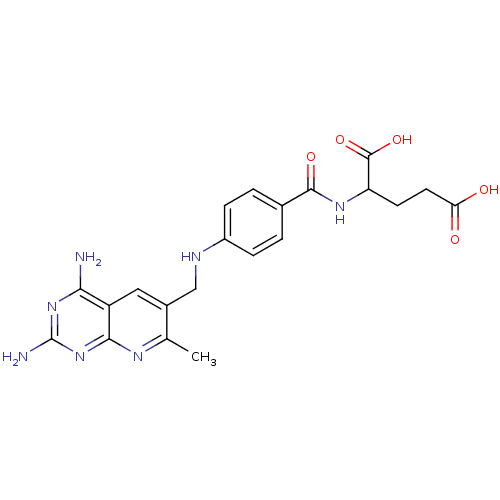
(2-{4-[(2,4-Diamino-7-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1nc2nc(N)nc(N)c2cc1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H23N7O5/c1-10-12(8-14-17(22)27-21(23)28-18(14)25-10)9-24-13-4-2-11(3-5-13)19(31)26-15(20(32)33)6-7-16(29)30/h2-5,8,15,24H,6-7,9H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023684
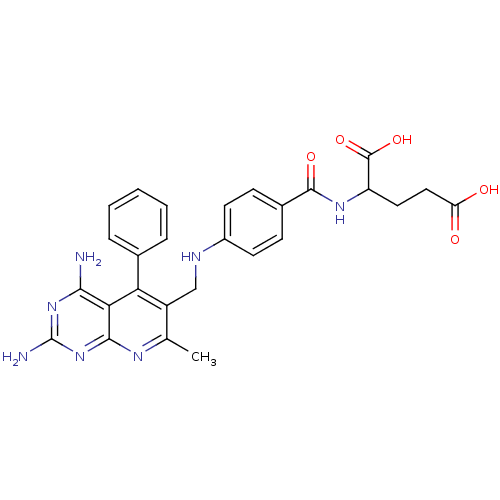
(2-{4-[(2,4-Diamino-7-methyl-5-phenyl-pyrido[2,3-d]...)Show SMILES Cc1nc2nc(N)nc(N)c2c(-c2ccccc2)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C27H27N7O5/c1-14-18(21(15-5-3-2-4-6-15)22-23(28)33-27(29)34-24(22)31-14)13-30-17-9-7-16(8-10-17)25(37)32-19(26(38)39)11-12-20(35)36/h2-10,19,30H,11-13H2,1H3,(H,32,37)(H,35,36)(H,38,39)(H4,28,29,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86492

(CAS_170713-75-4 | NSC_6324645 | Nociceptin)Show SMILES [#6]-[#6](-[#8])-[#6](-[#7]-[#6](=O)-[#6](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50546246
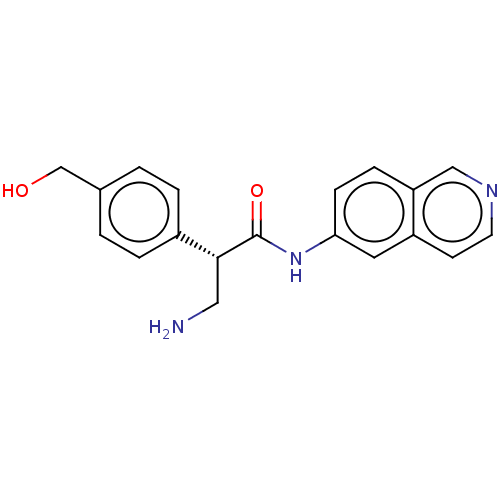
(CHEMBL4753043 | US11608319, Compound AR-13503)Show SMILES NC[C@@H](C(=O)Nc1ccc2cnccc2c1)c1ccc(CO)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50546246

(CHEMBL4753043 | US11608319, Compound AR-13503)Show SMILES NC[C@@H](C(=O)Nc1ccc2cnccc2c1)c1ccc(CO)cc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-3
(Homo sapiens (Human)) | BDBM50214664
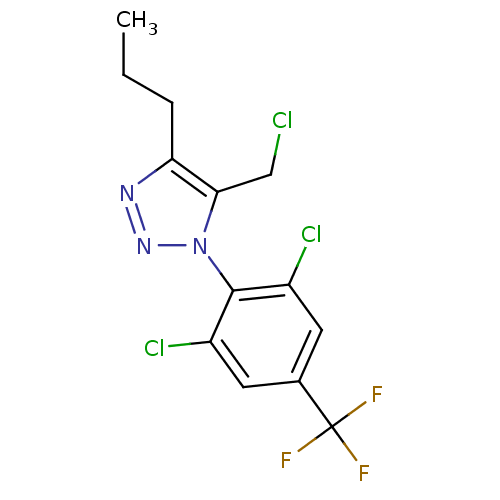
(5-chloromethyl-1-(2,6-dichloro-4-trifluoromethylph...)Show SMILES CCCc1nnn(c1CCl)-c1c(Cl)cc(cc1Cl)C(F)(F)F |(32.44,-26.02,;31.19,-25.11,;31.36,-23.58,;30.11,-22.67,;30.12,-21.13,;28.65,-20.65,;27.75,-21.9,;28.65,-23.14,;28.17,-24.61,;29.2,-25.75,;26.21,-21.89,;25.45,-23.23,;26.22,-24.56,;23.91,-23.23,;23.14,-21.89,;23.91,-20.56,;25.45,-20.56,;26.22,-19.23,;21.6,-21.89,;20.04,-21.96,;21.56,-20.35,;21.65,-23.43,)| Show InChI InChI=1S/C13H11Cl3F3N3/c1-2-3-10-11(6-14)22(21-20-10)12-8(15)4-7(5-9(12)16)13(17,18)19/h4-5H,2-3,6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.659 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shimane University
Curated by ChEMBL
| Assay Description
Displacement of [3H]EBOB from human recombinant GABAbeta3 receptor expressed in HEK293 cells |
Bioorg Med Chem 15: 5090-104 (2007)
Article DOI: 10.1016/j.bmc.2007.05.039
BindingDB Entry DOI: 10.7270/Q2K64HS0 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50156552
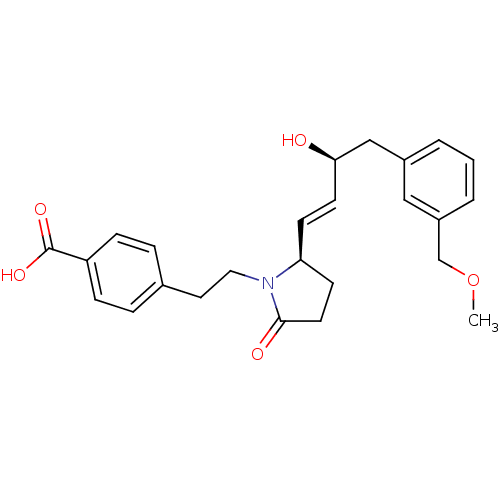
(4-(2-((R)-2-((S,E)-3-hydroxy-4-(3-(methoxymethyl)p...)Show SMILES COCc1cccc(C[C@H](O)\C=C\[C@H]2CCC(=O)N2CCc2ccc(cc2)C(O)=O)c1 |r| Show InChI InChI=1S/C25H29NO5/c1-31-17-20-4-2-3-19(15-20)16-23(27)11-9-22-10-12-24(28)26(22)14-13-18-5-7-21(8-6-18)25(29)30/h2-9,11,15,22-23,27H,10,12-14,16-17H2,1H3,(H,29,30)/b11-9+/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
J Med Chem 47: 6124-7 (2004)
Article DOI: 10.1021/jm049290a
BindingDB Entry DOI: 10.7270/Q2P84BBQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86491

(DiPOA | [8-(3,3-Diphenyl-propyl)-4-oxo-1-phenyl-1,...)Show SMILES OC(=O)CN1CN(c2ccccc2)C2(CCN(CCC(c3ccccc3)c3ccccc3)CC2)C1=O Show InChI InChI=1S/C30H33N3O3/c34-28(35)22-32-23-33(26-14-8-3-9-15-26)30(29(32)36)17-20-31(21-18-30)19-16-27(24-10-4-1-5-11-24)25-12-6-2-7-13-25/h1-15,27H,16-23H2,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50156551

(4-{2-[(2S)-2-[(3R)-3-[3-(4-chloro-2-methylphenyl)p...)Show SMILES Cc1cc(Cl)ccc1-c1cccc(c1)[C@H](O)CC[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C29H30ClNO4/c1-19-17-24(30)9-12-26(19)22-3-2-4-23(18-22)27(32)13-10-25-11-14-28(33)31(25)16-15-20-5-7-21(8-6-20)29(34)35/h2-9,12,17-18,25,27,32H,10-11,13-16H2,1H3,(H,34,35)/t25-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
J Med Chem 47: 6124-7 (2004)
Article DOI: 10.1021/jm049290a
BindingDB Entry DOI: 10.7270/Q2P84BBQ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50546247

(AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...)Show SMILES Cc1ccc(C(=O)OCc2ccc(cc2)[C@@H](CN)C(=O)Nc2ccc3cnccc3c2)c(C)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50156553

(4-(2-((R)-2-((S,E)-5-cyclobutyl-3-hydroxypent-1-en...)Show SMILES O[C@@H](CCC1CCC1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C22H29NO4/c24-20(11-6-16-2-1-3-16)12-9-19-10-13-21(25)23(19)15-14-17-4-7-18(8-5-17)22(26)27/h4-5,7-9,12,16,19-20,24H,1-3,6,10-11,13-15H2,(H,26,27)/b12-9+/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
J Med Chem 47: 6124-7 (2004)
Article DOI: 10.1021/jm049290a
BindingDB Entry DOI: 10.7270/Q2P84BBQ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50546247

(AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...)Show SMILES Cc1ccc(C(=O)OCc2ccc(cc2)[C@@H](CN)C(=O)Nc2ccc3cnccc3c2)c(C)c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50773

(1-[4-(2-methoxyphenyl)-1-piperazinyl]-3-(1-naphtha...)Show InChI InChI=1S/C24H28N2O3/c1-28-24-11-5-4-10-22(24)26-15-13-25(14-16-26)17-20(27)18-29-23-12-6-8-19-7-2-3-9-21(19)23/h2-12,20,27H,13-18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Displacement of [125I-HEAT from human alpha1D-adrenoreceptor expressed in CHOK1 cell membranes incubated for 60 mins |
Bioorg Med Chem Lett 28: 547-551 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.068
BindingDB Entry DOI: 10.7270/Q2057JHB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585443
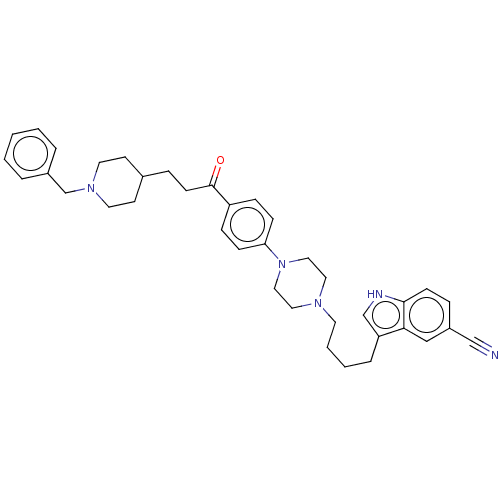
(CHEMBL5082250)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1ccc(cc1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse AChE assessed as inhibition constant using acetylthiocholine iodide as substrate measured every 2 mins by lineweaver-burk plot an... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-3
(Homo sapiens (Human)) | BDBM50214659
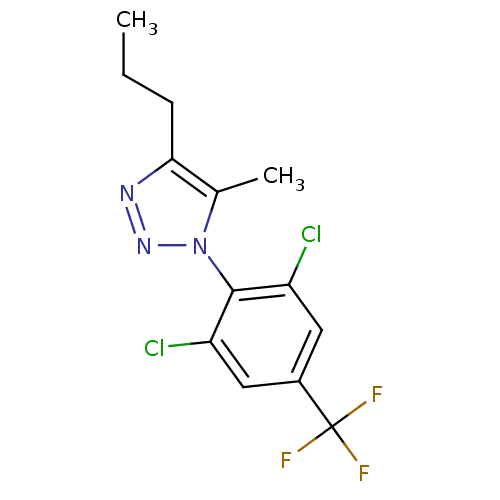
(1-(2,6-dichloro-4-trifluoromethylphenyl)-5-methyl-...)Show SMILES CCCc1nnn(c1C)-c1c(Cl)cc(cc1Cl)C(F)(F)F |(3.52,-8.6,;2.27,-7.69,;2.44,-6.16,;1.2,-5.26,;1.2,-3.72,;-.27,-3.23,;-1.17,-4.48,;-.27,-5.73,;-.75,-7.19,;-2.71,-4.48,;-3.47,-5.81,;-2.69,-7.14,;-5,-5.82,;-5.78,-4.48,;-5.01,-3.14,;-3.47,-3.14,;-2.7,-1.81,;-7.32,-4.48,;-8.87,-4.54,;-7.36,-2.94,;-7.27,-6.02,)| Show InChI InChI=1S/C13H12Cl2F3N3/c1-3-4-11-7(2)21(20-19-11)12-9(14)5-8(6-10(12)15)13(16,17)18/h5-6H,3-4H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shimane University
Curated by ChEMBL
| Assay Description
Displacement of [3H]EBOB from human recombinant GABAbeta3 receptor expressed in HEK293 cells |
Bioorg Med Chem 15: 5090-104 (2007)
Article DOI: 10.1016/j.bmc.2007.05.039
BindingDB Entry DOI: 10.7270/Q2K64HS0 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50181298
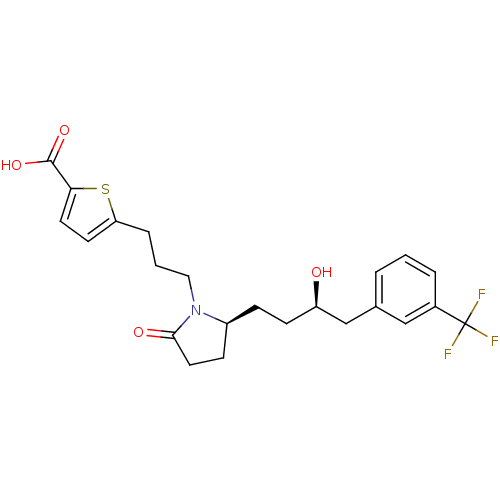
(5-(3-((S)-2-((R)-3-hydroxy-4-(3-(trifluoromethyl)p...)Show SMILES O[C@H](CC[C@H]1CCC(=O)N1CCCc1ccc(s1)C(O)=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H26F3NO4S/c24-23(25,26)16-4-1-3-15(13-16)14-18(28)8-6-17-7-11-21(29)27(17)12-2-5-19-9-10-20(32-19)22(30)31/h1,3-4,9-10,13,17-18,28H,2,5-8,11-12,14H2,(H,30,31)/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50230555

(CHEMBL5267078)Show SMILES [H][C@]12CCCC[C@@]1([H])c1cc(Cl)cc(C(=O)N[C@@]3([H])CN4CCC3CC4)c1O2 |wU:6.7,1.0,wD:17.18,(7.76,-5.43,;7.44,-3.92,;8.95,-4.25,;9.98,-3.08,;9.49,-1.63,;7.97,-1.31,;6.95,-2.47,;6.65,-.96,;5.43,-2.47,;4.41,-1.31,;2.9,-1.64,;1.87,-.49,;2.41,-3.08,;3.43,-4.24,;2.94,-5.69,;3.97,-6.85,;1.43,-5.99,;-.11,-5.92,;-.11,-7.46,;-1.02,-7.16,;-2.55,-7,;-3.16,-5.57,;-2.25,-4.34,;-.71,-4.52,;-.88,-6.04,;-2.4,-5.85,;4.95,-3.94,;6.2,-4.84,)| Show InChI InChI=1S/C20H25ClN2O2/c21-13-9-15-14-3-1-2-4-18(14)25-19(15)16(10-13)20(24)22-17-11-23-7-5-12(17)6-8-23/h9-10,12,14,17-18H,1-8,11H2,(H,22,24)/t14-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor determined as pA2 in guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50546247

(AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...)Show SMILES Cc1ccc(C(=O)OCc2ccc(cc2)[C@@H](CN)C(=O)Nc2ccc3cnccc3c2)c(C)c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50610937
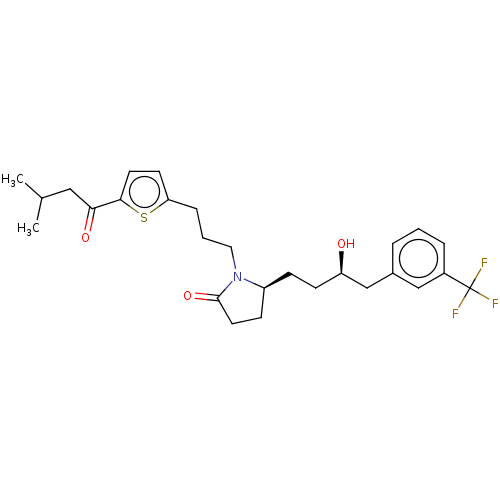
(CHEMBL5272661) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86258
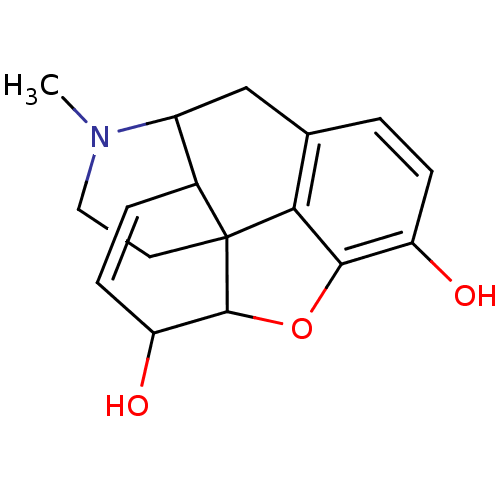
(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86493
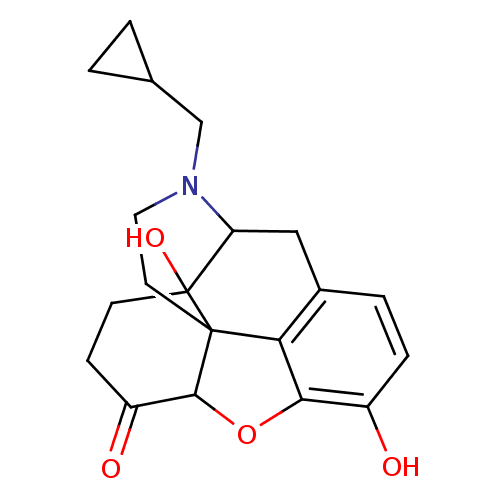
(CAS_27943 | NALTREXONE-HCl | NSC_27943 | Naltrexon...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(=O)CCC35O |TLB:22:23:7.12.13:5.4.18,THB:24:23:7.12.13:5.4.18| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-3
(Homo sapiens (Human)) | BDBM50214673
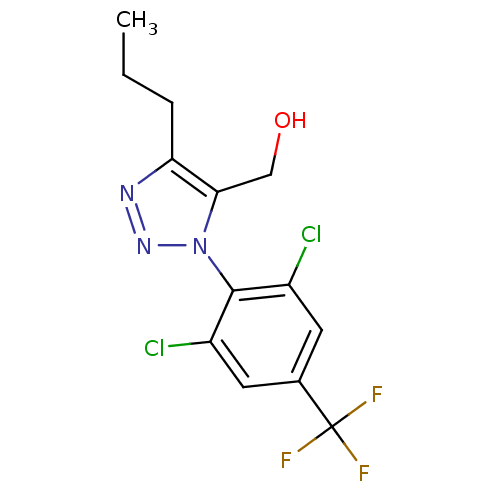
(1-(2,6-dichloro-4-trifluoromethylphenyl)-5-hydroxy...)Show SMILES CCCc1nnn(c1CO)-c1c(Cl)cc(cc1Cl)C(F)(F)F |(3.18,-26.58,;1.93,-25.67,;2.1,-24.14,;.85,-23.23,;.86,-21.69,;-.61,-21.21,;-1.51,-22.46,;-.61,-23.7,;-1.09,-25.17,;-.06,-26.31,;-3.05,-22.45,;-3.81,-23.79,;-3.04,-25.12,;-5.35,-23.79,;-6.12,-22.45,;-5.35,-21.12,;-3.81,-21.12,;-3.04,-19.79,;-7.66,-22.45,;-9.22,-22.52,;-7.7,-20.91,;-7.61,-23.99,)| Show InChI InChI=1S/C13H12Cl2F3N3O/c1-2-3-10-11(6-22)21(20-19-10)12-8(14)4-7(5-9(12)15)13(16,17)18/h4-5,22H,2-3,6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shimane University
Curated by ChEMBL
| Assay Description
Displacement of [3H]EBOB from human recombinant GABAbeta3 receptor expressed in HEK293 cells |
Bioorg Med Chem 15: 5090-104 (2007)
Article DOI: 10.1016/j.bmc.2007.05.039
BindingDB Entry DOI: 10.7270/Q2K64HS0 |
More data for this
Ligand-Target Pair | |
Menin
(Homo sapiens (Human)) | BDBM50489239
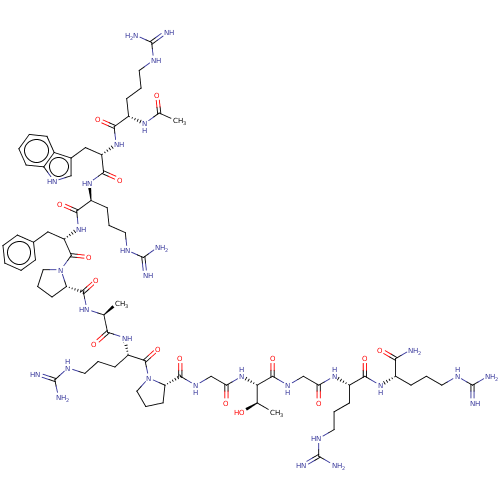
(CHEMBL2314823)Show SMILES C[C@@H](O)[C@H](NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C73H116N30O15/c1-39(59(109)98-50(24-13-31-89-73(83)84)67(117)102-32-14-25-53(102)64(114)91-38-56(107)101-57(40(2)104)66(116)92-37-55(106)95-48(22-11-29-87-71(79)80)61(111)96-46(58(74)108)20-9-27-85-69(75)76)93-65(115)54-26-15-33-103(54)68(118)52(34-42-16-5-4-6-17-42)100-62(112)49(23-12-30-88-72(81)82)97-63(113)51(35-43-36-90-45-19-8-7-18-44(43)45)99-60(110)47(94-41(3)105)21-10-28-86-70(77)78/h4-8,16-19,36,39-40,46-54,57,90,104H,9-15,20-35,37-38H2,1-3H3,(H2,74,108)(H,91,114)(H,92,116)(H,93,115)(H,94,105)(H,95,106)(H,96,111)(H,97,113)(H,98,109)(H,99,110)(H,100,112)(H,101,107)(H4,75,76,85)(H4,77,78,86)(H4,79,80,87)(H4,81,82,88)(H4,83,84,89)/t39-,40+,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to menin (unknown origin) after 60 mins by fluorescence polarization assay |
J Med Chem 56: 1113-23 (2013)
Article DOI: 10.1021/jm3015298
BindingDB Entry DOI: 10.7270/Q22B91XM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50156547
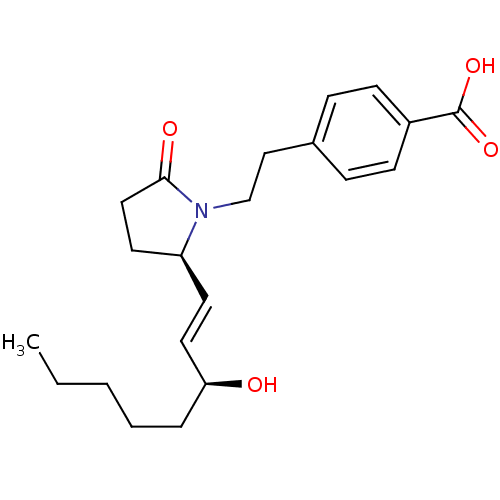
(4-(2-((R)-2-((S)-3-hydroxyoct-1-enyl)-5-oxopyrroli...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C21H29NO4/c1-2-3-4-5-19(23)12-10-18-11-13-20(24)22(18)15-14-16-6-8-17(9-7-16)21(25)26/h6-10,12,18-19,23H,2-5,11,13-15H2,1H3,(H,25,26)/b12-10+/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
J Med Chem 47: 6124-7 (2004)
Article DOI: 10.1021/jm049290a
BindingDB Entry DOI: 10.7270/Q2P84BBQ |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50096326
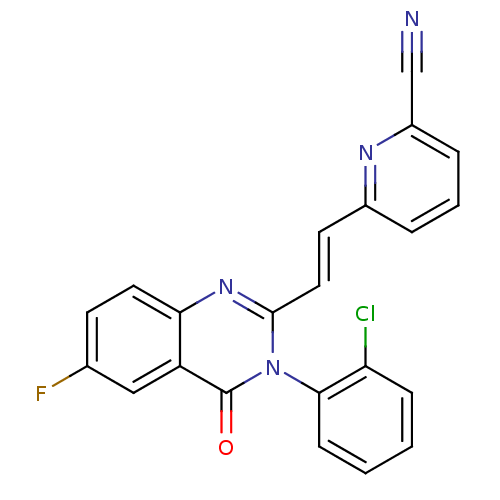
(6-{(E)-2-[3-(2-Chloro-phenyl)-6-fluoro-4-oxo-3,4-d...)Show SMILES Fc1ccc2nc(\C=C\c3cccc(n3)C#N)n(-c3ccccc3Cl)c(=O)c2c1 |(1.2,-4.46,;2.55,-5.21,;2.55,-6.75,;3.88,-7.52,;5.21,-6.75,;6.56,-7.52,;7.91,-6.73,;9.25,-7.5,;9.25,-9.04,;10.6,-9.81,;10.6,-11.35,;11.93,-12.12,;13.26,-11.35,;13.26,-9.78,;11.92,-9.04,;14.58,-8.99,;15.91,-8.22,;7.91,-5.18,;9.25,-4.41,;9.22,-2.88,;10.53,-2.1,;11.88,-2.85,;11.91,-4.39,;10.57,-5.18,;10.57,-6.72,;6.56,-4.41,;6.54,-2.87,;5.21,-5.19,;3.88,-4.42,)| Show InChI InChI=1S/C22H12ClFN4O/c23-18-6-1-2-7-20(18)28-21(11-9-15-4-3-5-16(13-25)26-15)27-19-10-8-14(24)12-17(19)22(28)29/h1-12H/b11-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50506546
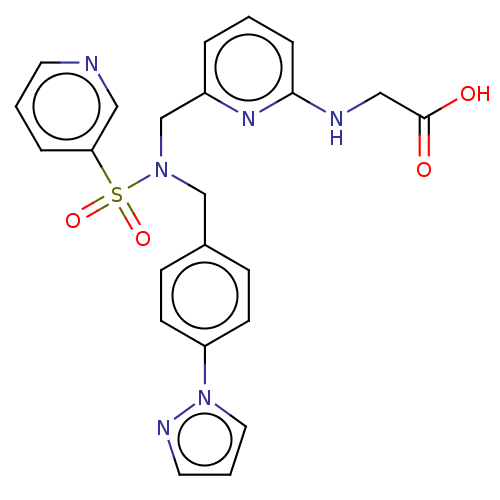
(Omidenepag | UR-7276)Show SMILES OC(=O)CNc1cccc(CN(Cc2ccc(cc2)-n2cccn2)S(=O)(=O)c2cccnc2)n1 Show InChI InChI=1S/C23H22N6O4S/c30-23(31)15-25-22-6-1-4-19(27-22)17-28(34(32,33)21-5-2-11-24-14-21)16-18-7-9-20(10-8-18)29-13-3-12-26-29/h1-14H,15-17H2,(H,25,27)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM21130
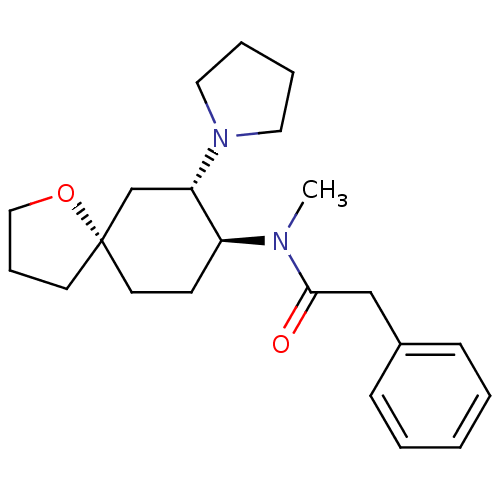
(N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C22H32N2O2/c1-23(21(25)16-18-8-3-2-4-9-18)19-10-12-22(11-7-15-26-22)17-20(19)24-13-5-6-14-24/h2-4,8-9,19-20H,5-7,10-17H2,1H3/t19-,20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50773

(1-[4-(2-methoxyphenyl)-1-piperazinyl]-3-(1-naphtha...)Show InChI InChI=1S/C24H28N2O3/c1-28-24-11-5-4-10-22(24)26-15-13-25(14-16-26)17-20(27)18-29-23-12-6-8-19-7-2-3-9-21(19)23/h2-12,20,27H,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Displacement of [125I-HEAT from human alpha1A-adrenoreceptor expressed in CHOK1 cell membranes incubated for 60 mins |
Bioorg Med Chem Lett 28: 547-551 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.068
BindingDB Entry DOI: 10.7270/Q2057JHB |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50156554
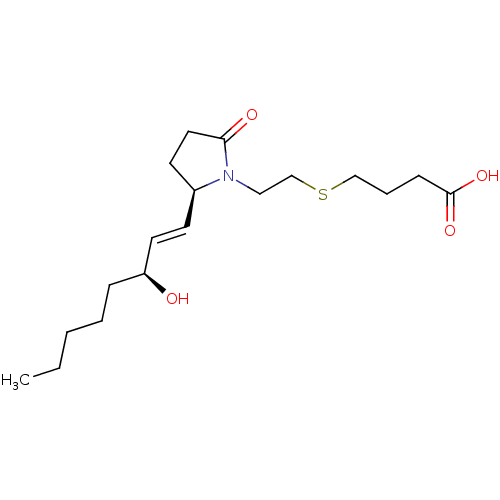
(4-(2-((R)-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxopyrro...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCSCCCC(O)=O |r| Show InChI InChI=1S/C18H31NO4S/c1-2-3-4-6-16(20)10-8-15-9-11-17(21)19(15)12-14-24-13-5-7-18(22)23/h8,10,15-16,20H,2-7,9,11-14H2,1H3,(H,22,23)/b10-8+/t15-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
J Med Chem 47: 6124-7 (2004)
Article DOI: 10.1021/jm049290a
BindingDB Entry DOI: 10.7270/Q2P84BBQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017021
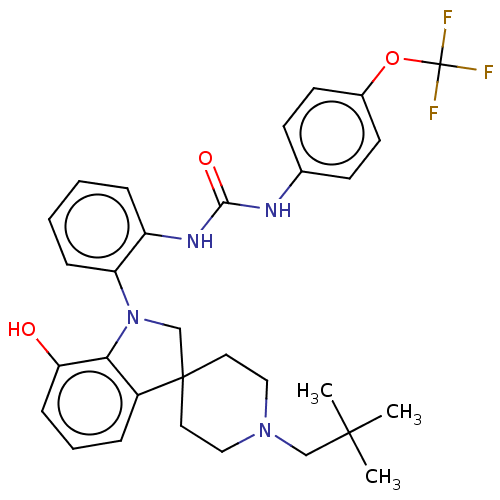
(CHEMBL3287047 | US9428504, 1)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2cccc3O)c2ccccc2NC(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C31H35F3N4O3/c1-29(2,3)19-37-17-15-30(16-18-37)20-38(27-23(30)7-6-10-26(27)39)25-9-5-4-8-24(25)36-28(40)35-21-11-13-22(14-12-21)41-31(32,33)34/h4-14,39H,15-20H2,1-3H3,(H2,35,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to P2Y1 receptor in human platelets |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50130973
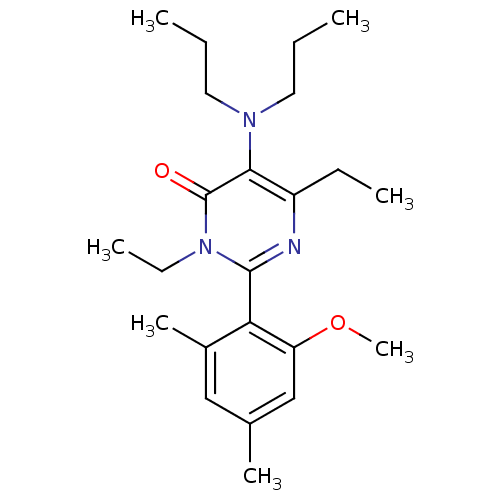
(5-Dipropylamino-3,6-diethyl-2-(2-methoxy-4,6-dimet...)Show SMILES CCCN(CCC)c1c(CC)nc(-c2c(C)cc(C)cc2OC)n(CC)c1=O |(.82,4.8,;.82,3.26,;-.51,2.49,;-.51,.95,;-1.84,.18,;-1.84,-1.36,;-3.17,-2.13,;.82,.18,;.82,-1.36,;-.51,-2.13,;-.51,-3.67,;2.15,-2.13,;3.48,-1.36,;4.83,-2.13,;4.81,-3.67,;3.48,-4.44,;6.16,-4.44,;7.49,-3.67,;8.83,-4.44,;7.47,-2.12,;6.14,-1.36,;6.16,.2,;7.47,.96,;3.48,.18,;4.81,.96,;4.81,2.5,;2.15,.95,;2.15,2.49,)| Show InChI InChI=1S/C23H35N3O2/c1-8-12-25(13-9-2)21-18(10-3)24-22(26(11-4)23(21)27)20-17(6)14-16(5)15-19(20)28-7/h14-15H,8-13H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Affinity for Corticotropin releasing factor receptor 1 on IMR-32 (human neuroblastoma) cells by [125I]-sauvagine displacement. |
Bioorg Med Chem Lett 13: 2497-500 (2003)
BindingDB Entry DOI: 10.7270/Q2C53K72 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-3
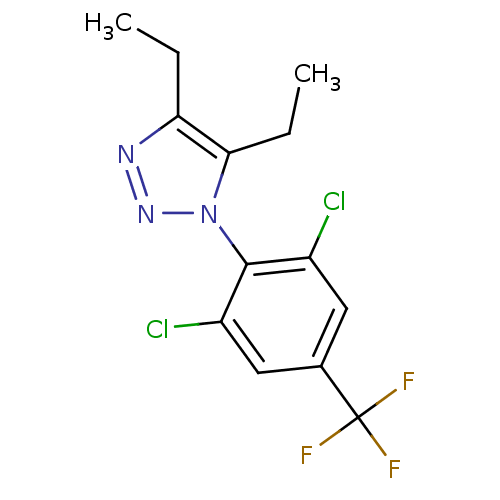
(Homo sapiens (Human)) | BDBM50214650
(1-(2,6-dichloro-4-trifluoromethylphenyl)-4,5-dieth...)Show SMILES CCc1nnn(c1CC)-c1c(Cl)cc(cc1Cl)C(F)(F)F |(31.28,.4,;31.44,1.93,;30.2,2.84,;30.21,4.38,;28.74,4.86,;27.84,3.62,;28.73,2.37,;28.25,.9,;29.28,-.24,;26.3,3.62,;25.53,2.29,;26.31,.95,;24,2.28,;23.22,3.62,;23.99,4.95,;25.53,4.95,;26.31,6.28,;21.68,3.62,;20.13,3.55,;21.65,5.16,;21.73,2.08,)| Show InChI InChI=1S/C13H12Cl2F3N3/c1-3-10-11(4-2)21(20-19-10)12-8(14)5-7(6-9(12)15)13(16,17)18/h5-6H,3-4H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shimane University
Curated by ChEMBL
| Assay Description
Displacement of [3H]EBOB from human recombinant GABAbeta3 receptor expressed in HEK293 cells |
Bioorg Med Chem 15: 5090-104 (2007)
Article DOI: 10.1016/j.bmc.2007.05.039
BindingDB Entry DOI: 10.7270/Q2K64HS0 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
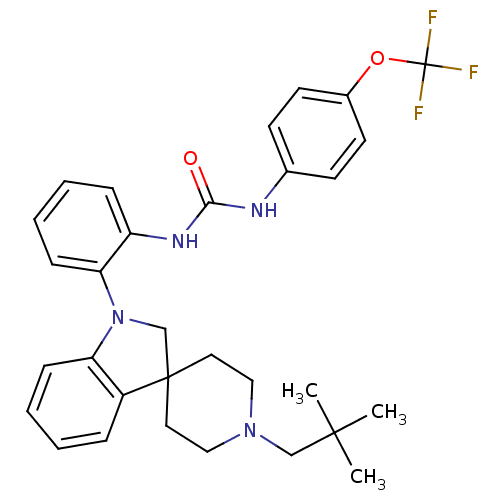
(Homo sapiens (Human)) | BDBM50445214
(CHEMBL3104636)Show SMILES CC(C)(C)CN1CCC2(CN(c3ccccc23)c2ccccc2NC(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C31H35F3N4O2/c1-29(2,3)20-37-18-16-30(17-19-37)21-38(26-10-6-4-8-24(26)30)27-11-7-5-9-25(27)36-28(39)35-22-12-14-23(15-13-22)40-31(32,33)34/h4-15H,16-21H2,1-3H3,(H2,35,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis |
J Med Chem 56: 9275-95 (2013)
Article DOI: 10.1021/jm4013906
BindingDB Entry DOI: 10.7270/Q2KW5HHP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50100388

(CHEMBL3325904)Show InChI InChI=1S/C24H26N2O/c1-26-13-12-20-14-23(25-16-18-8-4-2-5-9-18)24(27)15-21(20)22(17-26)19-10-6-3-7-11-19/h2-11,14-15,22,25,27H,12-13,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 cell membranes by liquid scintillation counting based competition bindin... |
Eur J Med Chem 85: 16-26 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.059
BindingDB Entry DOI: 10.7270/Q2PZ5BK4 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
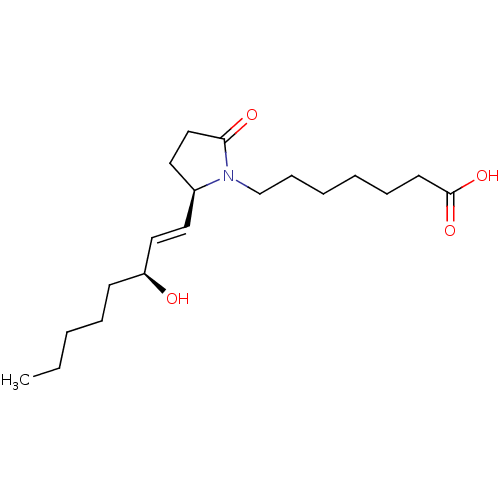
(Homo sapiens (Human)) | BDBM50142481
(7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCCCCCC(O)=O Show InChI InChI=1S/C19H33NO4/c1-2-3-6-9-17(21)13-11-16-12-14-18(22)20(16)15-8-5-4-7-10-19(23)24/h11,13,16-17,21H,2-10,12,14-15H2,1H3,(H,23,24)/b13-11+/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
J Med Chem 47: 6124-7 (2004)
Article DOI: 10.1021/jm049290a
BindingDB Entry DOI: 10.7270/Q2P84BBQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50445208
(CHEMBL3105199)Show SMILES CC(C)(C)CN1CCC2(CC1)CCN(c1ccccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1)c1ccccc21 Show InChI InChI=1S/C32H37F3N4O2/c1-30(2,3)22-38-19-16-31(17-20-38)18-21-39(27-10-6-4-8-25(27)31)28-11-7-5-9-26(28)37-29(40)36-23-12-14-24(15-13-23)41-32(33,34)35/h4-15H,16-22H2,1-3H3,(H2,36,37,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis |
J Med Chem 56: 9275-95 (2013)
Article DOI: 10.1021/jm4013906
BindingDB Entry DOI: 10.7270/Q2KW5HHP |
More data for this
Ligand-Target Pair | |
Menin
(Homo sapiens (Human)) | BDBM50489254
(CHEMBL2314819)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)C1(CCC1)NC(=O)[C@]1([H])CCCN1C(=O)[C@H](Cc1cc(F)cc(F)c1)NC(=O)[C@@H](N)CCCCCCCCNC2=O |r| Show InChI InChI=1S/C40H60F2N10O6/c41-26-21-25(22-27(42)24-26)23-30-37(57)52-20-9-14-32(52)35(55)50-40(15-10-16-40)38(58)49-29(12-7-18-47-39(44)45)36(56)51-19-8-13-31(51)34(54)46-17-6-4-2-1-3-5-11-28(43)33(53)48-30/h21-22,24,28-32H,1-20,23,43H2,(H,46,54)(H,48,53)(H,49,58)(H,50,55)(H4,44,45,47)/t28-,29-,30-,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to menin (unknown origin) after 60 mins by fluorescence polarization assay |
J Med Chem 56: 1113-23 (2013)
Article DOI: 10.1021/jm3015298
BindingDB Entry DOI: 10.7270/Q22B91XM |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50436912
(CHEMBL2401804)Show SMILES CCOC(=O)c1sc(Nc2cccnc2Oc2ccccc2C(C)(C)C)nc1C(F)(F)F Show InChI InChI=1S/C22H22F3N3O3S/c1-5-30-19(29)16-17(22(23,24)25)28-20(32-16)27-14-10-8-12-26-18(14)31-15-11-7-6-9-13(15)21(2,3)4/h6-12H,5H2,1-4H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis |
Bioorg Med Chem Lett 23: 4206-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.025
BindingDB Entry DOI: 10.7270/Q26M388Q |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50015290
(CHEMBL3263070 | US9120798, 30)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(F)ccc3O)c2ccccc2Nc2nnc(s2)-c2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C32H33F4N5O2S/c1-30(2,3)18-40-16-14-31(15-17-40)19-41(27-25(42)13-12-22(33)26(27)31)24-7-5-4-6-23(24)37-29-39-38-28(44-29)20-8-10-21(11-9-20)43-32(34,35)36/h4-13,42H,14-19H2,1-3H3,(H,37,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-MeS-ADP from human cloned P2Y1 receptor expressed in HEK293 cells by SPA analysis |
Bioorg Med Chem Lett 24: 2481-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.011
BindingDB Entry DOI: 10.7270/Q2R78GS0 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50445201
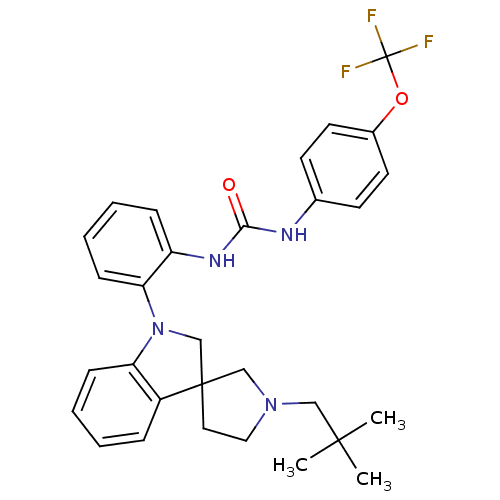
(CHEMBL3105201)Show SMILES CC(C)(C)CN1CCC2(C1)CN(c1ccccc21)c1ccccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C30H33F3N4O2/c1-28(2,3)18-36-17-16-29(19-36)20-37(25-10-6-4-8-23(25)29)26-11-7-5-9-24(26)35-27(38)34-21-12-14-22(15-13-21)39-30(31,32)33/h4-15H,16-20H2,1-3H3,(H2,34,35,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis |
J Med Chem 56: 9275-95 (2013)
Article DOI: 10.1021/jm4013906
BindingDB Entry DOI: 10.7270/Q2KW5HHP |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50445213
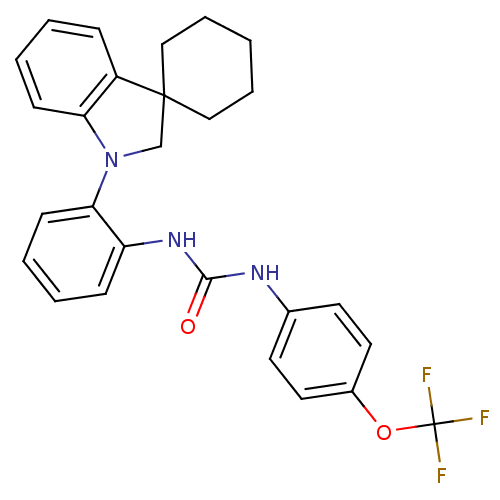
(CHEMBL3104624)Show SMILES FC(F)(F)Oc1ccc(NC(=O)Nc2ccccc2N2CC3(CCCCC3)c3ccccc23)cc1 Show InChI InChI=1S/C27H26F3N3O2/c28-27(29,30)35-20-14-12-19(13-15-20)31-25(34)32-22-9-3-5-11-24(22)33-18-26(16-6-1-7-17-26)21-8-2-4-10-23(21)33/h2-5,8-15H,1,6-7,16-18H2,(H2,31,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis |
J Med Chem 56: 9275-95 (2013)
Article DOI: 10.1021/jm4013906
BindingDB Entry DOI: 10.7270/Q2KW5HHP |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50429537
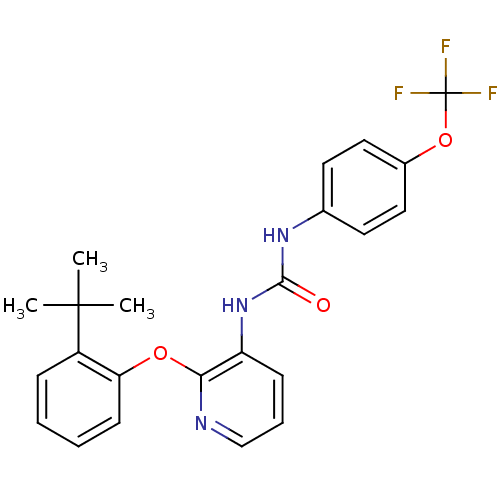
(CHEMBL2333770)Show SMILES CC(C)(C)c1ccccc1Oc1ncccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H22F3N3O3/c1-22(2,3)17-7-4-5-9-19(17)31-20-18(8-6-14-27-20)29-21(30)28-15-10-12-16(13-11-15)32-23(24,25)26/h4-14H,1-3H3,(H2,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells assessed as residual [beta-33P] bound to plate after 1 hr by... |
J Med Chem 56: 1704-14 (2013)
Article DOI: 10.1021/jm301708u
BindingDB Entry DOI: 10.7270/Q2ST7R6G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50429537

(CHEMBL2333770)Show SMILES CC(C)(C)c1ccccc1Oc1ncccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H22F3N3O3/c1-22(2,3)17-7-4-5-9-19(17)31-20-18(8-6-14-27-20)29-21(30)28-15-10-12-16(13-11-15)32-23(24,25)26/h4-14H,1-3H3,(H2,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis |
J Med Chem 56: 9275-95 (2013)
Article DOI: 10.1021/jm4013906
BindingDB Entry DOI: 10.7270/Q2KW5HHP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50436910
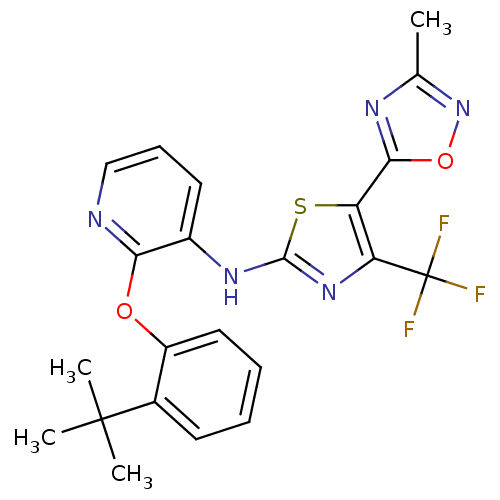
(CHEMBL2401853)Show SMILES Cc1noc(n1)-c1sc(Nc2cccnc2Oc2ccccc2C(C)(C)C)nc1C(F)(F)F Show InChI InChI=1S/C22H20F3N5O2S/c1-12-27-19(32-30-12)16-17(22(23,24)25)29-20(33-16)28-14-9-7-11-26-18(14)31-15-10-6-5-8-13(15)21(2,3)4/h5-11H,1-4H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis |
Bioorg Med Chem Lett 23: 4206-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.025
BindingDB Entry DOI: 10.7270/Q26M388Q |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50015278
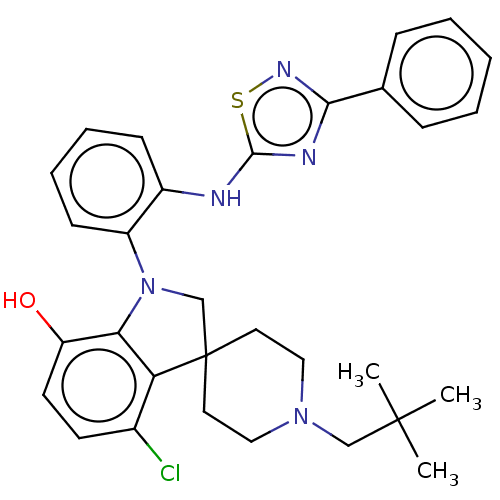
(CHEMBL3263058 | US9120798, 35)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(Cl)ccc3O)c2ccccc2Nc2nc(ns2)-c2ccccc2)CC1 Show InChI InChI=1S/C31H34ClN5OS/c1-30(2,3)19-36-17-15-31(16-18-36)20-37(27-25(38)14-13-22(32)26(27)31)24-12-8-7-11-23(24)33-29-34-28(35-39-29)21-9-5-4-6-10-21/h4-14,38H,15-20H2,1-3H3,(H,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-MeS-ADP from human cloned P2Y1 receptor expressed in HEK293 cells by SPA analysis |
Bioorg Med Chem Lett 24: 2481-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.011
BindingDB Entry DOI: 10.7270/Q2R78GS0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data