 Found 134 hits with Last Name = 'huang' and Initial = 'jc'
Found 134 hits with Last Name = 'huang' and Initial = 'jc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86492

(CAS_170713-75-4 | NSC_6324645 | Nociceptin)Show SMILES [#6]-[#6](-[#8])-[#6](-[#7]-[#6](=O)-[#6](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86491

(DiPOA | [8-(3,3-Diphenyl-propyl)-4-oxo-1-phenyl-1,...)Show SMILES OC(=O)CN1CN(c2ccccc2)C2(CCN(CCC(c3ccccc3)c3ccccc3)CC2)C1=O Show InChI InChI=1S/C30H33N3O3/c34-28(35)22-32-23-33(26-14-8-3-9-15-26)30(29(32)36)17-20-31(21-18-30)19-16-27(24-10-4-1-5-11-24)25-12-6-2-7-13-25/h1-15,27H,16-23H2,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86258
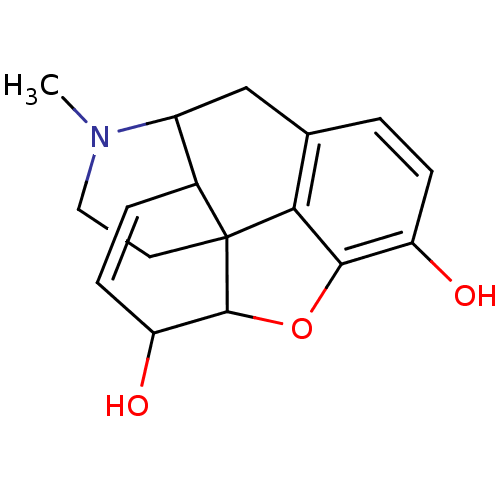
(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86493
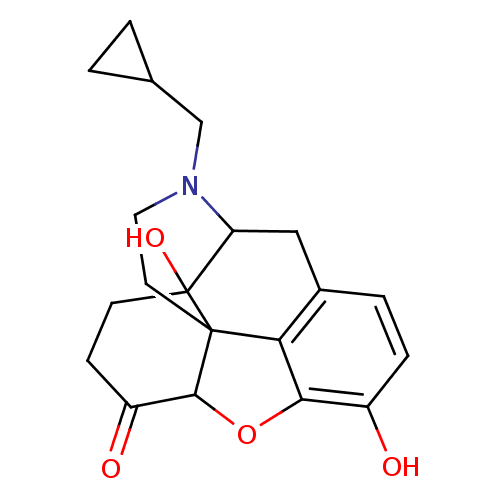
(CAS_27943 | NALTREXONE-HCl | NSC_27943 | Naltrexon...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(=O)CCC35O |TLB:22:23:7.12.13:5.4.18,THB:24:23:7.12.13:5.4.18| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM21130
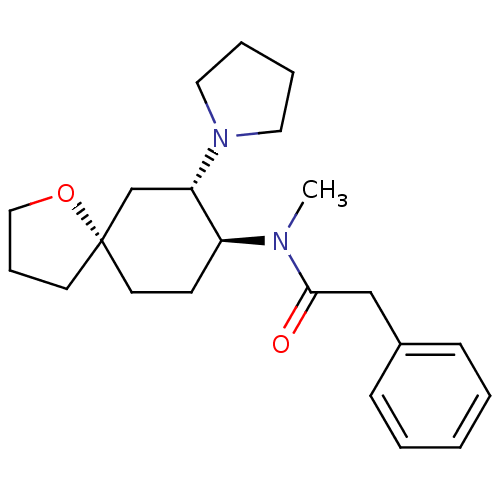
(N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C22H32N2O2/c1-23(21(25)16-18-8-3-2-4-9-18)19-10-12-22(11-7-15-26-22)17-20(19)24-13-5-6-14-24/h2-4,8-9,19-20H,5-7,10-17H2,1H3/t19-,20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86258

(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86491

(DiPOA | [8-(3,3-Diphenyl-propyl)-4-oxo-1-phenyl-1,...)Show SMILES OC(=O)CN1CN(c2ccccc2)C2(CCN(CCC(c3ccccc3)c3ccccc3)CC2)C1=O Show InChI InChI=1S/C30H33N3O3/c34-28(35)22-32-23-33(26-14-8-3-9-15-26)30(29(32)36)17-20-31(21-18-30)19-16-27(24-10-4-1-5-11-24)25-12-6-2-7-13-25/h1-15,27H,16-23H2,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86491

(DiPOA | [8-(3,3-Diphenyl-propyl)-4-oxo-1-phenyl-1,...)Show SMILES OC(=O)CN1CN(c2ccccc2)C2(CCN(CCC(c3ccccc3)c3ccccc3)CC2)C1=O Show InChI InChI=1S/C30H33N3O3/c34-28(35)22-32-23-33(26-14-8-3-9-15-26)30(29(32)36)17-20-31(21-18-30)19-16-27(24-10-4-1-5-11-24)25-12-6-2-7-13-25/h1-15,27H,16-23H2,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM86260

(CAS_465-65-6 | NALOXONE | NSC_10064 | Naloxone(-))Show SMILES Oc1ccc2CC3N(CC=C)CCC45C(Oc1c24)C(=O)CCC35O |TLB:21:22:7.11.12:5.4.17,THB:23:22:7.11.12:5.4.17| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 488 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM86258

(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86258

(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Caspase-9
(Homo sapiens (Human)) | BDBM50140543
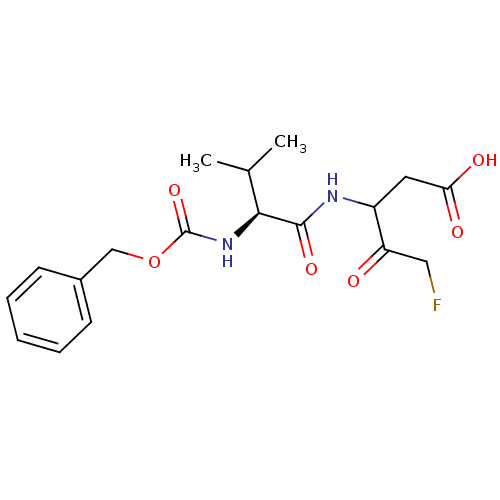
(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-9 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 1
(Homo sapiens (Human)) | BDBM50414894
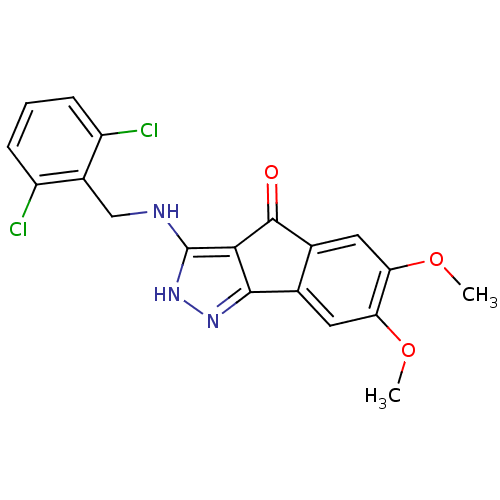
(CHEMBL573760)Show SMILES COc1cc2C(=O)c3c(NCc4c(Cl)cccc4Cl)[nH]nc3-c2cc1OC Show InChI InChI=1S/C19H15Cl2N3O3/c1-26-14-6-9-10(7-15(14)27-2)18(25)16-17(9)23-24-19(16)22-8-11-12(20)4-3-5-13(11)21/h3-7H,8H2,1-2H3,(H2,22,23,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human HRI kinase |
Bioorg Med Chem Lett 19: 6548-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.033
BindingDB Entry DOI: 10.7270/Q26Q1ZGZ |
More data for this
Ligand-Target Pair | |
Caspase-7
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-7 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50052635

(5,6,7-Trichloro-1H-quinoline-2,3,4-trione 3-oxime ...)Show InChI InChI=1S/C9H3Cl3N2O3/c10-2-1-3-4(6(12)5(2)11)8(15)7(14-17)9(16)13-3/h1H,(H2,13,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- DCKA binding to NMDA receptor of rat brain membranes |
J Med Chem 39: 3248-55 (1996)
Article DOI: 10.1021/jm960214k
BindingDB Entry DOI: 10.7270/Q2QC02KC |
More data for this
Ligand-Target Pair | |
Caspase-8
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-8 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 1
(Homo sapiens (Human)) | BDBM50414893
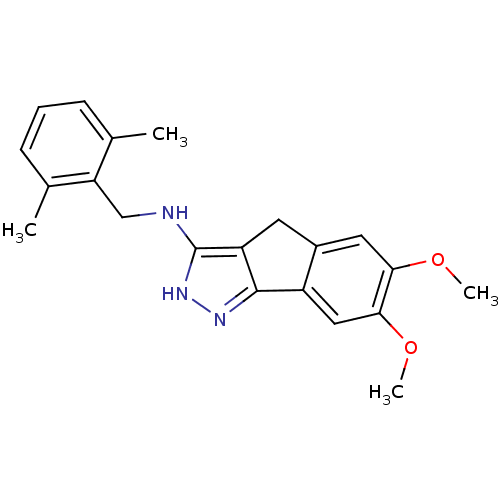
(CHEMBL578426)Show InChI InChI=1S/C21H23N3O2/c1-12-6-5-7-13(2)17(12)11-22-21-16-8-14-9-18(25-3)19(26-4)10-15(14)20(16)23-24-21/h5-7,9-10H,8,11H2,1-4H3,(H2,22,23,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human HRI kinase |
Bioorg Med Chem Lett 19: 6548-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.033
BindingDB Entry DOI: 10.7270/Q26Q1ZGZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50066544
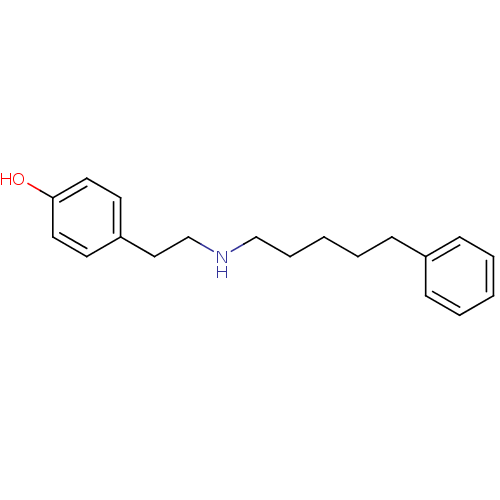
(4-[2-(5-Phenyl-pentylamino)-ethyl]-phenol | CHEMBL...)Show InChI InChI=1S/C19H25NO/c21-19-12-10-18(11-13-19)14-16-20-15-6-2-5-9-17-7-3-1-4-8-17/h1,3-4,7-8,10-13,20-21H,2,5-6,9,14-16H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Functional antagonism by electrical assays in Xenopus oocytes expressing the 1A/2B NMDA receptor |
J Med Chem 41: 3499-506 (1998)
Article DOI: 10.1021/jm980235+
BindingDB Entry DOI: 10.7270/Q29Z95MM |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50052629

(6,7-Dichloro-1H-quinoline-2,3,4-trione 3-oxime | C...)Show InChI InChI=1S/C9H4Cl2N2O3/c10-4-1-3-6(2-5(4)11)12-9(15)7(13-16)8(3)14/h1-2H,(H2,12,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- DCKA binding to NMDA receptor of rat brain membranes |
J Med Chem 39: 3248-55 (1996)
Article DOI: 10.1021/jm960214k
BindingDB Entry DOI: 10.7270/Q2QC02KC |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50066538
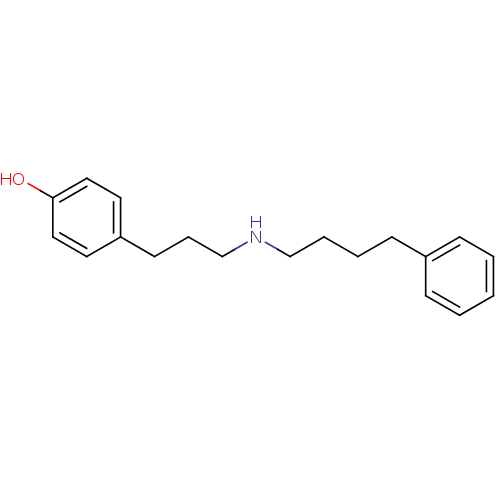
(4-[3-(4-Phenyl-butylamino)-propyl]-phenol | CHEMBL...)Show InChI InChI=1S/C19H25NO/c21-19-13-11-18(12-14-19)10-6-16-20-15-5-4-9-17-7-2-1-3-8-17/h1-3,7-8,11-14,20-21H,4-6,9-10,15-16H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Functional antagonism by electrical assays in Xenopus oocytes expressing the 1A/2B NMDA receptor |
J Med Chem 41: 3499-506 (1998)
Article DOI: 10.1021/jm980235+
BindingDB Entry DOI: 10.7270/Q29Z95MM |
More data for this
Ligand-Target Pair | |
Caspase-6
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-6 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 1
(Homo sapiens (Human)) | BDBM50414892

(CHEMBL576168)Show SMILES COc1cc2Cc3c(NCc4c(C)cccc4Cl)[nH]nc3-c2cc1OC Show InChI InChI=1S/C20H20ClN3O2/c1-11-5-4-6-16(21)15(11)10-22-20-14-7-12-8-17(25-2)18(26-3)9-13(12)19(14)23-24-20/h4-6,8-9H,7,10H2,1-3H3,(H2,22,23,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human HRI kinase |
Bioorg Med Chem Lett 19: 6548-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.033
BindingDB Entry DOI: 10.7270/Q26Q1ZGZ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-1 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50052627

(5,6,7-Trichloro-1,4-dihydro-quinoxaline-2,3-dione ...)Show InChI InChI=1S/C8H3Cl3N2O2/c9-2-1-3-6(5(11)4(2)10)13-8(15)7(14)12-3/h1H,(H,12,14)(H,13,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- DCKA binding to NMDA receptor of rat brain membranes |
J Med Chem 39: 3248-55 (1996)
Article DOI: 10.1021/jm960214k
BindingDB Entry DOI: 10.7270/Q2QC02KC |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 1
(Homo sapiens (Human)) | BDBM50414891
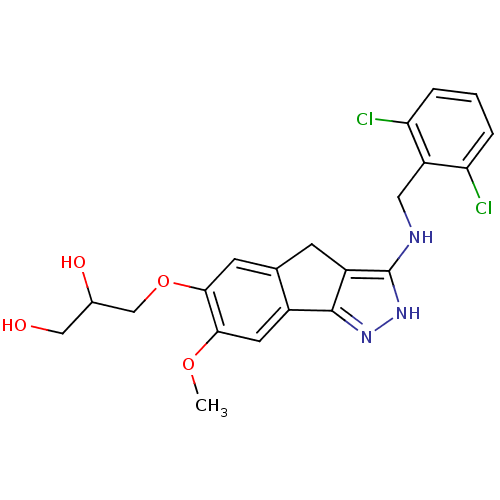
(CHEMBL574005)Show SMILES COc1cc-2c(Cc3c(NCc4c(Cl)cccc4Cl)[nH]nc-23)cc1OCC(O)CO Show InChI InChI=1S/C21H21Cl2N3O4/c1-29-18-7-13-11(6-19(18)30-10-12(28)9-27)5-14-20(13)25-26-21(14)24-8-15-16(22)3-2-4-17(15)23/h2-4,6-7,12,27-28H,5,8-10H2,1H3,(H2,24,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human HRI kinase |
Bioorg Med Chem Lett 19: 6548-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.033
BindingDB Entry DOI: 10.7270/Q26Q1ZGZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50052625

(5,7-Dichloro-1H-quinoline-2,3,4-trione 3-oxime | C...)Show InChI InChI=1S/C9H4Cl2N2O3/c10-3-1-4(11)6-5(2-3)12-9(15)7(13-16)8(6)14/h1-2H,(H2,12,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- DCKA binding to NMDA receptor of rat brain membranes |
J Med Chem 39: 3248-55 (1996)
Article DOI: 10.1021/jm960214k
BindingDB Entry DOI: 10.7270/Q2QC02KC |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50066547
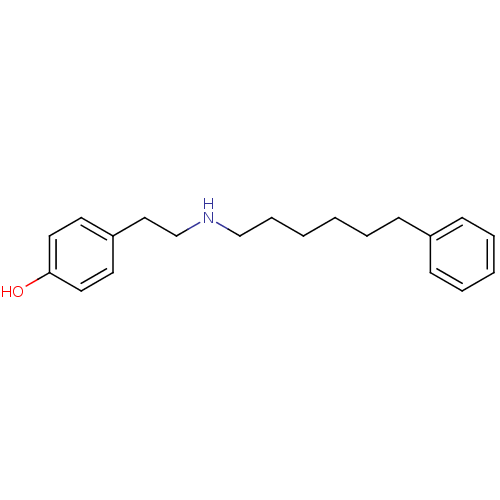
(4-[2-(6-Phenyl-hexylamino)-ethyl]-phenol | CHEMBL4...)Show InChI InChI=1S/C20H27NO/c22-20-13-11-19(12-14-20)15-17-21-16-7-2-1-4-8-18-9-5-3-6-10-18/h3,5-6,9-14,21-22H,1-2,4,7-8,15-17H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Functional antagonism by electrical assays in Xenopus oocytes expressing the 1A/2B NMDA receptor |
J Med Chem 41: 3499-506 (1998)
Article DOI: 10.1021/jm980235+
BindingDB Entry DOI: 10.7270/Q29Z95MM |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50052626
(5,7-Dimethyl-1H-quinoline-2,3,4-trione 3-oxime | C...)Show InChI InChI=1S/C11H10N2O3/c1-5-3-6(2)8-7(4-5)12-11(15)9(13-16)10(8)14/h3-4H,1-2H3,(H2,12,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- DCKA binding to NMDA receptor of rat brain membranes |
J Med Chem 39: 3248-55 (1996)
Article DOI: 10.1021/jm960214k
BindingDB Entry DOI: 10.7270/Q2QC02KC |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50066539
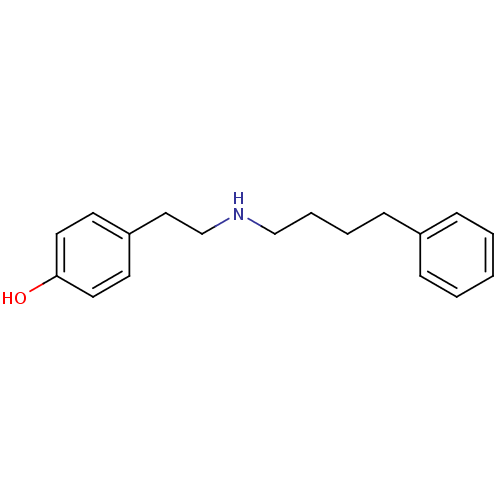
(4-[2-(4-Phenyl-butylamino)-ethyl]-phenol | CHEMBL1...)Show InChI InChI=1S/C18H23NO/c20-18-11-9-17(10-12-18)13-15-19-14-5-4-8-16-6-2-1-3-7-16/h1-3,6-7,9-12,19-20H,4-5,8,13-15H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Functional antagonism by electrical assays in Xenopus oocytes expressing the 1A/2B NMDA receptor |
J Med Chem 41: 3499-506 (1998)
Article DOI: 10.1021/jm980235+
BindingDB Entry DOI: 10.7270/Q29Z95MM |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50066546

(4-[3-(5-Phenyl-pentylamino)-propyl]-phenol | CHEMB...)Show InChI InChI=1S/C20H27NO/c22-20-14-12-19(13-15-20)11-7-17-21-16-6-2-5-10-18-8-3-1-4-9-18/h1,3-4,8-9,12-15,21-22H,2,5-7,10-11,16-17H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Functional antagonism by electrical assays in Xenopus oocytes expressing the 1A/2B NMDA receptor |
J Med Chem 41: 3499-506 (1998)
Article DOI: 10.1021/jm980235+
BindingDB Entry DOI: 10.7270/Q29Z95MM |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 1
(Homo sapiens (Human)) | BDBM50414895
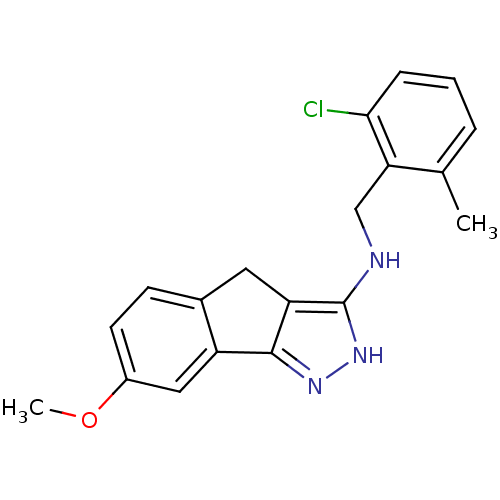
(CHEMBL578836)Show InChI InChI=1S/C19H18ClN3O/c1-11-4-3-5-17(20)16(11)10-21-19-15-8-12-6-7-13(24-2)9-14(12)18(15)22-23-19/h3-7,9H,8,10H2,1-2H3,(H2,21,22,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human HRI kinase |
Bioorg Med Chem Lett 19: 6548-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.033
BindingDB Entry DOI: 10.7270/Q26Q1ZGZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50052631
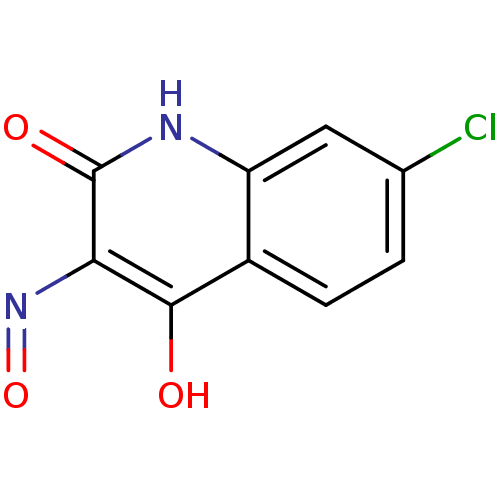
(7-Chloro-1H-quinoline-2,3,4-trione 3-oxime | CHEMB...)Show InChI InChI=1S/C9H5ClN2O3/c10-4-1-2-5-6(3-4)11-9(14)7(12-15)8(5)13/h1-3H,(H2,11,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- DCKA binding to NMDA receptor of rat brain membranes |
J Med Chem 39: 3248-55 (1996)
Article DOI: 10.1021/jm960214k
BindingDB Entry DOI: 10.7270/Q2QC02KC |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 1
(Homo sapiens (Human)) | BDBM50414890
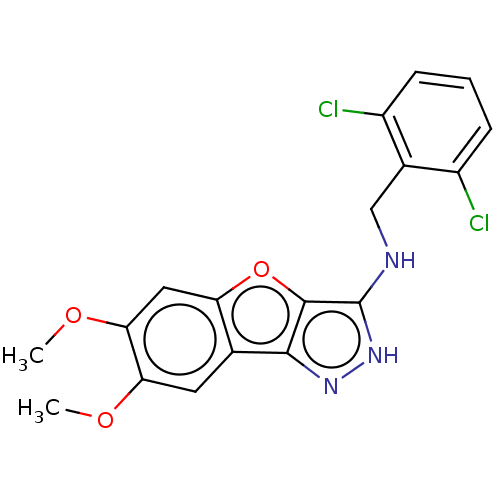
(CHEMBL565272)Show SMILES COc1cc2oc3c(NCc4c(Cl)cccc4Cl)[nH]nc3c2cc1OC | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human HRI kinase |
Bioorg Med Chem Lett 19: 6548-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.033
BindingDB Entry DOI: 10.7270/Q26Q1ZGZ |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 1
(Homo sapiens (Human)) | BDBM50414889
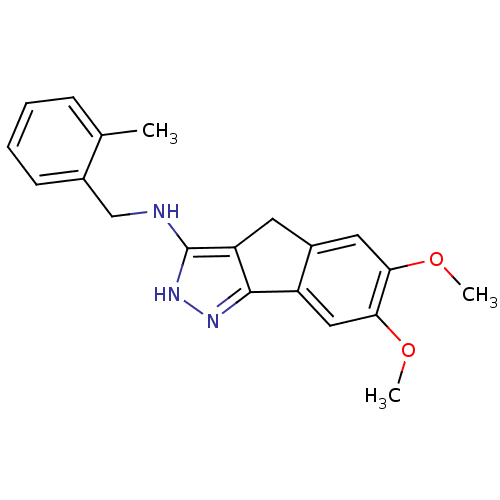
(CHEMBL574443)Show InChI InChI=1S/C20H21N3O2/c1-12-6-4-5-7-13(12)11-21-20-16-8-14-9-17(24-2)18(25-3)10-15(14)19(16)22-23-20/h4-7,9-10H,8,11H2,1-3H3,(H2,21,22,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human HRI kinase |
Bioorg Med Chem Lett 19: 6548-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.033
BindingDB Entry DOI: 10.7270/Q26Q1ZGZ |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50366934

(CHEMBL1794026)Show SMILES CC[C@H](C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-3-12(2)17(18(26)21-14(9-16(24)25)15(23)10-20)22-19(27)28-11-13-7-5-4-6-8-13/h4-8,12,14,17H,3,9-11H2,1-2H3,(H,21,26)(H,22,27)(H,24,25)/t12-,14?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 1
(Homo sapiens (Human)) | BDBM50414888
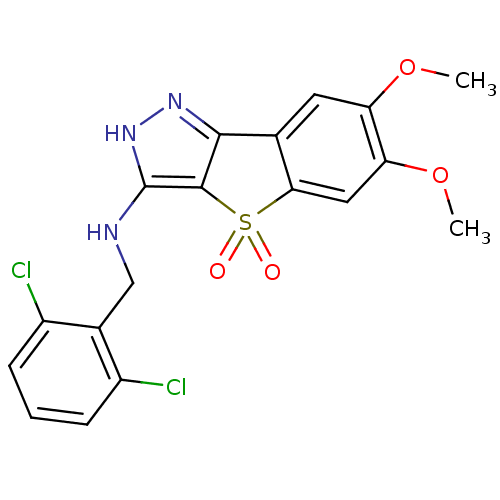
(CHEMBL583635)Show SMILES COc1cc2-c3n[nH]c(NCc4c(Cl)cccc4Cl)c3S(=O)(=O)c2cc1OC Show InChI InChI=1S/C18H15Cl2N3O4S/c1-26-13-6-9-15(7-14(13)27-2)28(24,25)17-16(9)22-23-18(17)21-8-10-11(19)4-3-5-12(10)20/h3-7H,8H2,1-2H3,(H2,21,22,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human HRI kinase |
Bioorg Med Chem Lett 19: 6548-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.033
BindingDB Entry DOI: 10.7270/Q26Q1ZGZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50066543
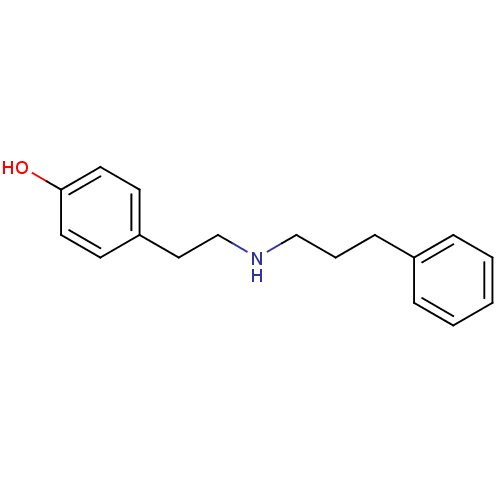
(4-[2-(3-Phenyl-propylamino)-ethyl]-phenol | CHEMBL...)Show InChI InChI=1S/C17H21NO/c19-17-10-8-16(9-11-17)12-14-18-13-4-7-15-5-2-1-3-6-15/h1-3,5-6,8-11,18-19H,4,7,12-14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Functional antagonism by electrical assays in Xenopus oocytes expressing the 1A/2B NMDA receptor |
J Med Chem 41: 3499-506 (1998)
Article DOI: 10.1021/jm980235+
BindingDB Entry DOI: 10.7270/Q29Z95MM |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140549

(3-((S)-2-Benzyloxycarbonylamino-2-cyclohexyl-acety...)Show SMILES OC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)C1CCCCC1)C(=O)CF Show InChI InChI=1S/C21H27FN2O6/c22-12-17(25)16(11-18(26)27)23-20(28)19(15-9-5-2-6-10-15)24-21(29)30-13-14-7-3-1-4-8-14/h1,3-4,7-8,15-16,19H,2,5-6,9-13H2,(H,23,28)(H,24,29)(H,26,27)/t16?,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140551

(3-((S)-2-Benzyloxycarbonylamino-2-phenyl-acetylami...)Show SMILES OC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)c1ccccc1)C(=O)CF Show InChI InChI=1S/C21H21FN2O6/c22-12-17(25)16(11-18(26)27)23-20(28)19(15-9-5-2-6-10-15)24-21(29)30-13-14-7-3-1-4-8-14/h1-10,16,19H,11-13H2,(H,23,28)(H,24,29)(H,26,27)/t16?,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140552
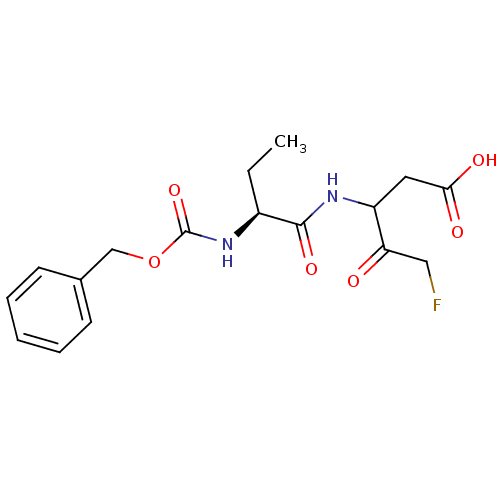
(3-((S)-2-Benzyloxycarbonylamino-butyrylamino)-5-fl...)Show SMILES CC[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C17H21FN2O6/c1-2-12(16(24)19-13(8-15(22)23)14(21)9-18)20-17(25)26-10-11-6-4-3-5-7-11/h3-7,12-13H,2,8-10H2,1H3,(H,19,24)(H,20,25)(H,22,23)/t12-,13?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50140548

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES COC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-12(2)17(22-19(26)28-11-13-7-5-4-6-8-13)18(25)21-14(15(23)10-20)9-16(24)27-3/h4-8,12,14,17H,9-11H2,1-3H3,(H,21,25)(H,22,26)/t14?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Calpain-1 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 1
(Homo sapiens (Human)) | BDBM50414887
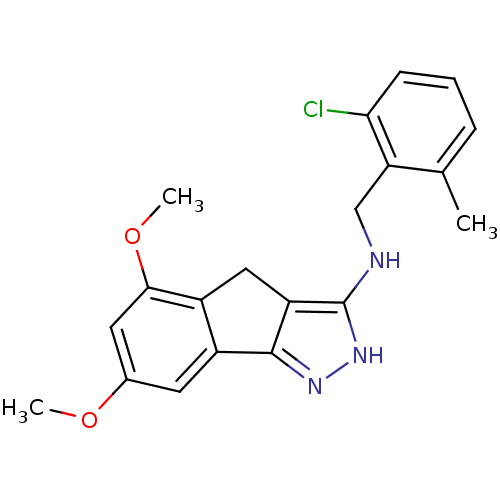
(CHEMBL574209)Show SMILES COc1cc-2c(Cc3c(NCc4c(C)cccc4Cl)[nH]nc-23)c(OC)c1 Show InChI InChI=1S/C20H20ClN3O2/c1-11-5-4-6-17(21)16(11)10-22-20-15-9-13-14(19(15)23-24-20)7-12(25-2)8-18(13)26-3/h4-8H,9-10H2,1-3H3,(H2,22,23,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human HRI kinase |
Bioorg Med Chem Lett 19: 6548-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.033
BindingDB Entry DOI: 10.7270/Q26Q1ZGZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM22778
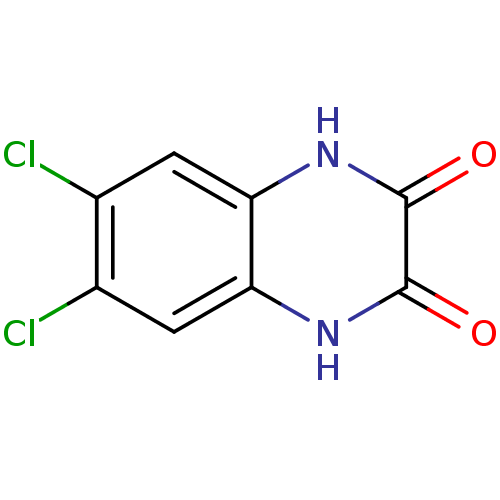
(6,7-Dichloro-1,4-dihydro-quinoxaline-2,3-dione | 6...)Show InChI InChI=1S/C8H4Cl2N2O2/c9-3-1-5-6(2-4(3)10)12-8(14)7(13)11-5/h1-2H,(H,11,13)(H,12,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- DCKA binding to NMDA receptor of rat brain membranes |
J Med Chem 39: 3248-55 (1996)
Article DOI: 10.1021/jm960214k
BindingDB Entry DOI: 10.7270/Q2QC02KC |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140550

(3-((S)-2-Benzyloxycarbonylamino-3-thiophen-2-yl-pr...)Show SMILES OC(=O)CC(NC(=O)[C@H](Cc1cccs1)NC(=O)OCc1ccccc1)C(=O)CF Show InChI InChI=1S/C20H21FN2O6S/c21-11-17(24)15(10-18(25)26)22-19(27)16(9-14-7-4-8-30-14)23-20(28)29-12-13-5-2-1-3-6-13/h1-8,15-16H,9-12H2,(H,22,27)(H,23,28)(H,25,26)/t15?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50052621
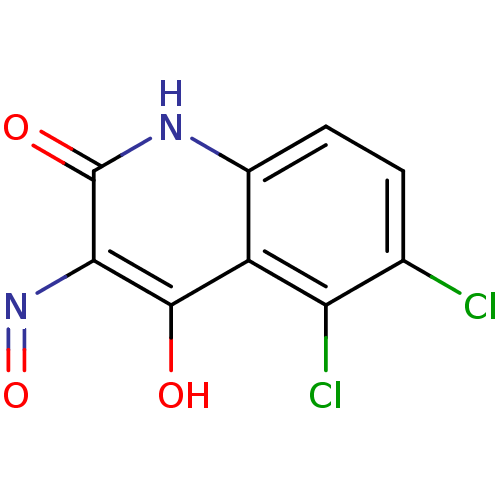
(5,6-Dichloro-1H-quinoline-2,3,4-trione 3-oxime | C...)Show InChI InChI=1S/C9H4Cl2N2O3/c10-3-1-2-4-5(6(3)11)8(14)7(13-16)9(15)12-4/h1-2H,(H2,12,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- DCKA binding to NMDA receptor of rat brain membranes |
J Med Chem 39: 3248-55 (1996)
Article DOI: 10.1021/jm960214k
BindingDB Entry DOI: 10.7270/Q2QC02KC |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50066545
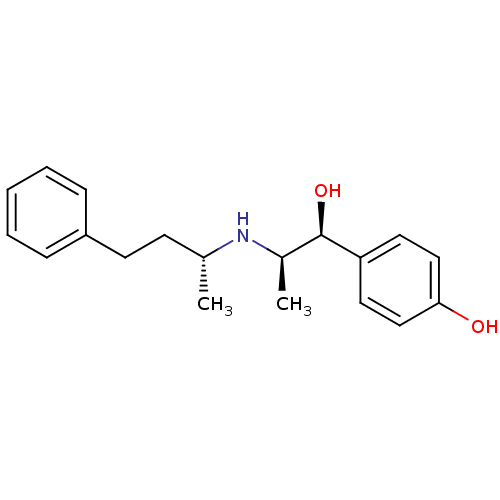
(4-[(1S,2R)-1-Hydroxy-2-((R)-1-methyl-3-phenyl-prop...)Show SMILES C[C@H](CCc1ccccc1)N[C@H](C)[C@@H](O)c1ccc(O)cc1 Show InChI InChI=1S/C19H25NO2/c1-14(8-9-16-6-4-3-5-7-16)20-15(2)19(22)17-10-12-18(21)13-11-17/h3-7,10-15,19-22H,8-9H2,1-2H3/t14-,15-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Functional antagonism by electrical assays in Xenopus oocytes expressing the 1A/2B NMDA receptor |
J Med Chem 41: 3499-506 (1998)
Article DOI: 10.1021/jm980235+
BindingDB Entry DOI: 10.7270/Q29Z95MM |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50066541
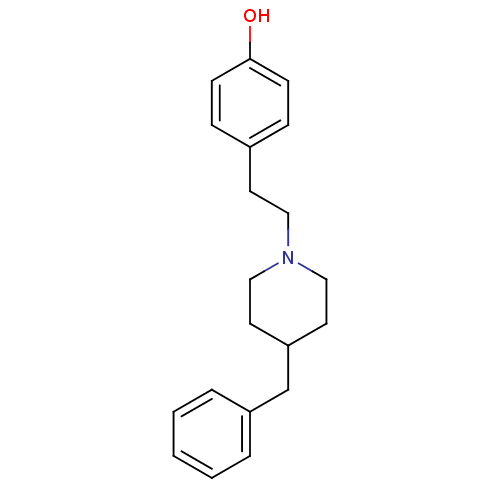
(4-[2-(4-Benzyl-piperidin-1-yl)-ethyl]-phenol; hydr...)Show InChI InChI=1S/C20H25NO/c22-20-8-6-17(7-9-20)10-13-21-14-11-19(12-15-21)16-18-4-2-1-3-5-18/h1-9,19,22H,10-16H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Functional antagonism by electrical assays in Xenopus oocytes expressing the 1A/2B NMDA receptor |
J Med Chem 41: 3499-506 (1998)
Article DOI: 10.1021/jm980235+
BindingDB Entry DOI: 10.7270/Q29Z95MM |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140544
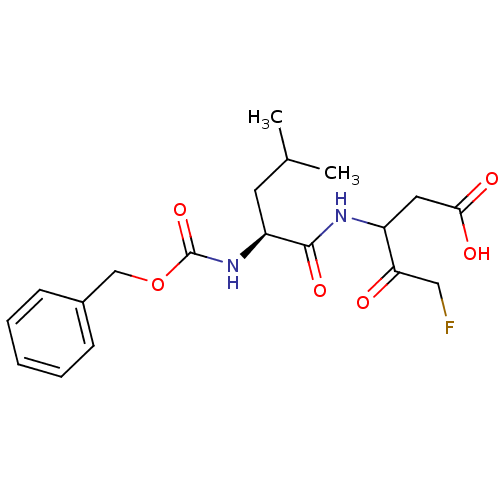
(3-((S)-2-Benzyloxycarbonylamino-4-methyl-pentanoyl...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-12(2)8-15(18(26)21-14(9-17(24)25)16(23)10-20)22-19(27)28-11-13-6-4-3-5-7-13/h3-7,12,14-15H,8-11H2,1-2H3,(H,21,26)(H,22,27)(H,24,25)/t14?,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 1
(Homo sapiens (Human)) | BDBM50414886
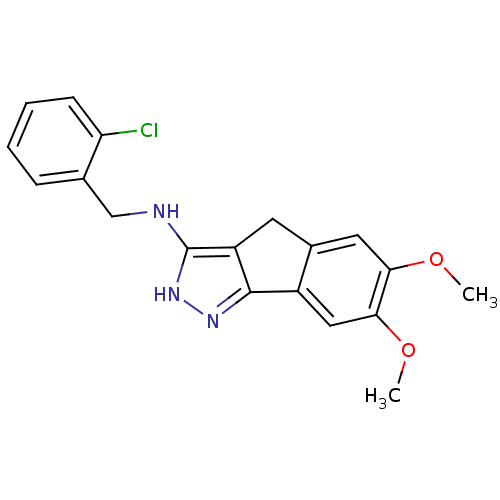
(CHEMBL573763)Show InChI InChI=1S/C19H18ClN3O2/c1-24-16-8-12-7-14-18(13(12)9-17(16)25-2)22-23-19(14)21-10-11-5-3-4-6-15(11)20/h3-6,8-9H,7,10H2,1-2H3,(H2,21,22,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human HRI kinase |
Bioorg Med Chem Lett 19: 6548-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.033
BindingDB Entry DOI: 10.7270/Q26Q1ZGZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data