

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptase delta (Homo sapiens (Human)) | BDBM50217812 (CHEMBL322526) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217627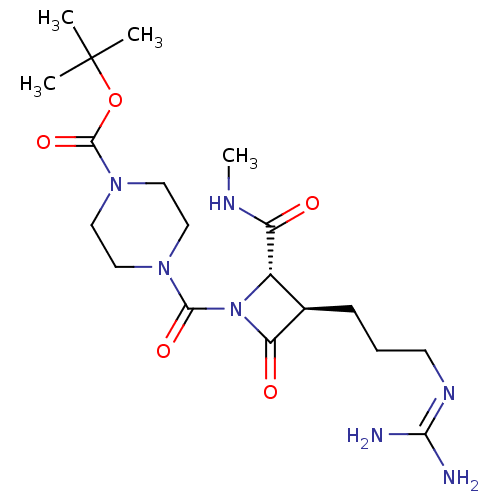 (CHEMBL110061) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217626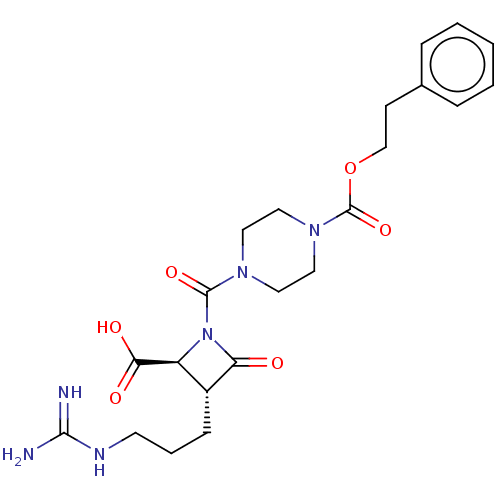 (CHEMBL322538) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217801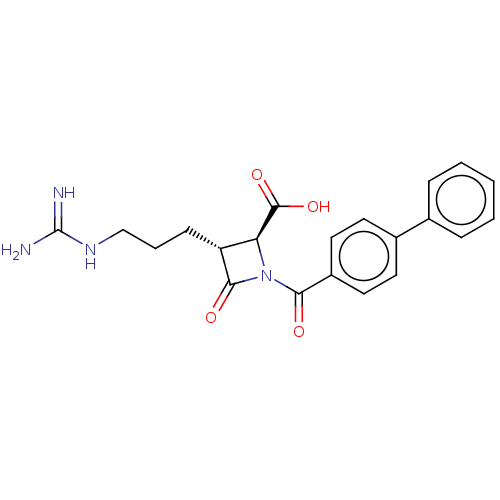 (CHEMBL111250) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144535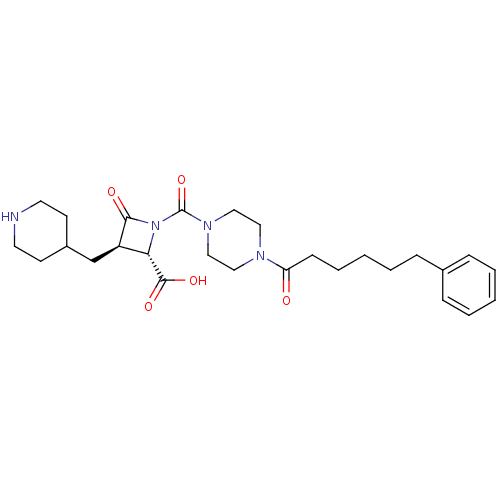 ((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217599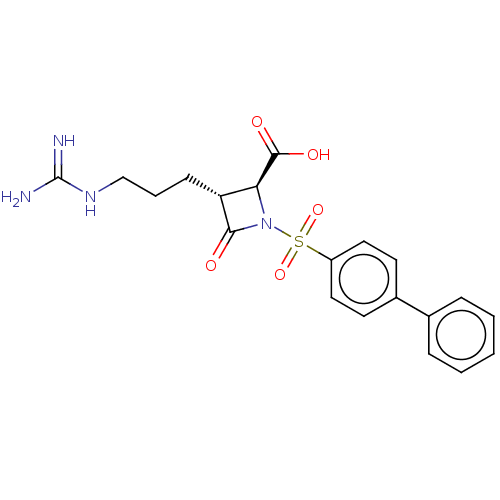 (CHEMBL109888) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217622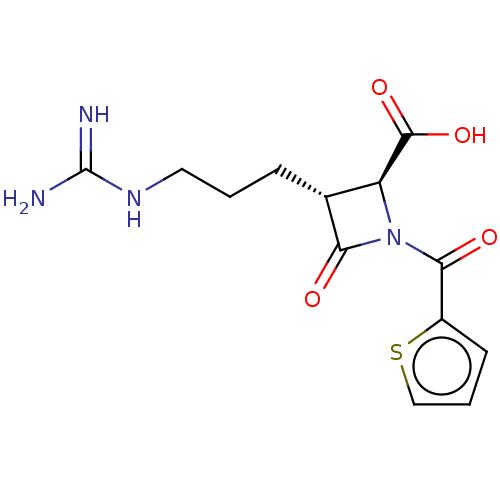 (CHEMBL443539) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50221046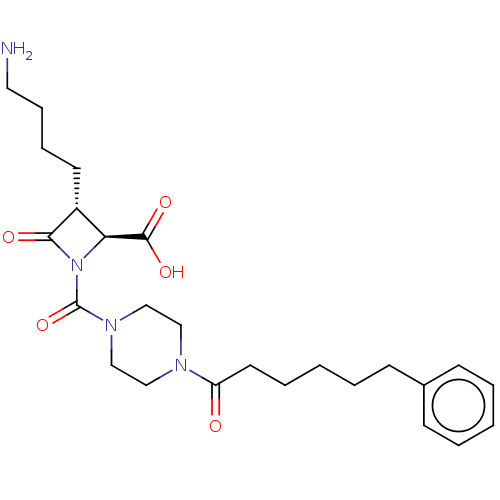 (CHEMBL72282) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217818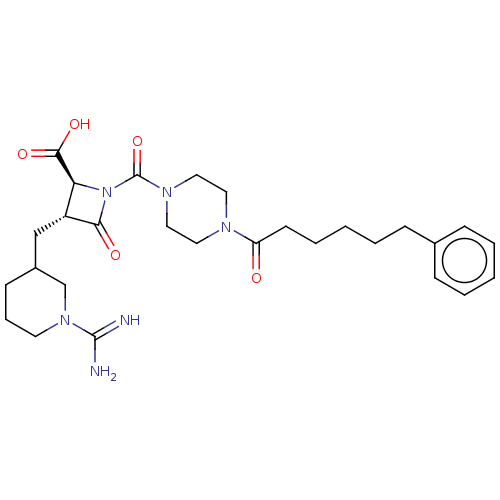 (CHEMBL440515) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217817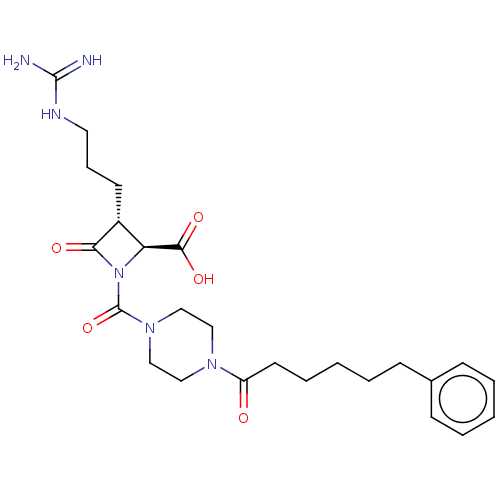 (CHEMBL326209) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217822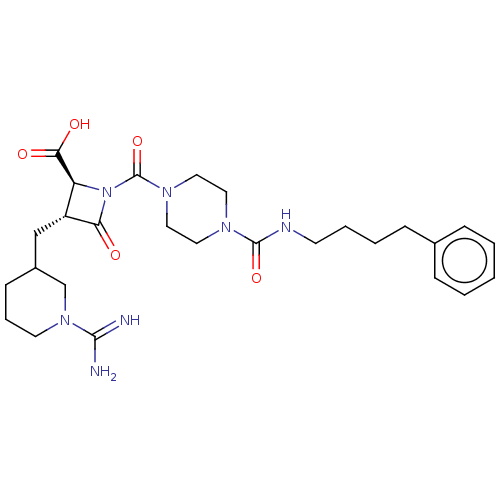 (CHEMBL111173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50120387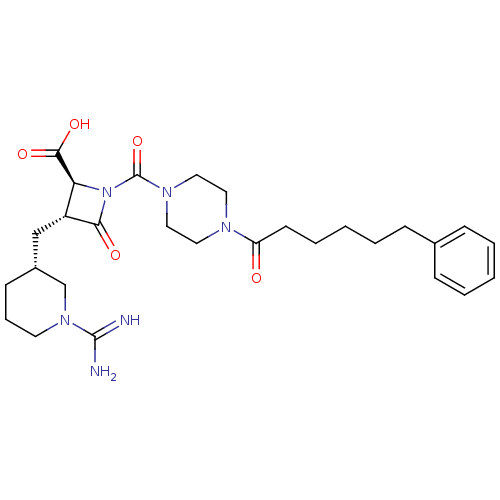 ((2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217823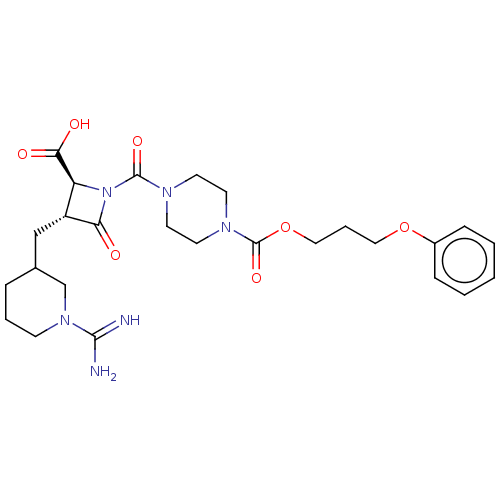 (CHEMBL111630) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217824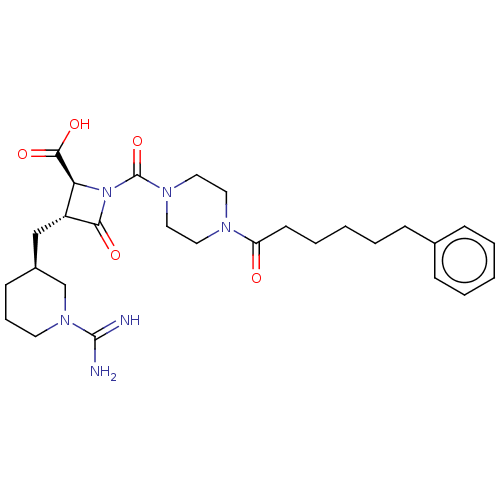 (BMS-363130 | CHEMBL70738) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50217824 (BMS-363130 | CHEMBL70738) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50120387 ((2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144532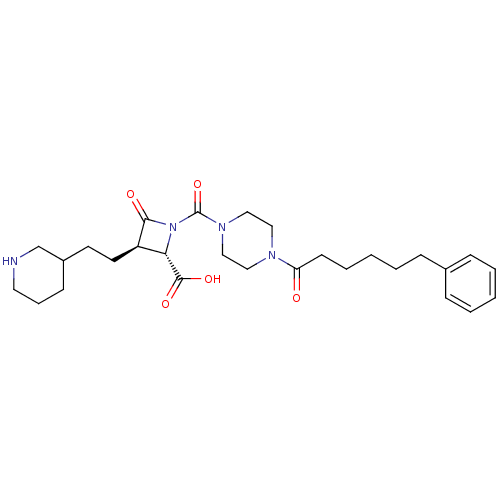 ((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217819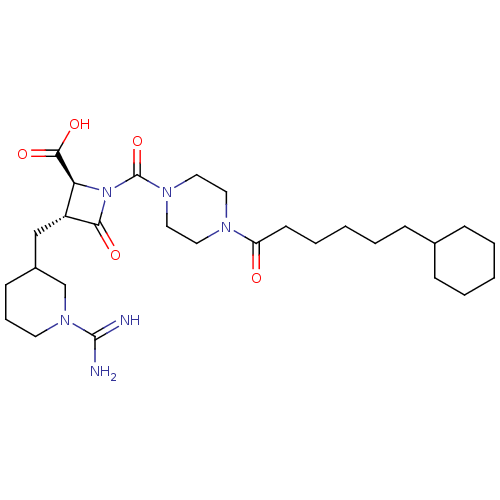 (CHEMBL109504) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217628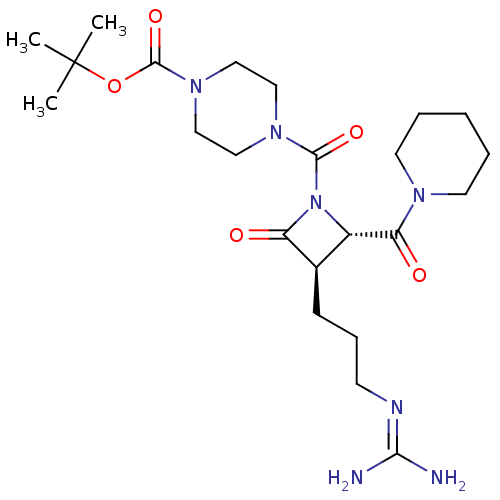 (CHEMBL107493) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217813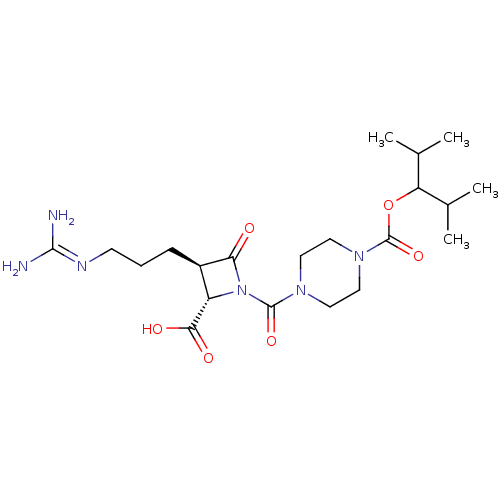 (CHEMBL302058) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217624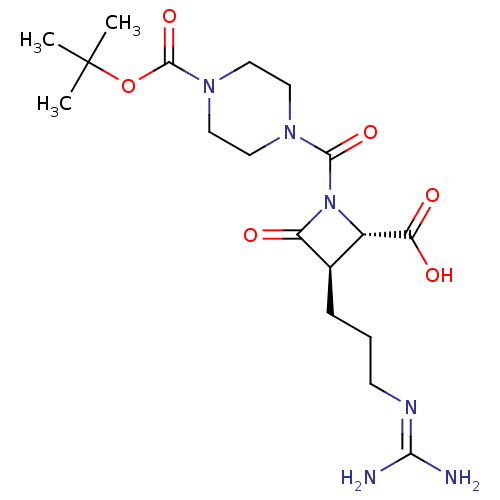 (CHEMBL321622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50217813 (CHEMBL302058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50120368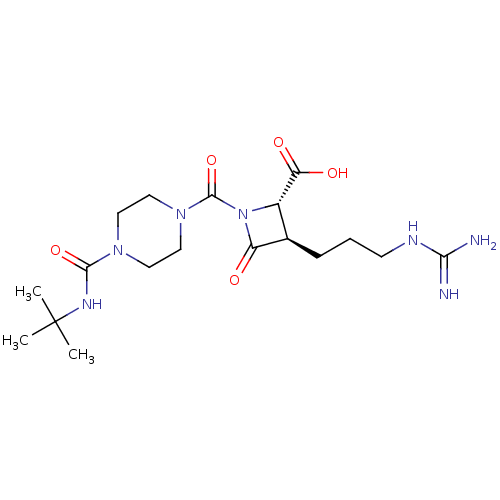 ((2S,3R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50220841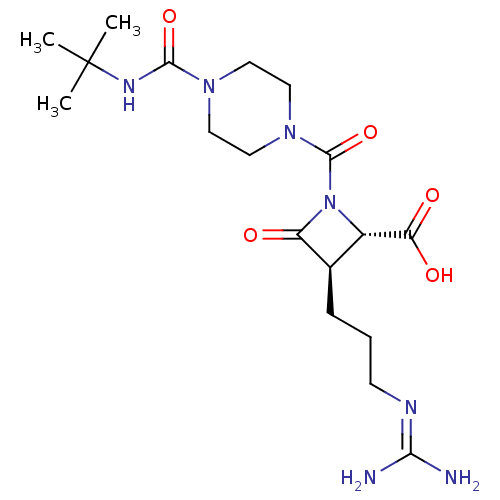 (BMS-262084 | CHEMBL71037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50120368 ((2S,3R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217820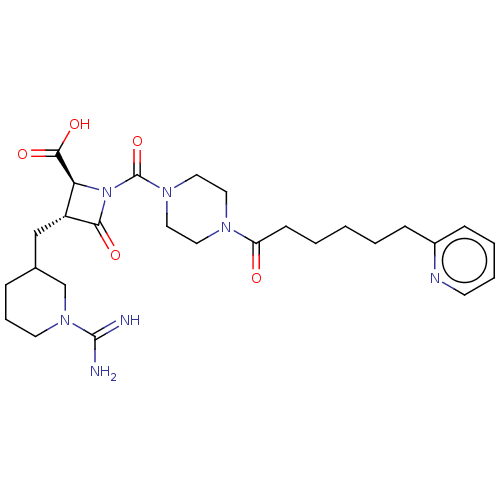 (CHEMBL447534) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217623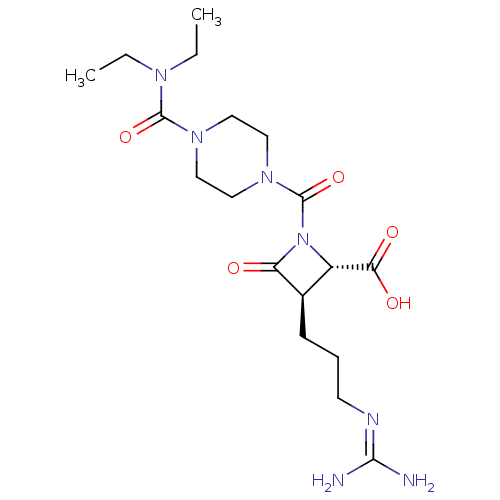 (CHEMBL109947) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217805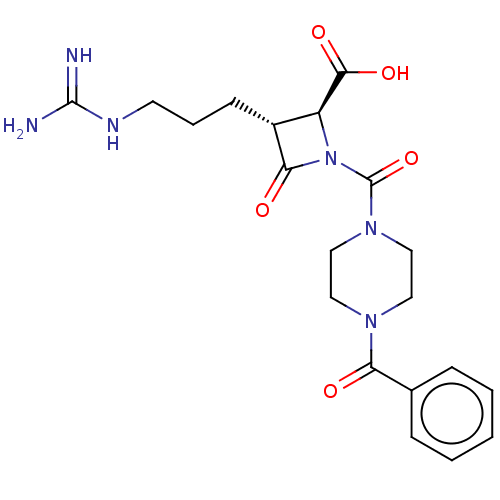 (CHEMBL111548) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217601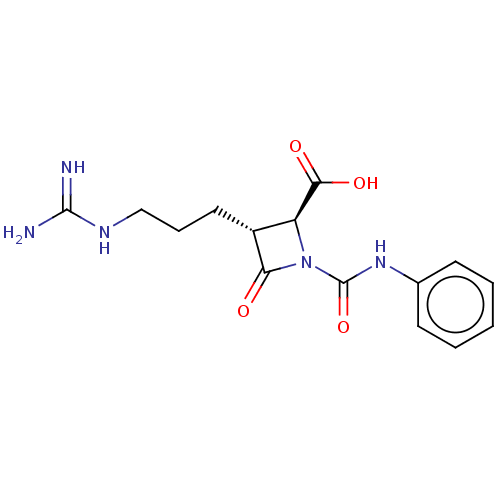 (CHEMBL320744) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217815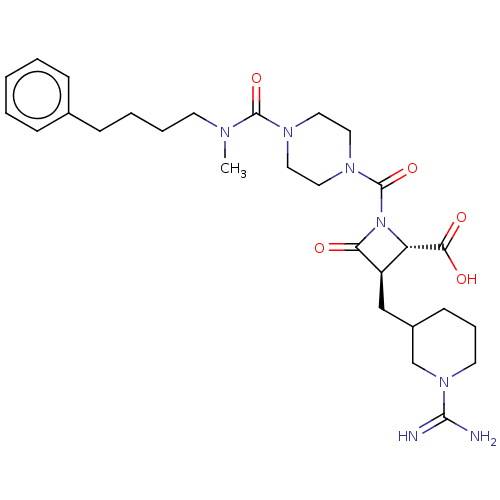 (CHEMBL113591) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217601 (CHEMBL320744) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217625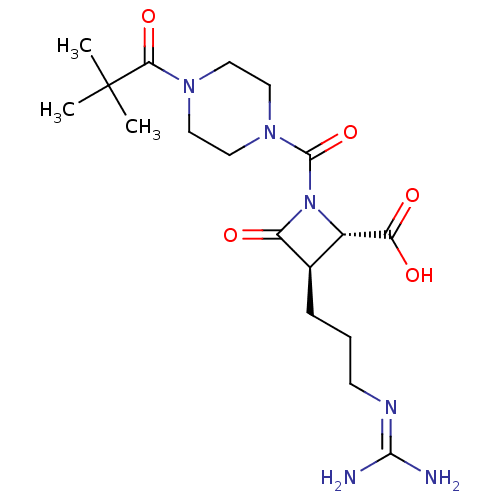 (CHEMBL326480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50220827 (CHEMBL441447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217629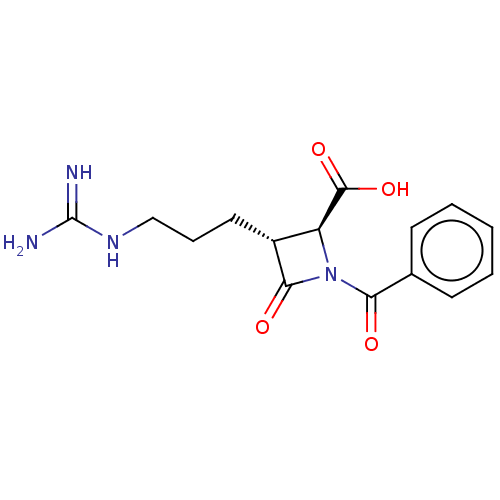 (CHEMBL111270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217816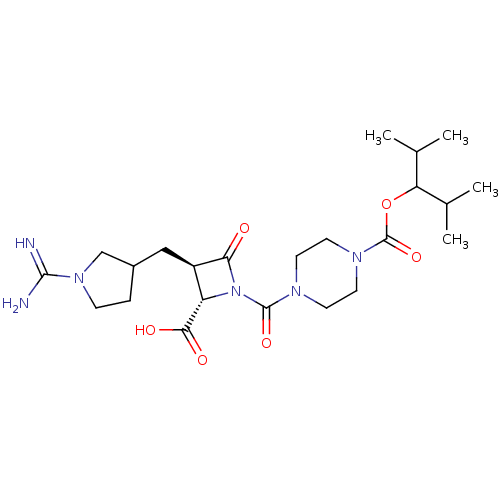 (CHEMBL432835) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217800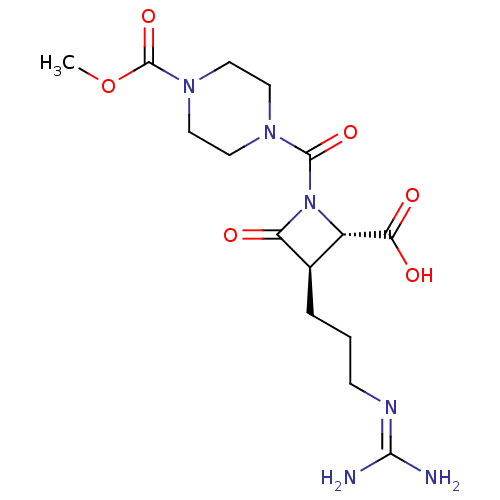 (CHEMBL109254) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217811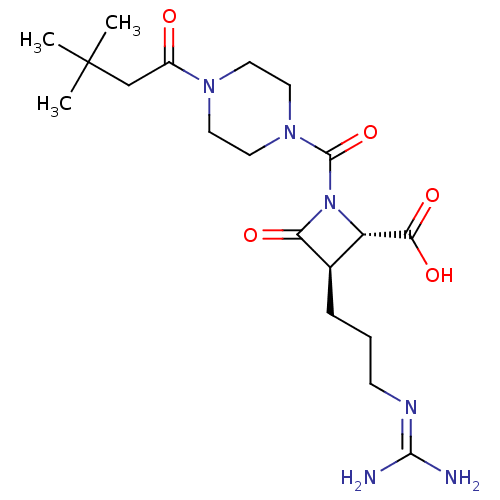 (CHEMBL109882) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217821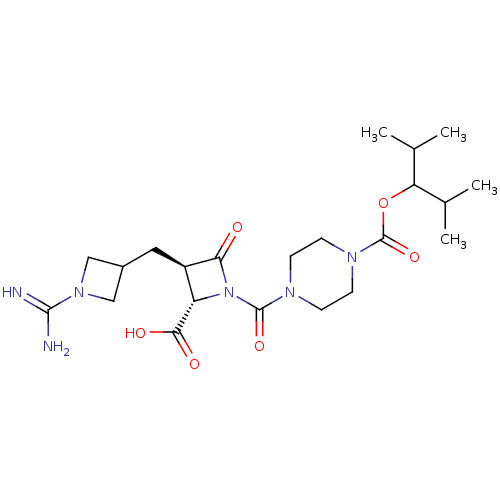 (CHEMBL109733) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217600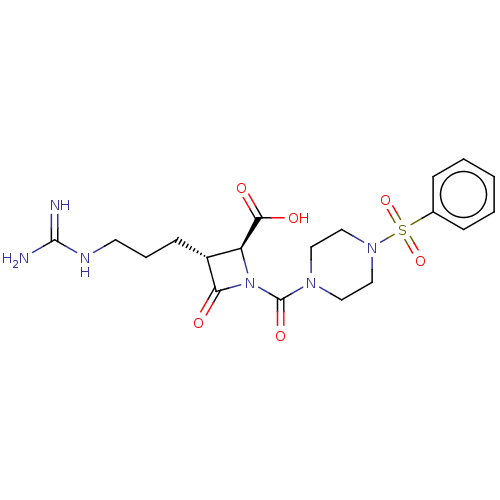 (CHEMBL109602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217808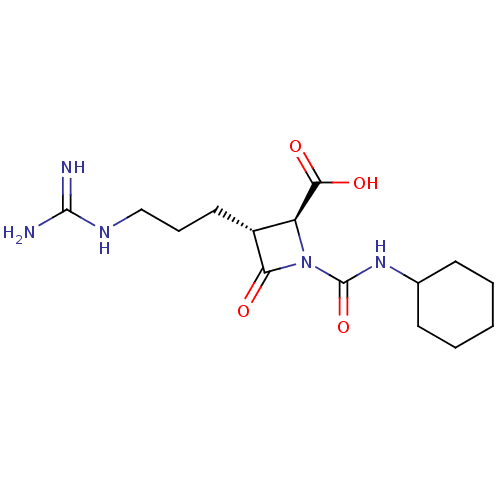 (CHEMBL111141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50220830 (CHEMBL306696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144531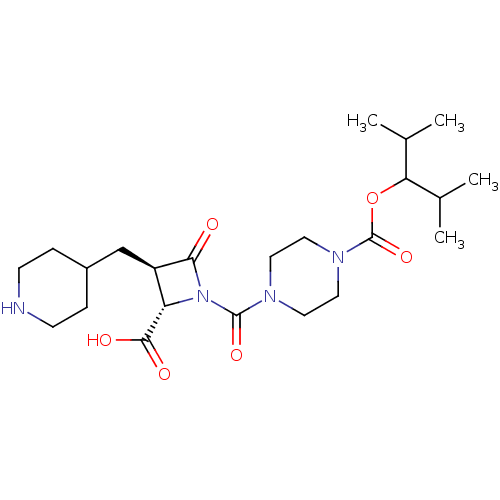 (4-((2S,3R)-2-Carboxy-4-oxo-3-piperidin-4-ylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM686 (BMS-186318 analog 23 | [1S-[1R*,2S*(2S*,3R*)]]-[3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50220737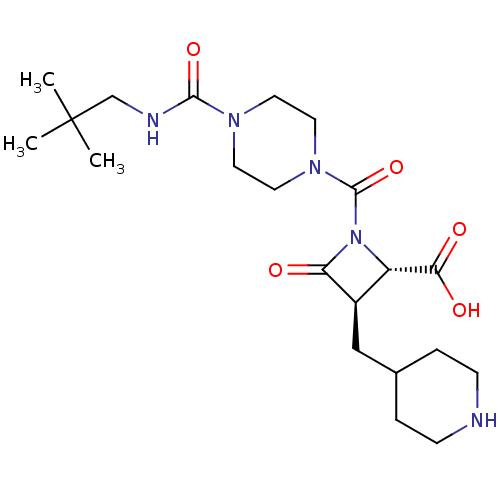 (CHEMBL308109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50221047 (CHEMBL70529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50220837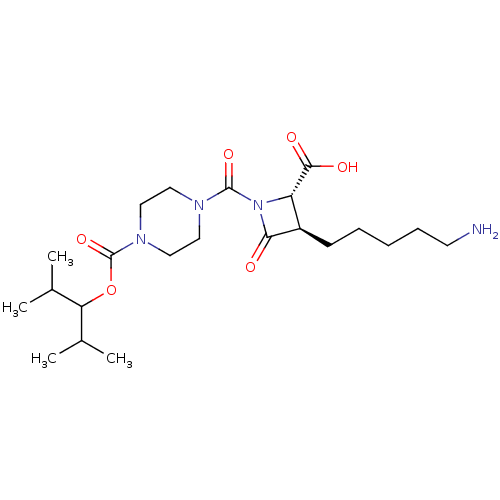 (CHEMBL70666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50220831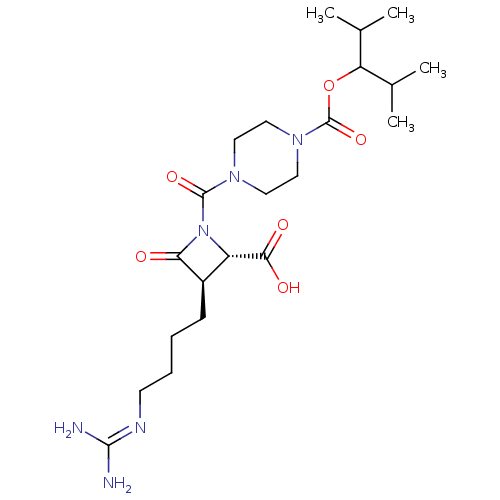 (CHEMBL70964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM680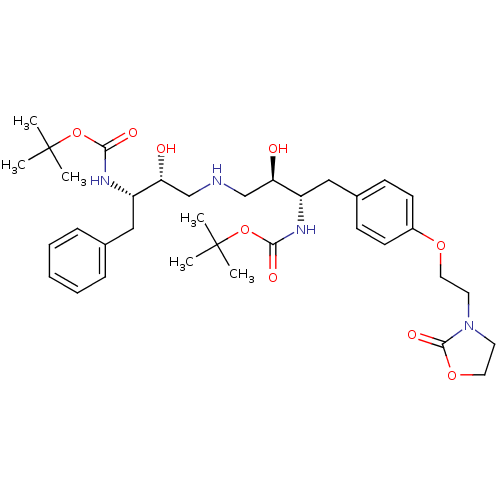 ((phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM679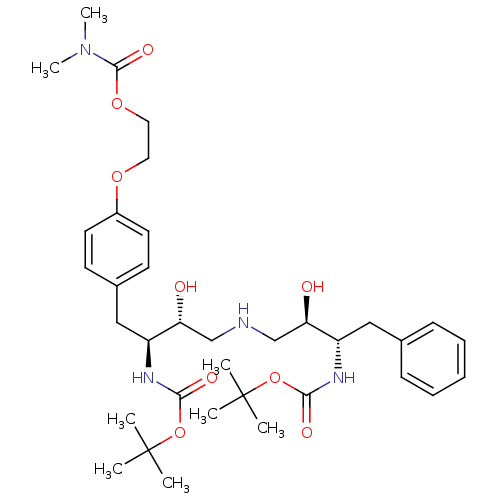 (BMS-186318 analog 16 | [1S-[1R*,2S*(2S*,3R*)]]-[3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM676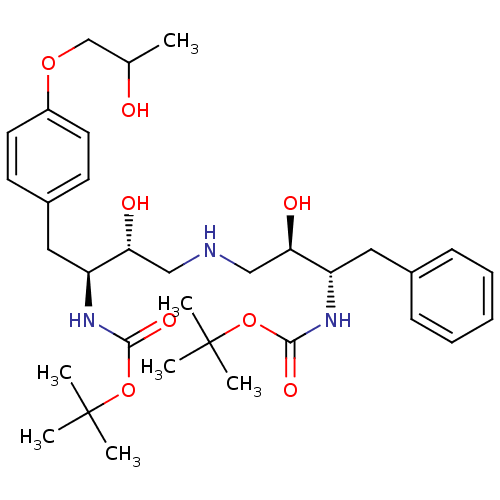 (BMS-186318 analog 13 | [1S-[1R*, 2S*(2S*, 3R*)]-[3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |