 Found 289 hits with Last Name = 'jeong' and Initial = 'jh'
Found 289 hits with Last Name = 'jeong' and Initial = 'jh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261110

(CHEMBL493982 | Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H33FN2O4/c1-3-35-29(34)32-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)33)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,32,34)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443584
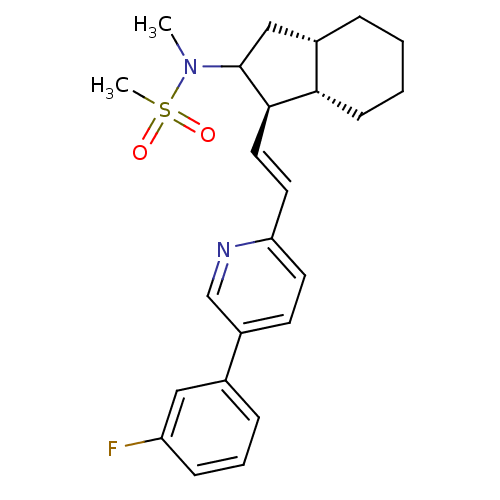
(CHEMBL3091980)Show SMILES CN(C1C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1)S(C)(=O)=O |r| Show InChI InChI=1S/C24H29FN2O2S/c1-27(30(2,28)29)24-15-18-6-3-4-9-22(18)23(24)13-12-21-11-10-19(16-26-21)17-7-5-8-20(25)14-17/h5,7-8,10-14,16,18,22-24H,3-4,6,9,15H2,1-2H3/b13-12+/t18-,22-,23+,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261110

(CHEMBL493982 | Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H33FN2O4/c1-3-35-29(34)32-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)33)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,32,34)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 in washed human platelets assessed as inhibition of haTRAP-induced platelet aggregation preincubated for 1 hr followed by... |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443584

(CHEMBL3091980)Show SMILES CN(C1C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1)S(C)(=O)=O |r| Show InChI InChI=1S/C24H29FN2O2S/c1-27(30(2,28)29)24-15-18-6-3-4-9-22(18)23(24)13-12-21-11-10-19(16-26-21)17-7-5-8-20(25)14-17/h5,7-8,10-14,16,18,22-24H,3-4,6,9,15H2,1-2H3/b13-12+/t18-,22-,23+,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 in washed human platelets assessed as inhibition of haTRAP-induced platelet aggregation preincubated for 1 hr followed by... |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443580

(CHEMBL3091993)Show SMILES CC1(C[C@@H]2CCCC[C@@H]2[C@H]1C=Cc1ccc(cn1)-c1cccc(F)c1)OC(N)=O |r,w:11.13| Show InChI InChI=1S/C24H27FN2O2/c1-24(29-23(26)28)14-17-5-2-3-8-21(17)22(24)12-11-20-10-9-18(15-27-20)16-6-4-7-19(25)13-16/h4,6-7,9-13,15,17,21-22H,2-3,5,8,14H2,1H3,(H2,26,28)/t17-,21-,22+,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443580

(CHEMBL3091993)Show SMILES CC1(C[C@@H]2CCCC[C@@H]2[C@H]1C=Cc1ccc(cn1)-c1cccc(F)c1)OC(N)=O |r,w:11.13| Show InChI InChI=1S/C24H27FN2O2/c1-24(29-23(26)28)14-17-5-2-3-8-21(17)22(24)12-11-20-10-9-18(15-27-20)16-6-4-7-19(25)13-16/h4,6-7,9-13,15,17,21-22H,2-3,5,8,14H2,1H3,(H2,26,28)/t17-,21-,22+,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 in washed human platelets assessed as inhibition of haTRAP-induced platelet aggregation preincubated for 1 hr followed by... |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50464556
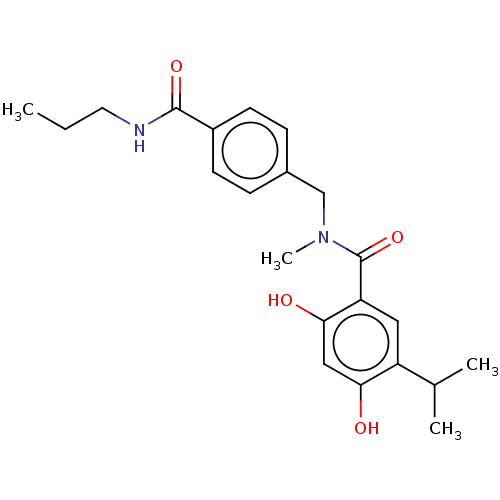
(CHEMBL4289811 | US10464907, Compound 21f | US10889...)Show SMILES CCCNC(=O)c1ccc(CN(C)C(=O)c2cc(C(C)C)c(O)cc2O)cc1 Show InChI InChI=1S/C22H28N2O4/c1-5-10-23-21(27)16-8-6-15(7-9-16)13-24(4)22(28)18-11-17(14(2)3)19(25)12-20(18)26/h6-9,11-12,14,25-26H,5,10,13H2,1-4H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-geldanamycin binding to N-terminal domain of recombinant full-length HSP90alpha (unknown origin) after 16 hrs by fluorescence pola... |
Eur J Med Chem 143: 390-401 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.054
BindingDB Entry DOI: 10.7270/Q29C713M |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443581

(CHEMBL3091991)Show SMILES CN(C1C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1)C(C)=O |r| Show InChI InChI=1S/C25H29FN2O/c1-17(29)28(2)25-15-19-6-3-4-9-23(19)24(25)13-12-22-11-10-20(16-27-22)18-7-5-8-21(26)14-18/h5,7-8,10-14,16,19,23-25H,3-4,6,9,15H2,1-2H3/b13-12+/t19-,23-,24+,25?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443586
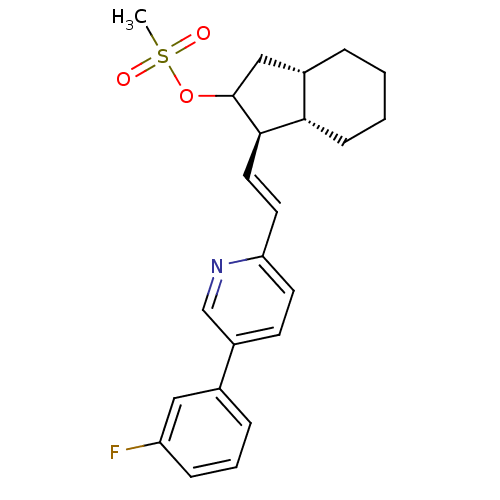
(CHEMBL3091975)Show SMILES CS(=O)(=O)OC1C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C23H26FNO3S/c1-29(26,27)28-23-14-17-5-2-3-8-21(17)22(23)12-11-20-10-9-18(15-25-20)16-6-4-7-19(24)13-16/h4,6-7,9-13,15,17,21-23H,2-3,5,8,14H2,1H3/b12-11+/t17-,21-,22+,23?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 in washed human platelets assessed as inhibition of haTRAP-induced platelet aggregation preincubated for 1 hr followed by... |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443586

(CHEMBL3091975)Show SMILES CS(=O)(=O)OC1C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C23H26FNO3S/c1-29(26,27)28-23-14-17-5-2-3-8-21(17)22(23)12-11-20-10-9-18(15-25-20)16-6-4-7-19(24)13-16/h4,6-7,9-13,15,17,21-23H,2-3,5,8,14H2,1H3/b12-11+/t17-,21-,22+,23?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50464557
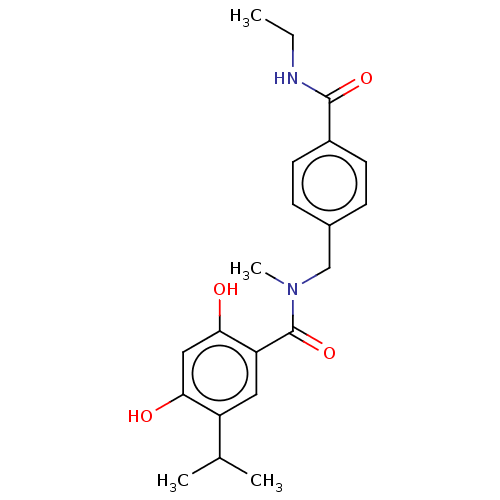
(CHEMBL4279132 | US10464907, Compound 21e | US10889...)Show SMILES CCNC(=O)c1ccc(CN(C)C(=O)c2cc(C(C)C)c(O)cc2O)cc1 Show InChI InChI=1S/C21H26N2O4/c1-5-22-20(26)15-8-6-14(7-9-15)12-23(4)21(27)17-10-16(13(2)3)18(24)11-19(17)25/h6-11,13,24-25H,5,12H2,1-4H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-geldanamycin binding to N-terminal domain of recombinant full-length HSP90alpha (unknown origin) after 16 hrs by fluorescence pola... |
Eur J Med Chem 143: 390-401 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.054
BindingDB Entry DOI: 10.7270/Q29C713M |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443583
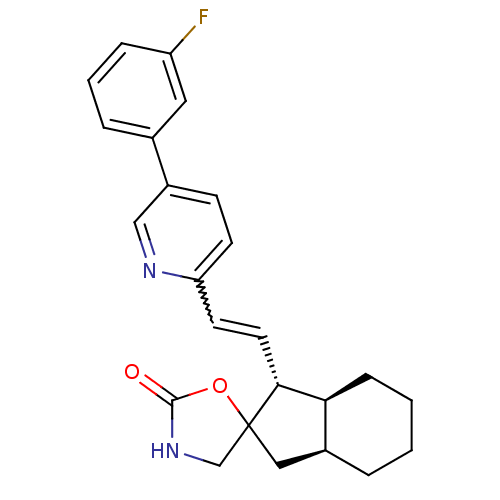
(CHEMBL3091981)Show SMILES Fc1cccc(c1)-c1ccc(C=C[C@@H]2[C@H]3CCCC[C@H]3CC22CNC(=O)O2)nc1 |r,w:11.11| Show InChI InChI=1S/C24H25FN2O2/c25-19-6-3-5-16(12-19)18-8-9-20(26-14-18)10-11-22-21-7-2-1-4-17(21)13-24(22)15-27-23(28)29-24/h3,5-6,8-12,14,17,21-22H,1-2,4,7,13,15H2,(H,27,28)/t17-,21-,22+,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at PAR1 in washed human platelets assessed as inhibition of haTRAP-induced platelet aggregation preincubated for 1 hr followed by... |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072672
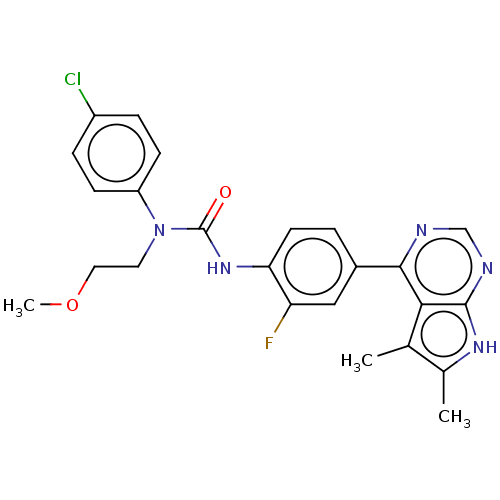
(CHEMBL3410056)Show SMILES COCCN(C(=O)Nc1ccc(cc1F)-c1ncnc2[nH]c(C)c(C)c12)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H23ClFN5O2/c1-14-15(2)29-23-21(14)22(27-13-28-23)16-4-9-20(19(26)12-16)30-24(32)31(10-11-33-3)18-7-5-17(25)6-8-18/h4-9,12-13H,10-11H2,1-3H3,(H,30,32)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443583

(CHEMBL3091981)Show SMILES Fc1cccc(c1)-c1ccc(C=C[C@@H]2[C@H]3CCCC[C@H]3CC22CNC(=O)O2)nc1 |r,w:11.11| Show InChI InChI=1S/C24H25FN2O2/c25-19-6-3-5-16(12-19)18-8-9-20(26-14-18)10-11-22-21-7-2-1-4-17(21)13-24(22)15-27-23(28)29-24/h3,5-6,8-12,14,17,21-22H,1-2,4,7,13,15H2,(H,27,28)/t17-,21-,22+,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50464562

(CHEMBL4293186 | US10464907, Compound 21d | US10889...)Show SMILES CNC(=O)c1ccc(CN(C)C(=O)c2cc(C(C)C)c(O)cc2O)cc1 Show InChI InChI=1S/C20H24N2O4/c1-12(2)15-9-16(18(24)10-17(15)23)20(26)22(4)11-13-5-7-14(8-6-13)19(25)21-3/h5-10,12,23-24H,11H2,1-4H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-geldanamycin binding to N-terminal domain of recombinant full-length HSP90alpha (unknown origin) after 16 hrs by fluorescence pola... |
Eur J Med Chem 143: 390-401 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.054
BindingDB Entry DOI: 10.7270/Q29C713M |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50008059
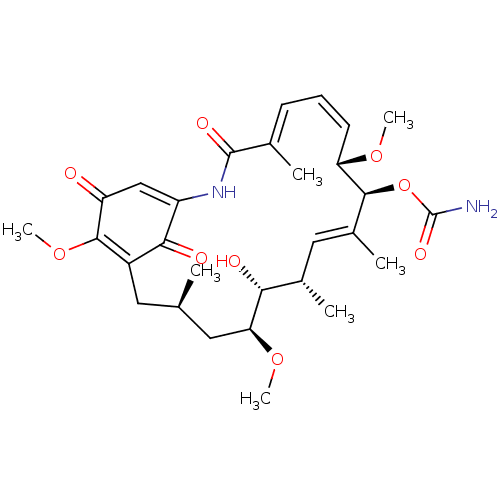
(CHEBI:5292 | GELDANAMYCIN)Show SMILES CO[C@H]1C[C@H](C)CC2=C(OC)C(=O)C=C(NC(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)C2=O |r,c:7,21,t:13,19,32| Show InChI InChI=1S/C29H40N2O9/c1-15-11-19-25(34)20(14-21(32)27(19)39-7)31-28(35)16(2)9-8-10-22(37-5)26(40-29(30)36)18(4)13-17(3)24(33)23(12-15)38-6/h8-10,13-15,17,22-24,26,33H,11-12H2,1-7H3,(H2,30,36)(H,31,35)/b10-8-,16-9+,18-13+/t15-,17+,22+,23+,24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University
Curated by ChEMBL
| Assay Description
Inhibition of FITC-geldanamycin binding to N-terminal domain of recombinant full-length HSP90alpha (unknown origin) after 16 hrs by fluorescence pola... |
Eur J Med Chem 143: 390-401 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.054
BindingDB Entry DOI: 10.7270/Q29C713M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50008059

(CHEBI:5292 | GELDANAMYCIN)Show SMILES CO[C@H]1C[C@H](C)CC2=C(OC)C(=O)C=C(NC(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)C2=O |r,c:7,21,t:13,19,32| Show InChI InChI=1S/C29H40N2O9/c1-15-11-19-25(34)20(14-21(32)27(19)39-7)31-28(35)16(2)9-8-10-22(37-5)26(40-29(30)36)18(4)13-17(3)24(33)23(12-15)38-6/h8-10,13-15,17,22-24,26,33H,11-12H2,1-7H3,(H2,30,36)(H,31,35)/b10-8-,16-9+,18-13+/t15-,17+,22+,23+,24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein isothiocyanate labeled geldanamycin binding to N-terminal domain of full length human recombinant Hsp90alpha expressed in E... |
Eur J Med Chem 124: 1069-1080 (2016)
Article DOI: 10.1016/j.ejmech.2016.10.038
BindingDB Entry DOI: 10.7270/Q29G5PZZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072662
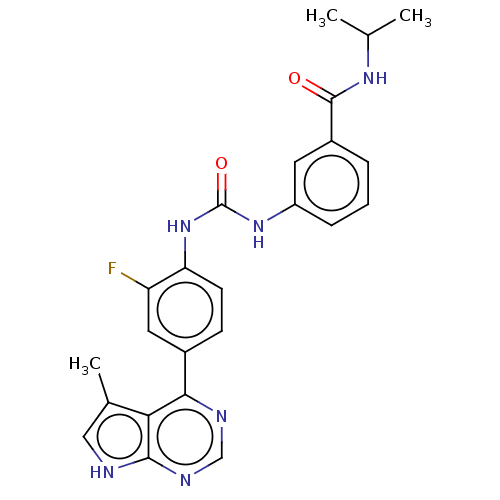
(CHEMBL3407526)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2F)-c2ncnc3[nH]cc(C)c23)c1 Show InChI InChI=1S/C24H23FN6O2/c1-13(2)29-23(32)16-5-4-6-17(9-16)30-24(33)31-19-8-7-15(10-18(19)25)21-20-14(3)11-26-22(20)28-12-27-21/h4-13H,1-3H3,(H,29,32)(H,26,27,28)(H2,30,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072671
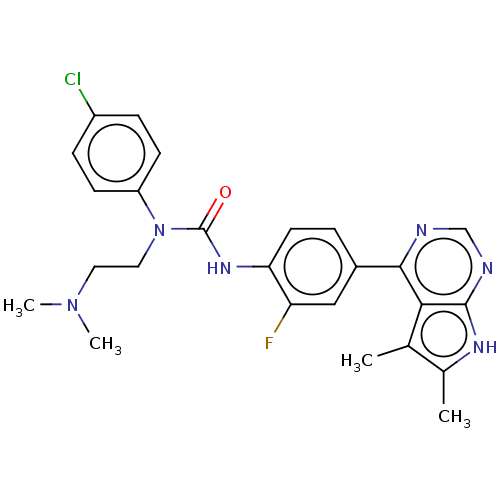
(CHEMBL3410055)Show SMILES CN(C)CCN(C(=O)Nc1ccc(cc1F)-c1ncnc2[nH]c(C)c(C)c12)c1ccc(Cl)cc1 Show InChI InChI=1S/C25H26ClFN6O/c1-15-16(2)30-24-22(15)23(28-14-29-24)17-5-10-21(20(27)13-17)31-25(34)33(12-11-32(3)4)19-8-6-18(26)7-9-19/h5-10,13-14H,11-12H2,1-4H3,(H,31,34)(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072728
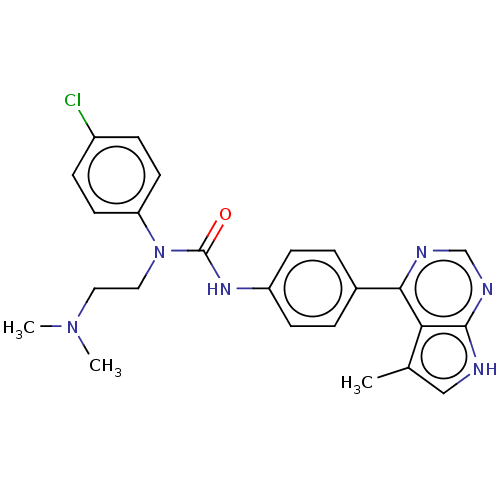
(CHEMBL3410052)Show SMILES CN(C)CCN(C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H25ClN6O/c1-16-14-26-23-21(16)22(27-15-28-23)17-4-8-19(9-5-17)29-24(32)31(13-12-30(2)3)20-10-6-18(25)7-11-20/h4-11,14-15H,12-13H2,1-3H3,(H,29,32)(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072730
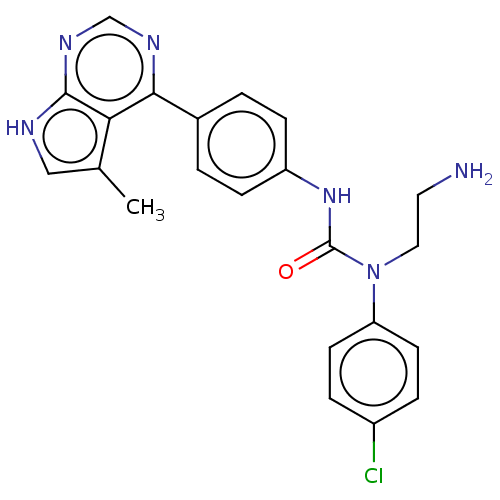
(CHEMBL3410050)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCN)c4ccc(Cl)cc4)cc3)c12 Show InChI InChI=1S/C22H21ClN6O/c1-14-12-25-21-19(14)20(26-13-27-21)15-2-6-17(7-3-15)28-22(30)29(11-10-24)18-8-4-16(23)5-9-18/h2-9,12-13H,10-11,24H2,1H3,(H,28,30)(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072670
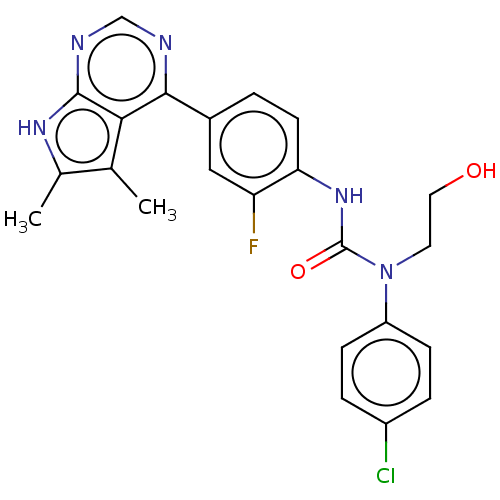
(CHEMBL3410053)Show SMILES Cc1[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(Cl)cc4)c(F)c3)c2c1C Show InChI InChI=1S/C23H21ClFN5O2/c1-13-14(2)28-22-20(13)21(26-12-27-22)15-3-8-19(18(25)11-15)29-23(32)30(9-10-31)17-6-4-16(24)5-7-17/h3-8,11-12,31H,9-10H2,1-2H3,(H,29,32)(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072727
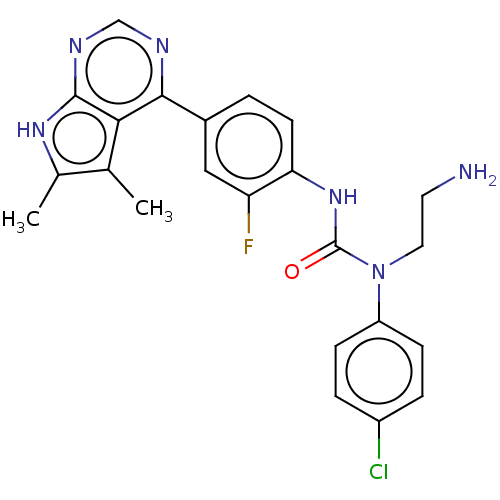
(CHEMBL3410054)Show SMILES Cc1[nH]c2ncnc(-c3ccc(NC(=O)N(CCN)c4ccc(Cl)cc4)c(F)c3)c2c1C Show InChI InChI=1S/C23H22ClFN6O/c1-13-14(2)29-22-20(13)21(27-12-28-22)15-3-8-19(18(25)11-15)30-23(32)31(10-9-26)17-6-4-16(24)5-7-17/h3-8,11-12H,9-10,26H2,1-2H3,(H,30,32)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072667
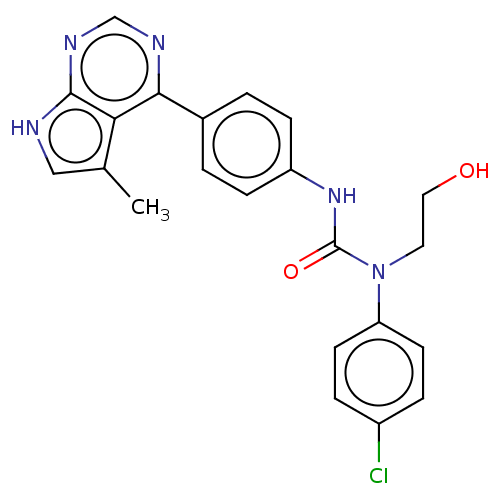
(CHEMBL3410042)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(Cl)cc4)cc3)c12 Show InChI InChI=1S/C22H20ClN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-2-6-17(7-3-15)27-22(30)28(10-11-29)18-8-4-16(23)5-9-18/h2-9,12-13,29H,10-11H2,1H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443585

(CHEMBL3091994)Show SMILES Fc1cccc(c1)-c1ccc(\C=C\[C@@H]2[C@H]3CCCC[C@H]3CC22OCCO2)nc1 |r| Show InChI InChI=1S/C24H26FNO2/c25-20-6-3-5-17(14-20)19-8-9-21(26-16-19)10-11-23-22-7-2-1-4-18(22)15-24(23)27-12-13-28-24/h3,5-6,8-11,14,16,18,22-23H,1-2,4,7,12-13,15H2/b11-10+/t18-,22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072731
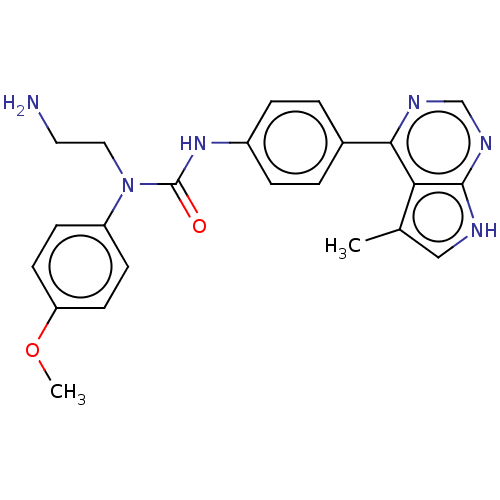
(CHEMBL3410049)Show SMILES COc1ccc(cc1)N(CCN)C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12 Show InChI InChI=1S/C23H24N6O2/c1-15-13-25-22-20(15)21(26-14-27-22)16-3-5-17(6-4-16)28-23(30)29(12-11-24)18-7-9-19(31-2)10-8-18/h3-10,13-14H,11-12,24H2,1-2H3,(H,28,30)(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50085442
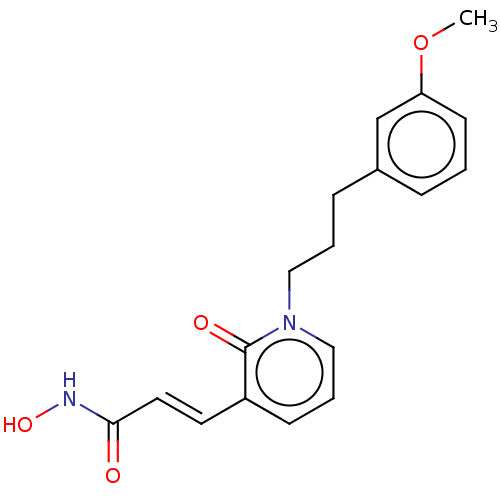
(CHEMBL3427664)Show InChI InChI=1S/C18H20N2O4/c1-24-16-8-2-5-14(13-16)6-3-11-20-12-4-7-15(18(20)22)9-10-17(21)19-23/h2,4-5,7-10,12-13,23H,3,6,11H2,1H3,(H,19,21)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using Fluor de Lys as substrate incubated with compound for 30 mins by microtiter-plate reading... |
J Med Chem 58: 3512-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00062
BindingDB Entry DOI: 10.7270/Q2GB25R3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50085318

(CHEMBL3427648)Show InChI InChI=1S/C18H15N3O3/c22-17(20-24)10-8-14-5-3-11-21(18(14)23)12-15-9-7-13-4-1-2-6-16(13)19-15/h1-11,24H,12H2,(H,20,22)/b10-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using Fluor de Lys as substrate incubated with compound for 30 mins by microtiter-plate reading... |
J Med Chem 58: 3512-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00062
BindingDB Entry DOI: 10.7270/Q2GB25R3 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443589

(CHEMBL3091992)Show SMILES CC1(O)C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C23H26FNO/c1-23(26)14-17-5-2-3-8-21(17)22(23)12-11-20-10-9-18(15-25-20)16-6-4-7-19(24)13-16/h4,6-7,9-13,15,17,21-22,26H,2-3,5,8,14H2,1H3/b12-11+/t17-,21-,22+,23?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072723
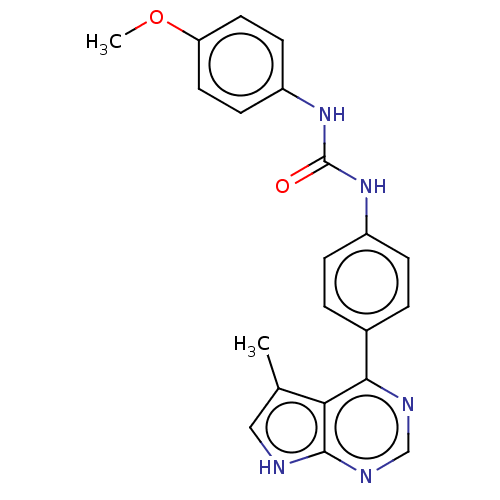
(CHEMBL3410032)Show SMILES COc1ccc(NC(=O)Nc2ccc(cc2)-c2ncnc3[nH]cc(C)c23)cc1 Show InChI InChI=1S/C21H19N5O2/c1-13-11-22-20-18(13)19(23-12-24-20)14-3-5-15(6-4-14)25-21(27)26-16-7-9-17(28-2)10-8-16/h3-12H,1-2H3,(H,22,23,24)(H2,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072668
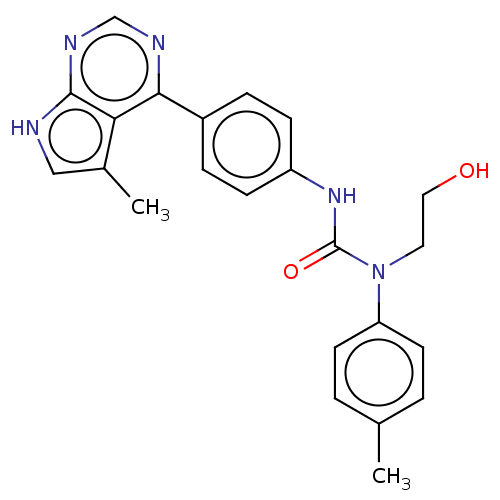
(CHEMBL3410045)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(C)cc4)cc3)c12 Show InChI InChI=1S/C23H23N5O2/c1-15-3-9-19(10-4-15)28(11-12-29)23(30)27-18-7-5-17(6-8-18)21-20-16(2)13-24-22(20)26-14-25-21/h3-10,13-14,29H,11-12H2,1-2H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072664
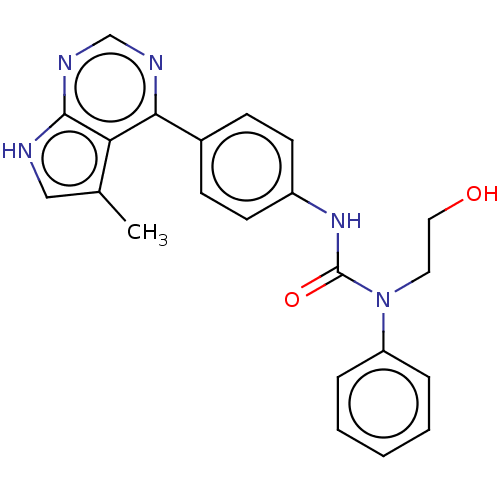
(CHEMBL3410036)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccccc4)cc3)c12 Show InChI InChI=1S/C22H21N5O2/c1-15-13-23-21-19(15)20(24-14-25-21)16-7-9-17(10-8-16)26-22(29)27(11-12-28)18-5-3-2-4-6-18/h2-10,13-14,28H,11-12H2,1H3,(H,26,29)(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443597
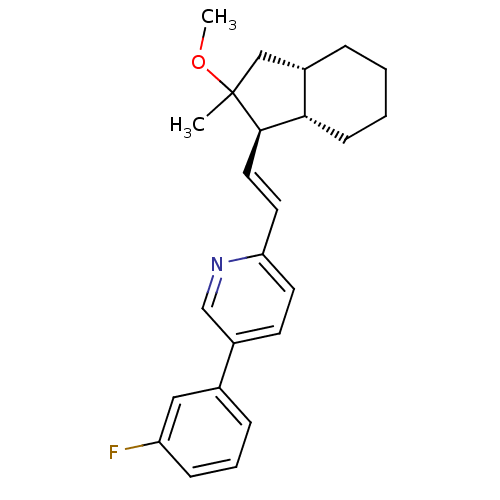
(CHEMBL3091982)Show SMILES COC1(C)C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C24H28FNO/c1-24(27-2)15-18-6-3-4-9-22(18)23(24)13-12-21-11-10-19(16-26-21)17-7-5-8-20(25)14-17/h5,7-8,10-14,16,18,22-23H,3-4,6,9,15H2,1-2H3/b13-12+/t18-,22-,23+,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50072675
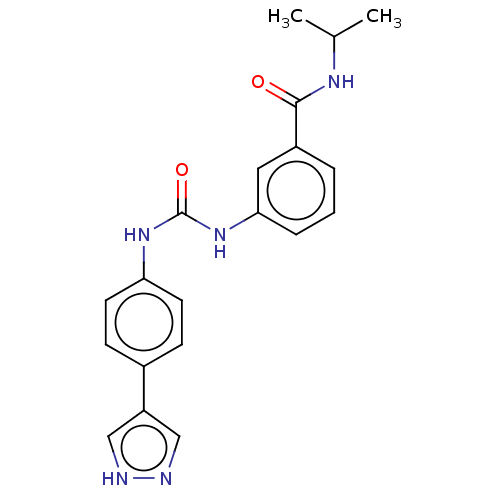
(CHEMBL3410022)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2cn[nH]c2)c1 Show InChI InChI=1S/C20H21N5O2/c1-13(2)23-19(26)15-4-3-5-18(10-15)25-20(27)24-17-8-6-14(7-9-17)16-11-21-22-12-16/h3-13H,1-2H3,(H,21,22)(H,23,26)(H2,24,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ROCK2 using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK peptide substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056799
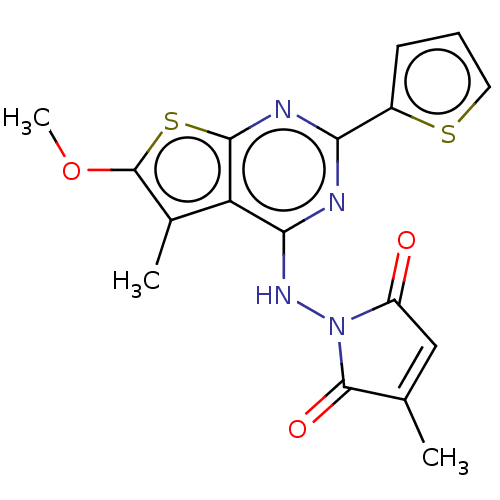
(CHEMBL3326029)Show SMILES COc1sc2nc(nc(NN3C(=O)C=C(C)C3=O)c2c1C)-c1cccs1 |t:13| Show InChI InChI=1S/C17H14N4O3S2/c1-8-7-11(22)21(16(8)23)20-14-12-9(2)17(24-3)26-15(12)19-13(18-14)10-5-4-6-25-10/h4-7H,1-3H3,(H,18,19,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT D835Y mutant using 10 uM ATP |
Eur J Med Chem 85: 399-407 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.001
BindingDB Entry DOI: 10.7270/Q2X35038 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072729
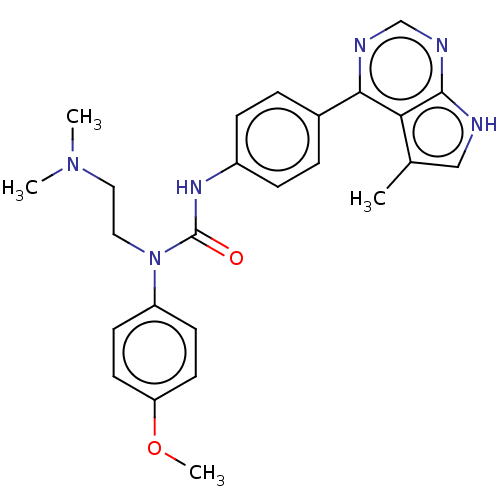
(CHEMBL3410051)Show SMILES COc1ccc(cc1)N(CCN(C)C)C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12 Show InChI InChI=1S/C25H28N6O2/c1-17-15-26-24-22(17)23(27-16-28-24)18-5-7-19(8-6-18)29-25(32)31(14-13-30(2)3)20-9-11-21(33-4)12-10-20/h5-12,15-16H,13-14H2,1-4H3,(H,29,32)(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443582
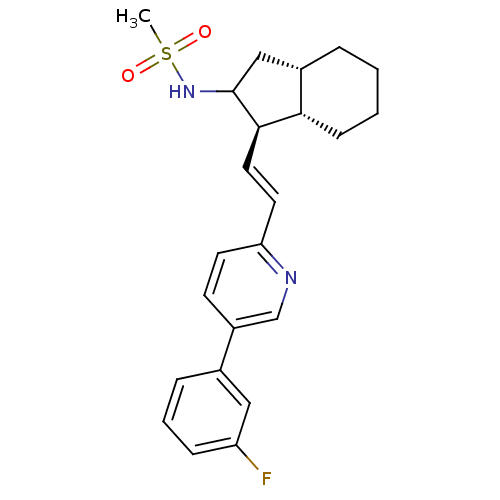
(CHEMBL3091989)Show SMILES CS(=O)(=O)NC1C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C23H27FN2O2S/c1-29(27,28)26-23-14-17-5-2-3-8-21(17)22(23)12-11-20-10-9-18(15-25-20)16-6-4-7-19(24)13-16/h4,6-7,9-13,15,17,21-23,26H,2-3,5,8,14H2,1H3/b12-11+/t17-,21-,22+,23?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50210933
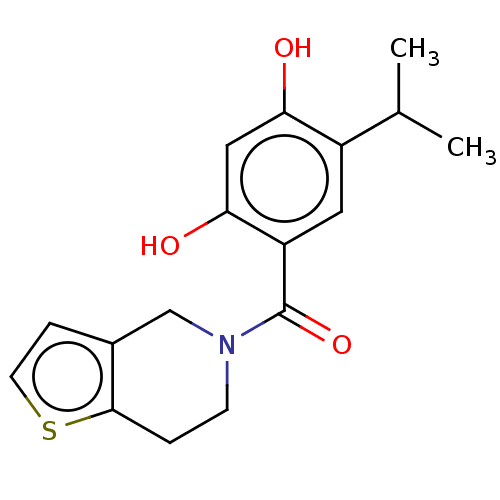
(CHEMBL3935883)Show InChI InChI=1S/C17H19NO3S/c1-10(2)12-7-13(15(20)8-14(12)19)17(21)18-5-3-16-11(9-18)4-6-22-16/h4,6-8,10,19-20H,3,5,9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein isothiocyanate labeled geldanamycin binding to N-terminal domain of full length human recombinant Hsp90alpha expressed in E... |
Eur J Med Chem 124: 1069-1080 (2016)
Article DOI: 10.1016/j.ejmech.2016.10.038
BindingDB Entry DOI: 10.7270/Q29G5PZZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50085444
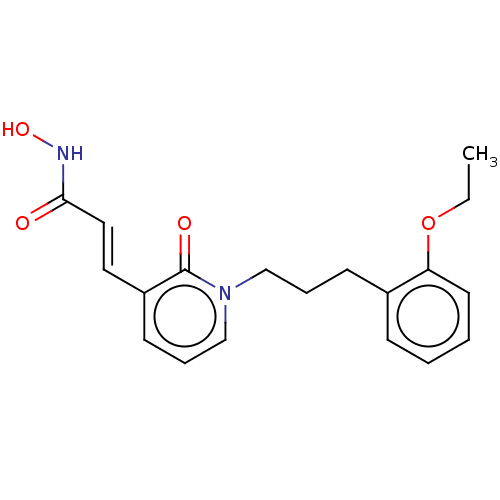
(CHEMBL3427666)Show InChI InChI=1S/C19H22N2O4/c1-2-25-17-10-4-3-7-15(17)8-5-13-21-14-6-9-16(19(21)23)11-12-18(22)20-24/h3-4,6-7,9-12,14,24H,2,5,8,13H2,1H3,(H,20,22)/b12-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using Fluor de Lys as substrate incubated with compound for 30 mins by microtiter-plate reading... |
J Med Chem 58: 3512-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00062
BindingDB Entry DOI: 10.7270/Q2GB25R3 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443598

(CHEMBL3091979)Show SMILES CC(=O)NC1C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C24H27FN2O/c1-16(28)27-24-14-18-5-2-3-8-22(18)23(24)12-11-21-10-9-19(15-26-21)17-6-4-7-20(25)13-17/h4,6-7,9-13,15,18,22-24H,2-3,5,8,14H2,1H3,(H,27,28)/b12-11+/t18-,22-,23+,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072669
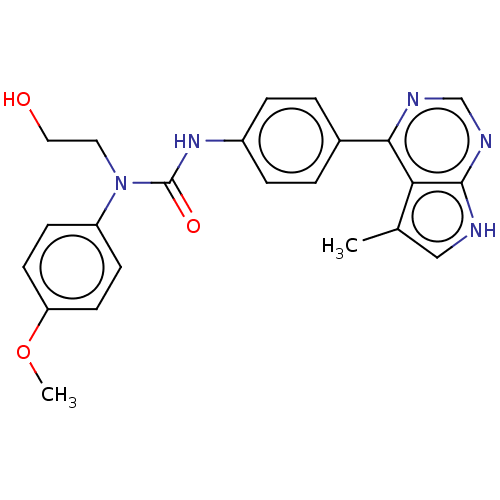
(CHEMBL3410048)Show SMILES COc1ccc(cc1)N(CCO)C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12 Show InChI InChI=1S/C23H23N5O3/c1-15-13-24-22-20(15)21(25-14-26-22)16-3-5-17(6-4-16)27-23(30)28(11-12-29)18-7-9-19(31-2)10-8-18/h3-10,13-14,29H,11-12H2,1-2H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056799

(CHEMBL3326029)Show SMILES COc1sc2nc(nc(NN3C(=O)C=C(C)C3=O)c2c1C)-c1cccs1 |t:13| Show InChI InChI=1S/C17H14N4O3S2/c1-8-7-11(22)21(16(8)23)20-14-12-9(2)17(24-3)26-15(12)19-13(18-14)10-5-4-6-25-10/h4-7H,1-3H3,(H,18,19,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using 10 uM ATP |
Eur J Med Chem 85: 399-407 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.001
BindingDB Entry DOI: 10.7270/Q2X35038 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072665
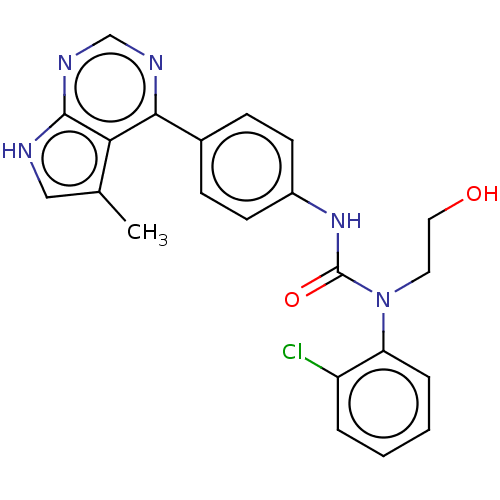
(CHEMBL3410040)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccccc4Cl)cc3)c12 Show InChI InChI=1S/C22H20ClN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-6-8-16(9-7-15)27-22(30)28(10-11-29)18-5-3-2-4-17(18)23/h2-9,12-13,29H,10-11H2,1H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072619
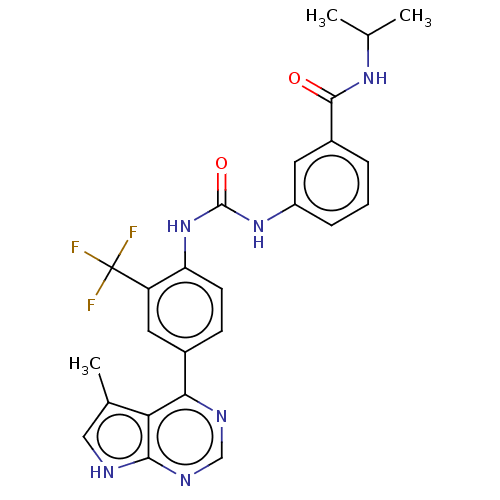
(CHEMBL3410026)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2C(F)(F)F)-c2ncnc3[nH]cc(C)c23)c1 Show InChI InChI=1S/C25H23F3N6O2/c1-13(2)32-23(35)16-5-4-6-17(9-16)33-24(36)34-19-8-7-15(10-18(19)25(26,27)28)21-20-14(3)11-29-22(20)31-12-30-21/h4-13H,1-3H3,(H,32,35)(H,29,30,31)(H2,33,34,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50364649
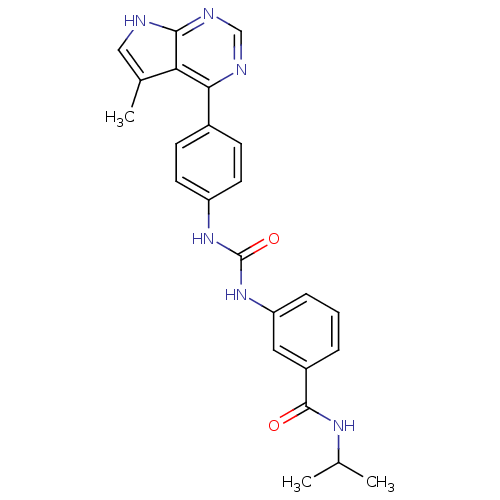
(CHEMBL1951346)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2ncnc3[nH]cc(C)c23)c1 Show InChI InChI=1S/C24H24N6O2/c1-14(2)28-23(31)17-5-4-6-19(11-17)30-24(32)29-18-9-7-16(8-10-18)21-20-15(3)12-25-22(20)27-13-26-21/h4-14H,1-3H3,(H,28,31)(H,25,26,27)(H2,29,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443594

(CHEMBL3091985)Show SMILES CC(=O)OC1C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C24H26FNO2/c1-16(27)28-24-14-18-5-2-3-8-22(18)23(24)12-11-21-10-9-19(15-26-21)17-6-4-7-20(25)13-17/h4,6-7,9-13,15,18,22-24H,2-3,5,8,14H2,1H3/b12-11+/t18-,22-,23+,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50443595
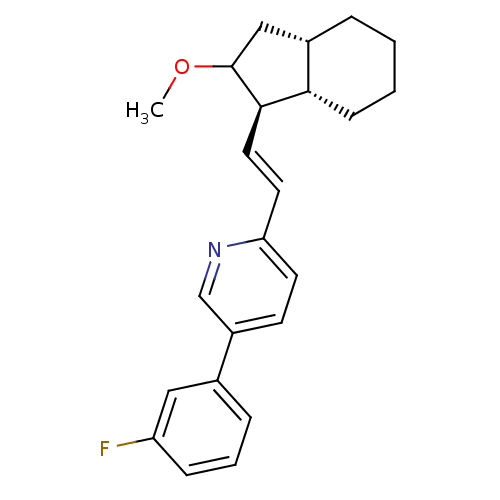
(CHEMBL3091984)Show SMILES COC1C[C@@H]2CCCC[C@@H]2[C@H]1\C=C\c1ccc(cn1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C23H26FNO/c1-26-23-14-17-5-2-3-8-21(17)22(23)12-11-20-10-9-18(15-25-20)16-6-4-7-19(24)13-16/h4,6-7,9-13,15,17,21-23H,2-3,5,8,14H2,1H3/b12-11+/t17-,21-,22+,23?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis |
ACS Med Chem Lett 4: 1054-8 (2013)
Article DOI: 10.1021/ml400235c
BindingDB Entry DOI: 10.7270/Q2KH0PSN |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072666
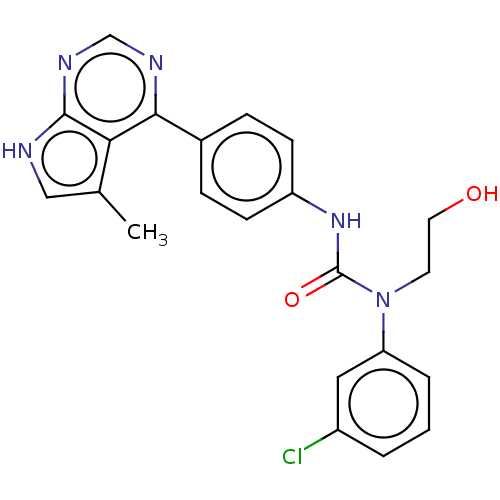
(CHEMBL3410041)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4cccc(Cl)c4)cc3)c12 Show InChI InChI=1S/C22H20ClN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-5-7-17(8-6-15)27-22(30)28(9-10-29)18-4-2-3-16(23)11-18/h2-8,11-13,29H,9-10H2,1H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056852
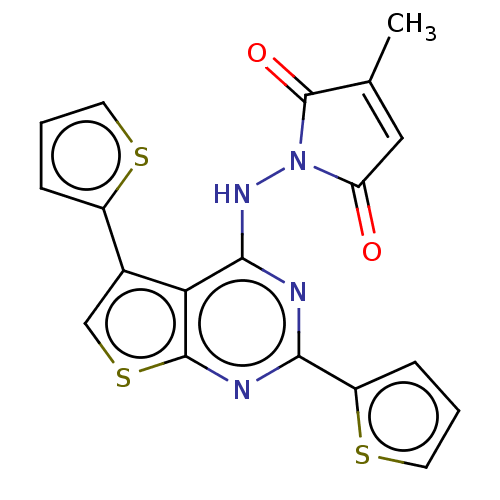
(CHEMBL3290618)Show SMILES CC1=CC(=O)N(Nc2nc(nc3scc(-c4cccs4)c23)-c2cccs2)C1=O |t:1| Show InChI InChI=1S/C19H12N4O2S3/c1-10-8-14(24)23(19(10)25)22-17-15-11(12-4-2-6-26-12)9-28-18(15)21-16(20-17)13-5-3-7-27-13/h2-9H,1H3,(H,20,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using 10 uM ATP |
Eur J Med Chem 85: 399-407 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.001
BindingDB Entry DOI: 10.7270/Q2X35038 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50085133
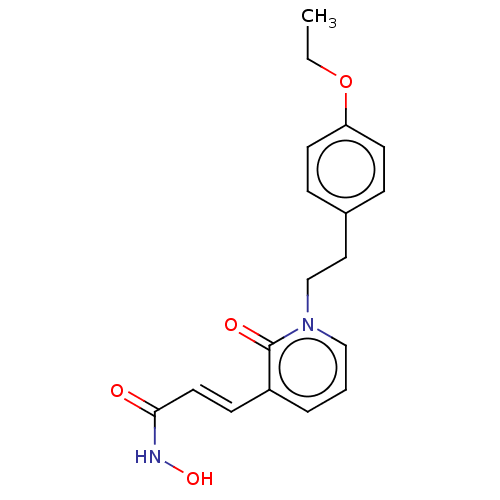
(CHEMBL3427656)Show InChI InChI=1S/C18H20N2O4/c1-2-24-16-8-5-14(6-9-16)11-13-20-12-3-4-15(18(20)22)7-10-17(21)19-23/h3-10,12,23H,2,11,13H2,1H3,(H,19,21)/b10-7+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using Fluor de Lys as substrate incubated with compound for 30 mins by microtiter-plate reading... |
J Med Chem 58: 3512-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00062
BindingDB Entry DOI: 10.7270/Q2GB25R3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data