 Found 3549 hits with Last Name = 'johnson' and Initial = 'b'
Found 3549 hits with Last Name = 'johnson' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
HIV-1 protease
(Human immunodeficiency virus) | BDBM36648
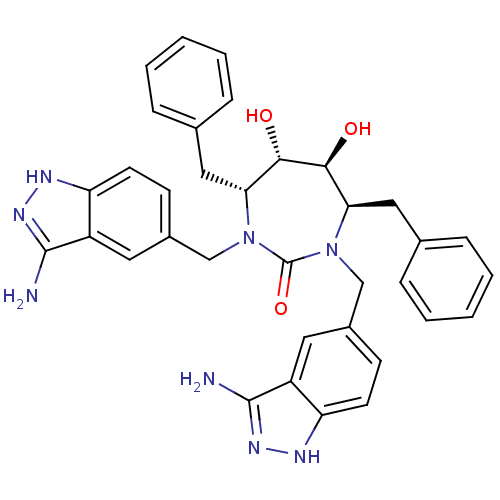
(3-alkylaminoindazole cyclic urea, (H))Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc(N)c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C35H36N8O3/c36-33-25-15-23(11-13-27(25)38-40-33)19-42-29(17-21-7-3-1-4-8-21)31(44)32(45)30(18-22-9-5-2-6-10-22)43(35(42)46)20-24-12-14-28-26(16-24)34(37)41-39-28/h1-16,29-32,44-45H,17-20H2,(H3,36,38,40)(H3,37,39,41)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50214385
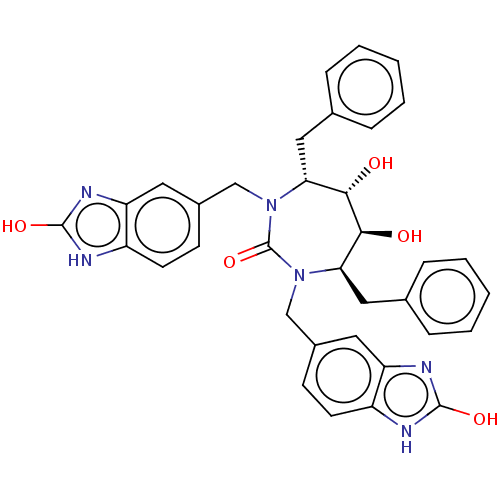
(CHEMBL316681)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(O)nc3c2)C(=O)N(Cc2ccc3[nH]c(O)nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O5/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)38-33(44)36-25)35(46)41(30(32(31)43)18-22-9-5-2-6-10-22)20-24-12-14-26-28(16-24)39-34(45)37-26/h1-16,29-32,42-43H,17-20H2,(H2,36,38,44)(H2,37,39,45)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I by using enzymatic assay at each of 6 substrate concentrations between 0.1 mM and... |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50073223
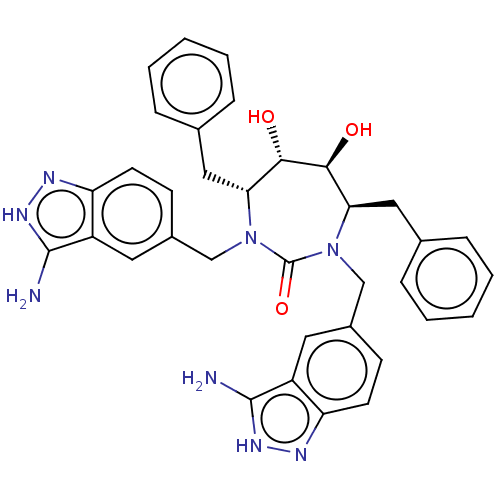
(CHEMBL73240)Show SMILES Nc1[nH]nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5n[nH]c(N)c5c4)C3=O)cc12 Show InChI InChI=1S/C41H60N6O6S/c1-26(2)36(44-40(51)53-23-31-25-54-39(42-31)27(3)4)38(50)43-33(18-28-12-9-8-10-13-28)35(48)21-47-17-16-46(20-34(47)37(49)45-41(5,6)7)19-29-14-11-15-30-22-52-24-32(29)30/h8-13,15,25-27,29,33-36,48H,14,16-24H2,1-7H3,(H,43,50)(H,44,51)(H,45,49)/t29?,33-,34-,35+,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36647
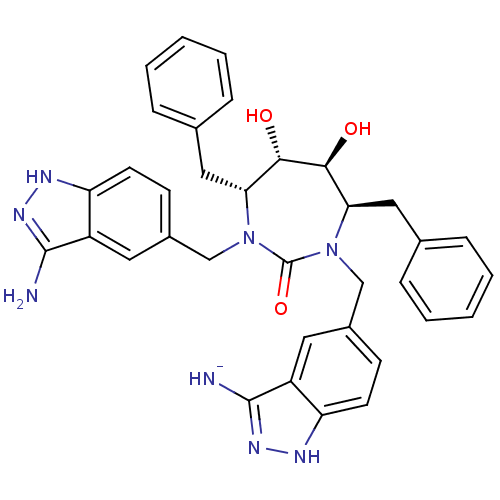
(3-Aminoindazole, 2)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc([NH-])c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C35H35N8O3/c36-33-25-15-23(11-13-27(25)38-40-33)19-42-29(17-21-7-3-1-4-8-21)31(44)32(45)30(18-22-9-5-2-6-10-22)43(35(42)46)20-24-12-14-28-26(16-24)34(37)41-39-28/h1-16,29-32,44-45H,17-20H2,(H5-,36,37,38,39,40,41)/q-1/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50214385

(CHEMBL316681)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(O)nc3c2)C(=O)N(Cc2ccc3[nH]c(O)nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O5/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)38-33(44)36-25)35(46)41(30(32(31)43)18-22-9-5-2-6-10-22)20-24-12-14-26-28(16-24)39-34(45)37-26/h1-16,29-32,42-43H,17-20H2,(H2,36,38,44)(H2,37,39,45)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36656

(Cyclobutylmethyl cyclic urea)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(CC4CCC4)C3=O)cc12 |r| Show InChI InChI=1S/C32H37N5O3/c33-31-25-16-24(14-15-26(25)34-35-31)20-37-28(18-22-10-5-2-6-11-22)30(39)29(38)27(17-21-8-3-1-4-9-21)36(32(37)40)19-23-12-7-13-23/h1-6,8-11,14-16,23,27-30,38-39H,7,12-13,17-20H2,(H3,33,34,35)/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288430

((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3c[nH]nc3c2)C(=O)N(Cc2ccc3c[nH]nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-27-19-36-38-29(27)15-25)35(44)41(22-26-12-14-28-20-37-39-30(28)16-26)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36649

(3-alkylaminoindazole cyclic urea, (Me))Show SMILES CNc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc(NC)c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C37H40N8O3/c1-38-35-27-17-25(13-15-29(27)40-42-35)21-44-31(19-23-9-5-3-6-10-23)33(46)34(47)32(20-24-11-7-4-8-12-24)45(37(44)48)22-26-14-16-30-28(18-26)36(39-2)43-41-30/h3-18,31-34,46-47H,19-22H2,1-2H3,(H2,38,40,42)(H2,39,41,43)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM161
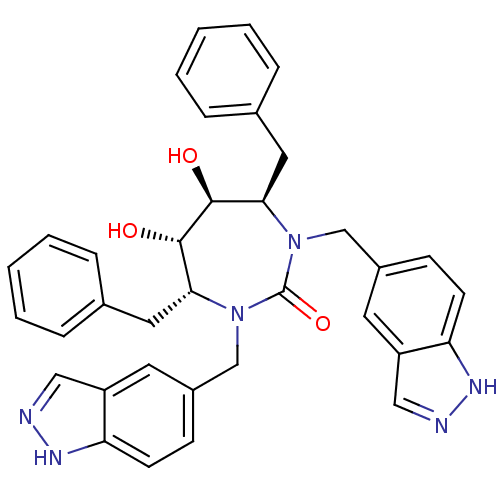
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM161

((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50069033
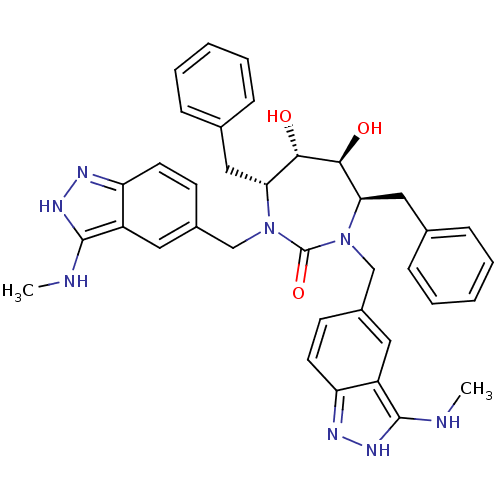
((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...)Show SMILES CNc1[nH]nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5n[nH]c(NC)c5c4)C3=O)cc12 Show InChI InChI=1S/C37H40N8O3/c1-38-35-27-17-25(13-15-29(27)40-42-35)21-44-31(19-23-9-5-3-6-10-23)33(46)34(47)32(20-24-11-7-4-8-12-24)45(37(44)48)22-26-14-16-30-28(18-26)36(39-2)43-41-30/h3-18,31-34,46-47H,19-22H2,1-2H3,(H2,38,40,42)(H2,39,41,43)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM161

((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36655
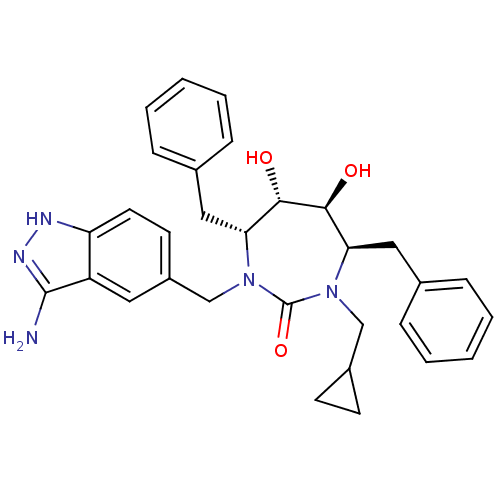
(Cyclopropylmethyl cyclic urea)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(CC4CC4)C3=O)cc12 |r| Show InChI InChI=1S/C31H35N5O3/c32-30-24-15-23(13-14-25(24)33-34-30)19-36-27(17-21-9-5-2-6-10-21)29(38)28(37)26(16-20-7-3-1-4-8-20)35(31(36)39)18-22-11-12-22/h1-10,13-15,22,26-29,37-38H,11-12,16-19H2,(H3,32,33,34)/t26-,27-,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM50124714
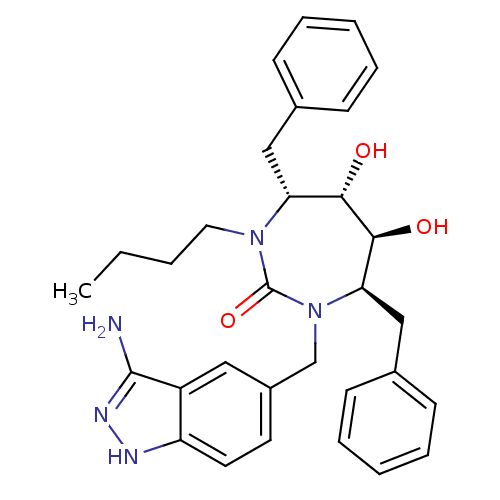
((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-4,...)Show SMILES CCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]nc(N)c3c2)C1=O Show InChI InChI=1S/C31H37N5O3/c1-2-3-16-35-26(18-21-10-6-4-7-11-21)28(37)29(38)27(19-22-12-8-5-9-13-22)36(31(35)39)20-23-14-15-25-24(17-23)30(32)34-33-25/h4-15,17,26-29,37-38H,2-3,16,18-20H2,1H3,(H3,32,33,34)/t26-,27-,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36657

(2-Naphthylmethyl cyclic urea)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5ccccc5c4)C3=O)cc12 |r| Show InChI InChI=1S/C38H37N5O3/c39-37-31-20-28(16-18-32(31)40-41-37)24-43-34(22-26-11-5-2-6-12-26)36(45)35(44)33(21-25-9-3-1-4-10-25)42(38(43)46)23-27-15-17-29-13-7-8-14-30(29)19-27/h1-20,33-36,44-45H,21-24H2,(H3,39,40,41)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50065064
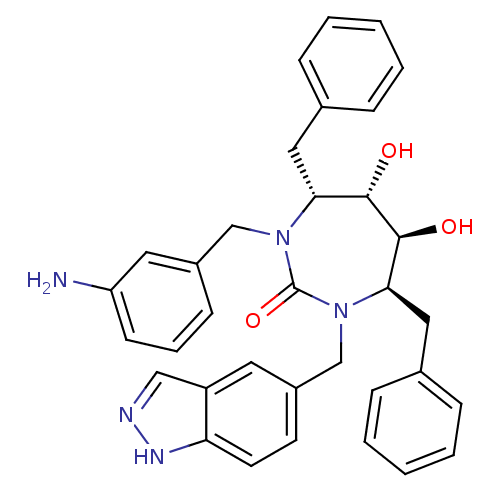
((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...)Show SMILES Nc1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3ccc4[nH]ncc4c3)C2=O)c1 Show InChI InChI=1S/C34H35N5O3/c35-28-13-7-12-25(17-28)21-38-30(18-23-8-3-1-4-9-23)32(40)33(41)31(19-24-10-5-2-6-11-24)39(34(38)42)22-26-14-15-29-27(16-26)20-36-37-29/h1-17,20,30-33,40-41H,18-19,21-22,35H2,(H,36,37)/t30-,31-,32+,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV -1 Protease |
Bioorg Med Chem Lett 9: 3217-20 (1999)
BindingDB Entry DOI: 10.7270/Q2B27TG8 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36646

(DMP850)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccccc4)C3=O)cc12 |r| Show InChI InChI=1S/C34H35N5O3/c35-33-27-18-26(16-17-28(27)36-37-33)22-39-30(20-24-12-6-2-7-13-24)32(41)31(40)29(19-23-10-4-1-5-11-23)38(34(39)42)21-25-14-8-3-9-15-25/h1-18,29-32,40-41H,19-22H2,(H3,35,36,37)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311157
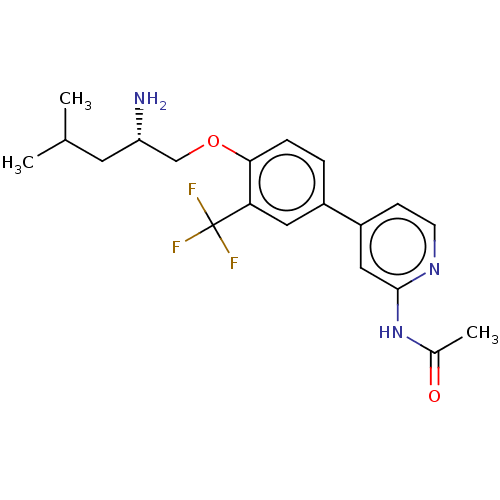
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-(trifl...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1C(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24F3N3O2/c1-12(2)8-16(24)11-28-18-5-4-14(9-17(18)20(21,22)23)15-6-7-25-19(10-15)26-13(3)27/h4-7,9-10,12,16H,8,11,24H2,1-3H3,(H,25,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311180
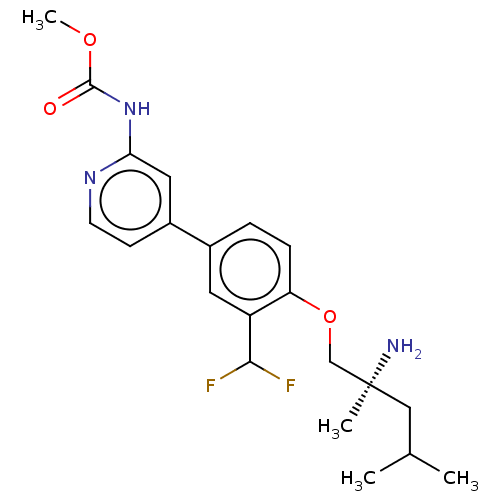
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C(F)F |r| Show InChI InChI=1S/C21H27F2N3O3/c1-13(2)11-21(3,24)12-29-17-6-5-14(9-16(17)19(22)23)15-7-8-25-18(10-15)26-20(27)28-4/h5-10,13,19H,11-12,24H2,1-4H3,(H,25,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311170
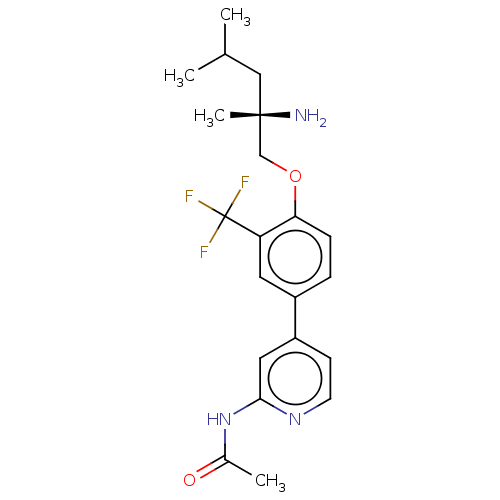
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(t...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1C(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H26F3N3O2/c1-13(2)11-20(4,25)12-29-18-6-5-15(9-17(18)21(22,23)24)16-7-8-26-19(10-16)27-14(3)28/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311155
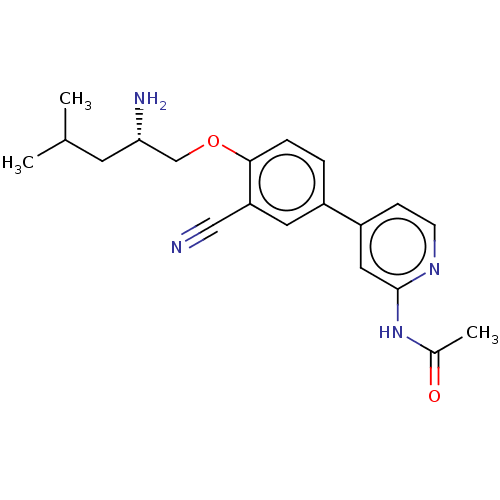
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-cyanop...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1C#N)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24N4O2/c1-13(2)8-18(22)12-26-19-5-4-15(9-17(19)11-21)16-6-7-23-20(10-16)24-14(3)25/h4-7,9-10,13,18H,8,12,22H2,1-3H3,(H,23,24,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311182

((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(OC(F)(F)F)c1 |r| Show InChI InChI=1S/C21H26F3N3O4/c1-13(2)11-20(3,25)12-30-16-6-5-14(9-17(16)31-21(22,23)24)15-7-8-26-18(10-15)27-19(28)29-4/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311158
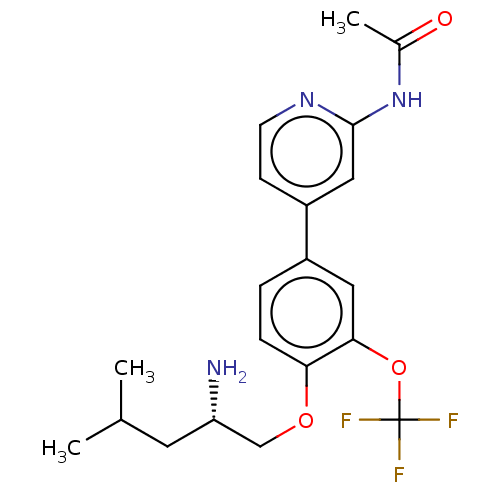
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-(trifl...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1OC(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24F3N3O3/c1-12(2)8-16(24)11-28-17-5-4-14(9-18(17)29-20(21,22)23)15-6-7-25-19(10-15)26-13(3)27/h4-7,9-10,12,16H,8,11,24H2,1-3H3,(H,25,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36658

(n-Pentyl cyclic urea)Show SMILES CCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]nc(N)c3c2)C1=O |r| Show InChI InChI=1S/C32H39N5O3/c1-2-3-10-17-36-27(19-22-11-6-4-7-12-22)29(38)30(39)28(20-23-13-8-5-9-14-23)37(32(36)40)21-24-15-16-26-25(18-24)31(33)35-34-26/h4-9,11-16,18,27-30,38-39H,2-3,10,17,19-21H2,1H3,(H3,33,34,35)/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36650
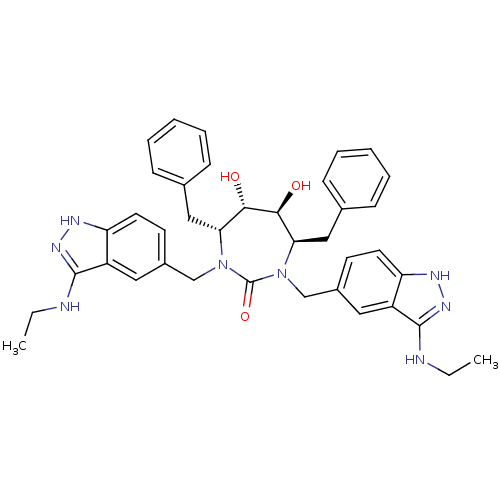
(3-alkylaminoindazole cyclic urea, (Et))Show SMILES CCNc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc(NCC)c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C39H44N8O3/c1-3-40-37-29-19-27(15-17-31(29)42-44-37)23-46-33(21-25-11-7-5-8-12-25)35(48)36(49)34(22-26-13-9-6-10-14-26)47(39(46)50)24-28-16-18-32-30(20-28)38(41-4-2)45-43-32/h5-20,33-36,48-49H,3-4,21-24H2,1-2H3,(H2,40,42,44)(H2,41,43,45)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50069029
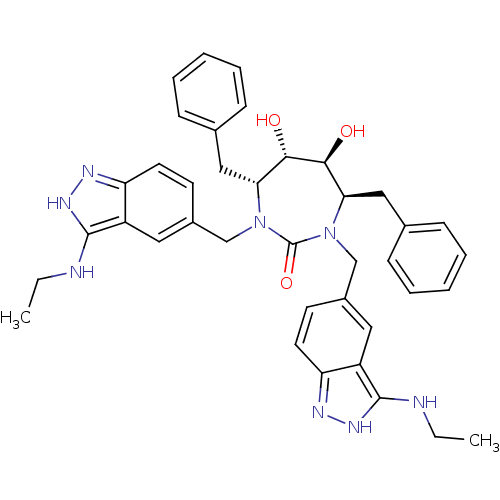
((4R,5S,6S,7R)-4,7-Dibenzyl-1,3-bis-(3-ethylamino-1...)Show SMILES CCNc1[nH]nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5n[nH]c(NCC)c5c4)C3=O)cc12 Show InChI InChI=1S/C39H44N8O3/c1-3-40-37-29-19-27(15-17-31(29)42-44-37)23-46-33(21-25-11-7-5-8-12-25)35(48)36(49)34(22-26-13-9-6-10-14-26)47(39(46)50)24-28-16-18-32-30(20-28)38(41-4-2)45-43-32/h5-20,33-36,48-49H,3-4,21-24H2,1-2H3,(H2,40,42,44)(H2,41,43,45)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311179
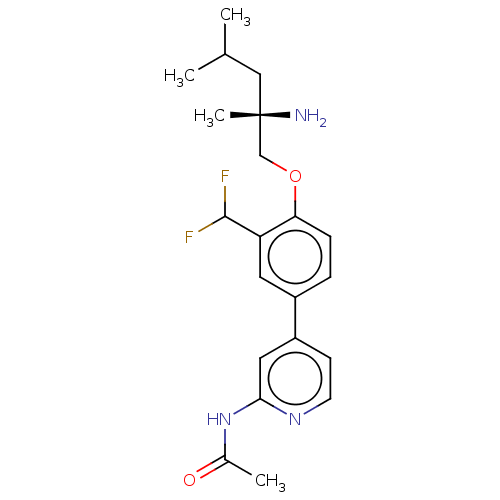
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(d...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1C(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H27F2N3O2/c1-13(2)11-21(4,24)12-28-18-6-5-15(9-17(18)20(22)23)16-7-8-25-19(10-16)26-14(3)27/h5-10,13,20H,11-12,24H2,1-4H3,(H,25,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311176
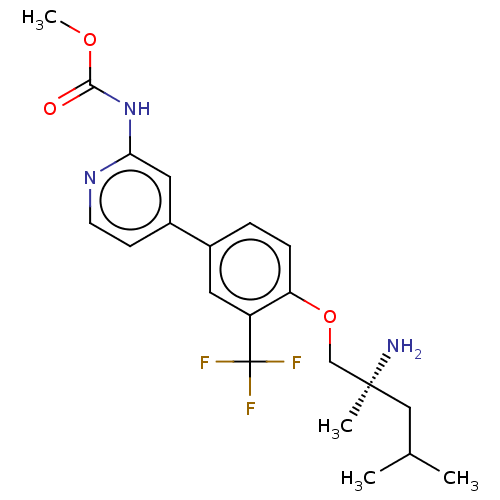
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H26F3N3O3/c1-13(2)11-20(3,25)12-30-17-6-5-14(9-16(17)21(22,23)24)15-7-8-26-18(10-15)27-19(28)29-4/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311189
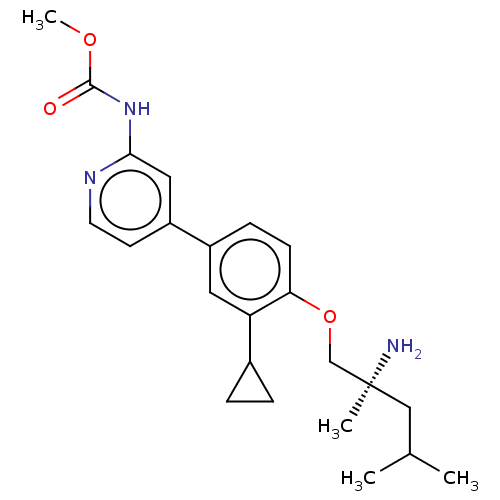
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C1CC1 |r| Show InChI InChI=1S/C23H31N3O3/c1-15(2)13-23(3,24)14-29-20-8-7-17(11-19(20)16-5-6-16)18-9-10-25-21(12-18)26-22(27)28-4/h7-12,15-16H,5-6,13-14,24H2,1-4H3,(H,25,26,27)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311268
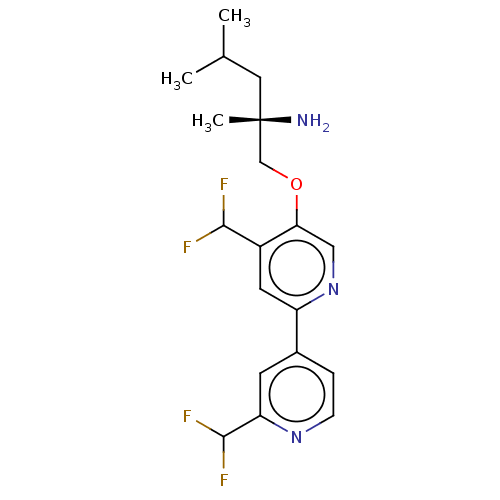
((S)-1-((2′,4-bis(difluoromethyl)-[2,4′...)Show SMILES CC(C)C[C@](C)(N)COc1cnc(cc1C(F)F)-c1ccnc(c1)C(F)F |r| Show InChI InChI=1S/C19H23F4N3O/c1-11(2)8-19(3,24)10-27-16-9-26-14(7-13(16)17(20)21)12-4-5-25-15(6-12)18(22)23/h4-7,9,11,17-18H,8,10,24H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50082401

((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...)Show SMILES Nc1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3ccc4[nH]nc(-c5cc[nH]n5)c4c3)C2=O)c1 Show InChI InChI=1S/C37H37N7O3/c38-28-13-7-12-26(18-28)22-43-32(20-24-8-3-1-4-9-24)35(45)36(46)33(21-25-10-5-2-6-11-25)44(37(43)47)23-27-14-15-30-29(19-27)34(42-41-30)31-16-17-39-40-31/h1-19,32-33,35-36,45-46H,20-23,38H2,(H,39,40)(H,41,42)/t32-,33-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV -1 Protease |
Bioorg Med Chem Lett 9: 3217-20 (1999)
BindingDB Entry DOI: 10.7270/Q2B27TG8 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311160
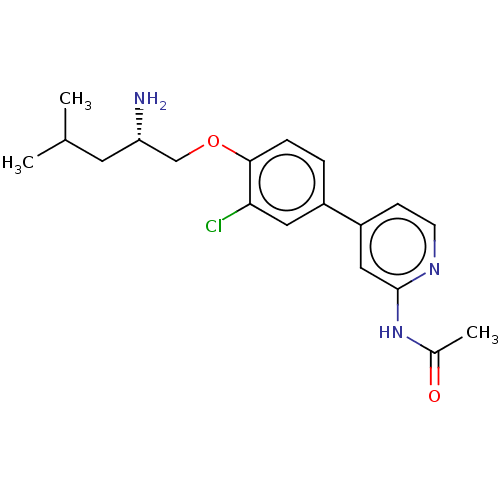
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-chloro...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1Cl)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C19H24ClN3O2/c1-12(2)8-16(21)11-25-18-5-4-14(9-17(18)20)15-6-7-22-19(10-15)23-13(3)24/h4-7,9-10,12,16H,8,11,21H2,1-3H3,(H,22,23,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311181
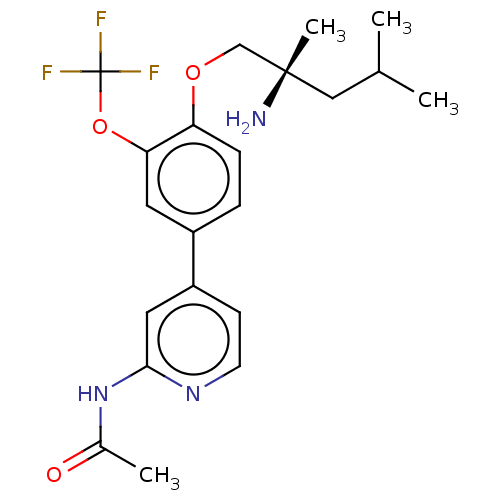
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(t...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1OC(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H26F3N3O3/c1-13(2)11-20(4,25)12-29-17-6-5-15(9-18(17)30-21(22,23)24)16-7-8-26-19(10-16)27-14(3)28/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311264
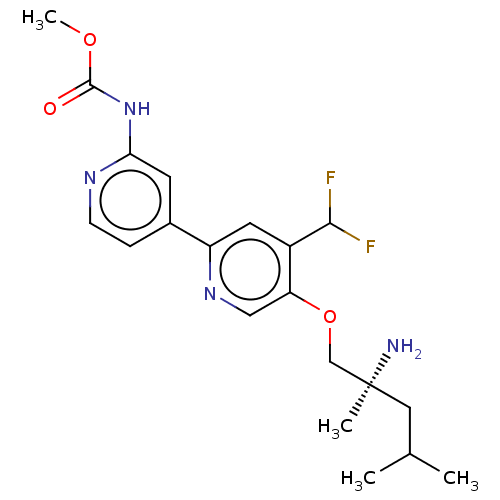
((S)-methyl (5-((2-amino-2,4-dimethylpentyl)oxy)-4-...)Show SMILES COC(=O)Nc1cc(ccn1)-c1cc(C(F)F)c(OC[C@@](C)(N)CC(C)C)cn1 |r| Show InChI InChI=1S/C20H26F2N4O3/c1-12(2)9-20(3,23)11-29-16-10-25-15(8-14(16)18(21)22)13-5-6-24-17(7-13)26-19(27)28-4/h5-8,10,12,18H,9,11,23H2,1-4H3,(H,24,26,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311261
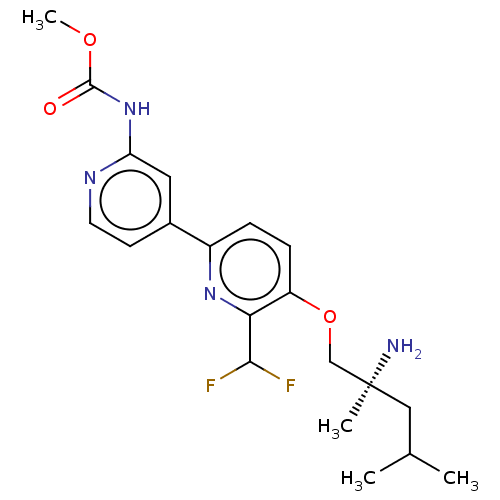
((S)-methyl (5-((2-amino-2,4-dimethylpentyl)oxy)-6-...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(n1)C(F)F |r| Show InChI InChI=1S/C20H26F2N4O3/c1-12(2)10-20(3,23)11-29-15-6-5-14(25-17(15)18(21)22)13-7-8-24-16(9-13)26-19(27)28-4/h5-9,12,18H,10-11,23H2,1-4H3,(H,24,26,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311178
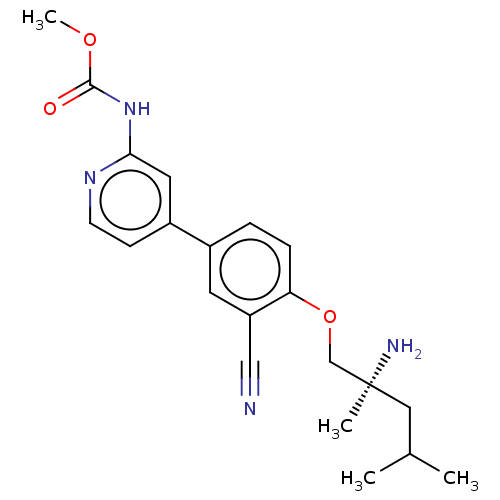
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C#N |r| Show InChI InChI=1S/C21H26N4O3/c1-14(2)11-21(3,23)13-28-18-6-5-15(9-17(18)12-22)16-7-8-24-19(10-16)25-20(26)27-4/h5-10,14H,11,13,23H2,1-4H3,(H,24,25,26)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311159
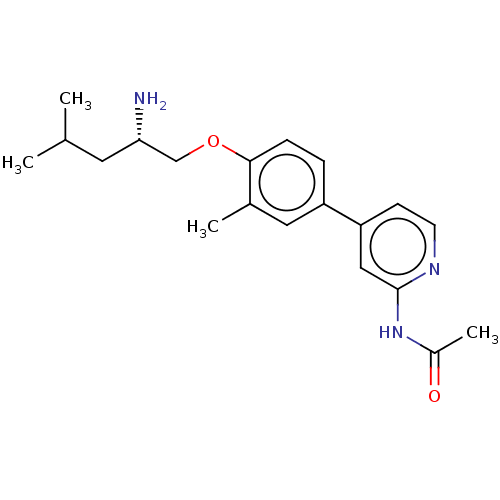
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-methyl...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1C)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)9-18(21)12-25-19-6-5-16(10-14(19)3)17-7-8-22-20(11-17)23-15(4)24/h5-8,10-11,13,18H,9,12,21H2,1-4H3,(H,22,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM50604135
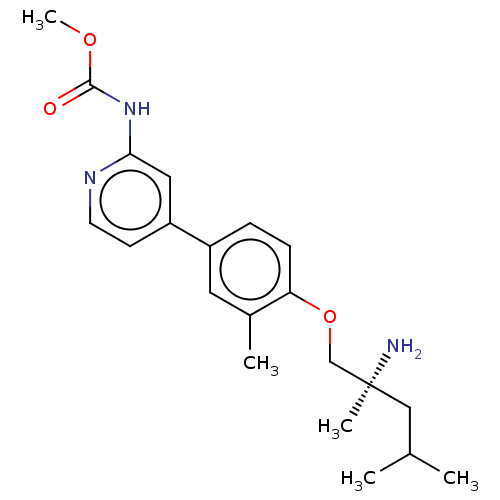
(CHEMBL5198877)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(C)c1 |r| | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311165
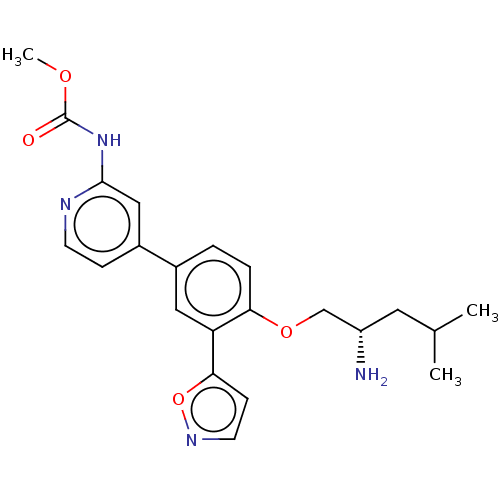
((S)-methyl (4-(4-((2-amino-4-methylpentyl)oxy)-3-(...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@H](N)CC(C)C)c(c1)-c1ccno1 |r| Show InChI InChI=1S/C22H26N4O4/c1-14(2)10-17(23)13-29-19-5-4-15(11-18(19)20-7-9-25-30-20)16-6-8-24-21(12-16)26-22(27)28-3/h4-9,11-12,14,17H,10,13,23H2,1-3H3,(H,24,26,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50069030

((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...)Show SMILES CC(C)Nc1[nH]nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5n[nH]c(NC(C)C)c5c4)C3=O)cc12 Show InChI InChI=1S/C41H48N8O3/c1-25(2)42-39-31-19-29(15-17-33(31)44-46-39)23-48-35(21-27-11-7-5-8-12-27)37(50)38(51)36(22-28-13-9-6-10-14-28)49(41(48)52)24-30-16-18-34-32(20-30)40(47-45-34)43-26(3)4/h5-20,25-26,35-38,50-51H,21-24H2,1-4H3,(H2,42,44,46)(H2,43,45,47)/t35-,36-,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36651
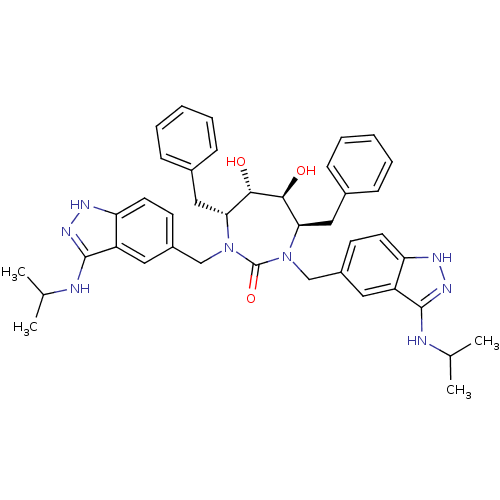
(3-alkylaminoindazole cyclic urea, (i-Pr))Show SMILES CC(C)Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc(NC(C)C)c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C41H48N8O3/c1-25(2)42-39-31-19-29(15-17-33(31)44-46-39)23-48-35(21-27-11-7-5-8-12-27)37(50)38(51)36(22-28-13-9-6-10-14-28)49(41(48)52)24-30-16-18-34-32(20-30)40(47-45-34)43-26(3)4/h5-20,25-26,35-38,50-51H,21-24H2,1-4H3,(H2,42,44,46)(H2,43,45,47)/t35-,36-,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50082400
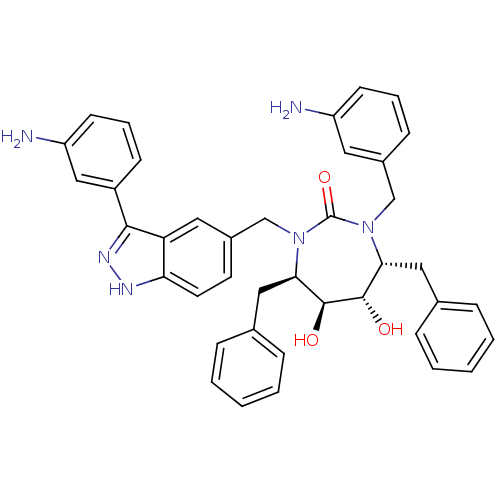
((4R,5S,6S,7R)-1-(3-Amino-benzyl)-3-[3-(3-amino-phe...)Show SMILES Nc1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3ccc4[nH]nc(-c5cccc(N)c5)c4c3)C2=O)c1 Show InChI InChI=1S/C40H40N6O3/c41-31-15-7-13-28(19-31)24-45-35(21-26-9-3-1-4-10-26)38(47)39(48)36(22-27-11-5-2-6-12-27)46(40(45)49)25-29-17-18-34-33(20-29)37(44-43-34)30-14-8-16-32(42)23-30/h1-20,23,35-36,38-39,47-48H,21-22,24-25,41-42H2,(H,43,44)/t35-,36-,38+,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV -1 Protease |
Bioorg Med Chem Lett 9: 3217-20 (1999)
BindingDB Entry DOI: 10.7270/Q2B27TG8 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM50604134
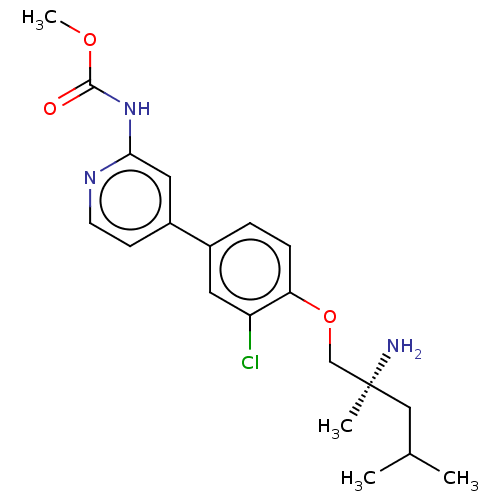
(CHEMBL5187778)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(Cl)c1 |r| | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288424

((4R,5S,6S,7R)-1,3-Bis-(1H-benzotriazol-5-ylmethyl)...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3nn[nH]c3c2)C(=O)N(Cc2ccc3nn[nH]c3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C33H32N8O3/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)36-38-34-25)33(44)41(20-24-12-14-26-28(16-24)37-39-35-26)30(32(31)43)18-22-9-5-2-6-10-22/h1-16,29-32,42-43H,17-20H2,(H,34,36,38)(H,35,37,39)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311177
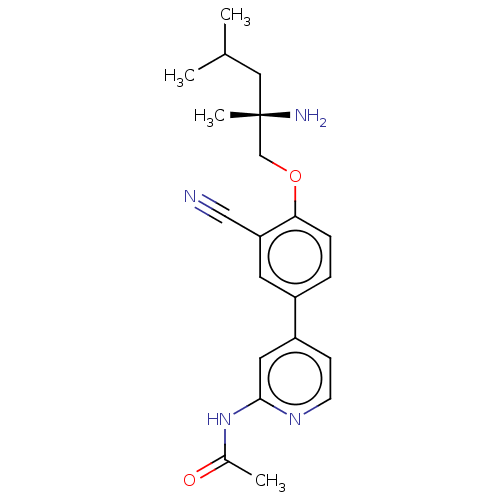
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-cy...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1C#N)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H26N4O2/c1-14(2)11-21(4,23)13-27-19-6-5-16(9-18(19)12-22)17-7-8-24-20(10-17)25-15(3)26/h5-10,14H,11,13,23H2,1-4H3,(H,24,25,26)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311380
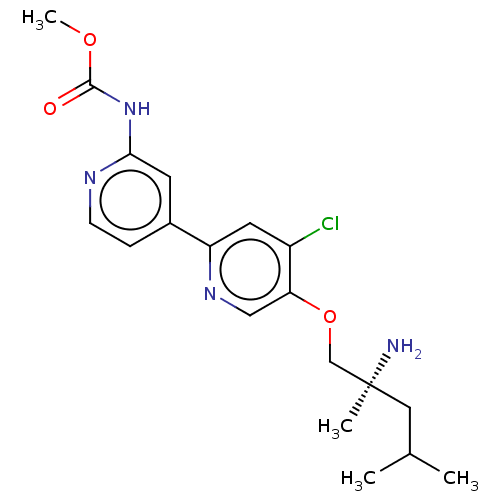
((S)-methyl (5-((2-amino-2,4-dimethylpentyl)oxy)-4-...)Show SMILES COC(=O)Nc1cc(ccn1)-c1cc(Cl)c(OC[C@@](C)(N)CC(C)C)cn1 |r| Show InChI InChI=1S/C19H25ClN4O3/c1-12(2)9-19(3,21)11-27-16-10-23-15(8-14(16)20)13-5-6-22-17(7-13)24-18(25)26-4/h5-8,10,12H,9,11,21H2,1-4H3,(H,22,24,25)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM50604141
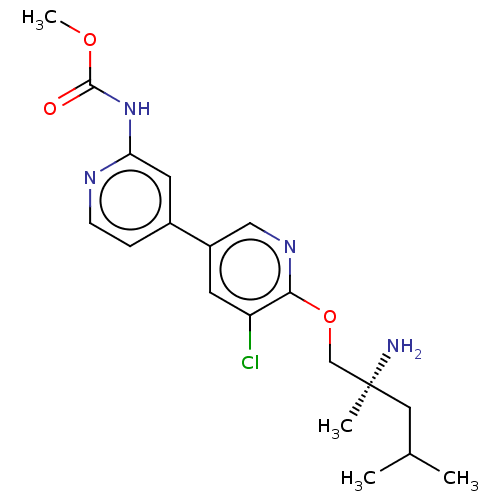
(CHEMBL5188433)Show SMILES COC(=O)Nc1cc(ccn1)-c1cnc(OC[C@@](C)(N)CC(C)C)c(Cl)c1 |r| | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50082397
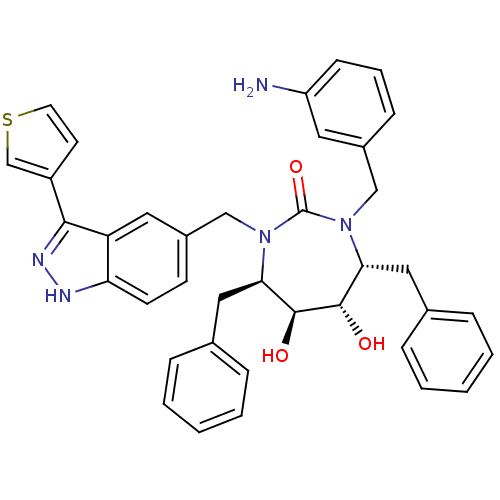
((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...)Show SMILES Nc1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3ccc4[nH]nc(-c5ccsc5)c4c3)C2=O)c1 Show InChI InChI=1S/C38H37N5O3S/c39-30-13-7-12-27(18-30)22-42-33(20-25-8-3-1-4-9-25)36(44)37(45)34(21-26-10-5-2-6-11-26)43(38(42)46)23-28-14-15-32-31(19-28)35(41-40-32)29-16-17-47-24-29/h1-19,24,33-34,36-37,44-45H,20-23,39H2,(H,40,41)/t33-,34-,36+,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV -1 Protease |
Bioorg Med Chem Lett 9: 3217-20 (1999)
BindingDB Entry DOI: 10.7270/Q2B27TG8 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36659
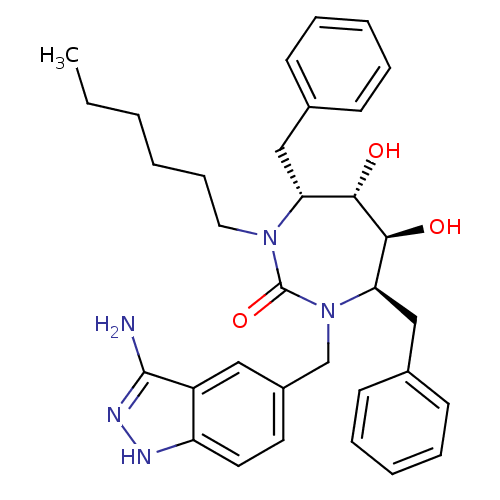
(n-Hexyl cyclic urea)Show SMILES CCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]nc(N)c3c2)C1=O |r| Show InChI InChI=1S/C33H41N5O3/c1-2-3-4-11-18-37-28(20-23-12-7-5-8-13-23)30(39)31(40)29(21-24-14-9-6-10-15-24)38(33(37)41)22-25-16-17-27-26(19-25)32(34)36-35-27/h5-10,12-17,19,28-31,39-40H,2-4,11,18,20-22H2,1H3,(H3,34,35,36)/t28-,29-,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288420

(5-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-(2-oxo-2,3-d...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3NC(=O)Cc3c2)C(=O)N(Cc2ccc3NC(=O)Cc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C37H36N4O5/c42-33-19-27-15-25(11-13-29(27)38-33)21-40-31(17-23-7-3-1-4-8-23)35(44)36(45)32(18-24-9-5-2-6-10-24)41(37(40)46)22-26-12-14-30-28(16-26)20-34(43)39-30/h1-16,31-32,35-36,44-45H,17-22H2,(H,38,42)(H,39,43)/t31-,32-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data