 Found 191 hits with Last Name = 'johnson' and Initial = 'md'
Found 191 hits with Last Name = 'johnson' and Initial = 'md' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50222915
(4'-[5-(4-pyrrolidin-1-ylmethyl-phenylamino)-1H-pyr...)Show SMILES Oc1ccc(c(O)c1)-c1ccc(cc1)-c1cc(Nc2ccc(CN3CCCC3)cc2)[nH]n1 Show InChI InChI=1S/C26H26N4O2/c31-22-11-12-23(25(32)15-22)19-5-7-20(8-6-19)24-16-26(29-28-24)27-21-9-3-18(4-10-21)17-30-13-1-2-14-30/h3-12,15-16,31-32H,1-2,13-14,17H2,(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 expressed in baculovirus/insect cell system |
J Med Chem 50: 5253-6 (2007)
Article DOI: 10.1021/jm0704604
BindingDB Entry DOI: 10.7270/Q2K0754D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50222916
(4'-{5-[4-(dimethylamino-methyl)-phenylamino]-2H-py...)Show SMILES CN(C)Cc1ccc(Nc2cc(n[nH]2)-c2ccc(cc2)-c2ccc(O)cc2O)cc1 Show InChI InChI=1S/C24H24N4O2/c1-28(2)15-16-3-9-19(10-4-16)25-24-14-22(26-27-24)18-7-5-17(6-8-18)21-12-11-20(29)13-23(21)30/h3-14,29-30H,15H2,1-2H3,(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 expressed in baculovirus/insect cell system |
J Med Chem 50: 5253-6 (2007)
Article DOI: 10.1021/jm0704604
BindingDB Entry DOI: 10.7270/Q2K0754D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50222920
(4'-{5-[6-(sec-butylamino-methyl)-pyridin-3-ylamino...)Show SMILES CCC(C)NCc1ccc(Nc2cc(n[nH]2)-c2ccc(cc2)-c2ccc(O)cc2O)cn1 Show InChI InChI=1S/C25H27N5O2/c1-3-16(2)26-14-19-8-9-20(15-27-19)28-25-13-23(29-30-25)18-6-4-17(5-7-18)22-11-10-21(31)12-24(22)32/h4-13,15-16,26,31-32H,3,14H2,1-2H3,(H2,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 expressed in baculovirus/insect cell system |
J Med Chem 50: 5253-6 (2007)
Article DOI: 10.1021/jm0704604
BindingDB Entry DOI: 10.7270/Q2K0754D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50222917
(4'-[5-(6-piperidin-1-ylmethyl-pyridin-3-ylamino)-1...)Show SMILES Oc1ccc(c(O)c1)-c1ccc(cc1)-c1cc(Nc2ccc(CN3CCCCC3)nc2)[nH]n1 Show InChI InChI=1S/C26H27N5O2/c32-22-10-11-23(25(33)14-22)18-4-6-19(7-5-18)24-15-26(30-29-24)28-20-8-9-21(27-16-20)17-31-12-2-1-3-13-31/h4-11,14-16,32-33H,1-3,12-13,17H2,(H2,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 expressed in baculovirus/insect cell system |
J Med Chem 50: 5253-6 (2007)
Article DOI: 10.1021/jm0704604
BindingDB Entry DOI: 10.7270/Q2K0754D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50222913

(4'-{5-[4-(isopropylamino-methyl)-phenylamino]-2H-p...)Show SMILES CC(C)NCc1ccc(Nc2cc(n[nH]2)-c2ccc(cc2)-c2ccc(O)cc2O)cc1 Show InChI InChI=1S/C25H26N4O2/c1-16(2)26-15-17-3-9-20(10-4-17)27-25-14-23(28-29-25)19-7-5-18(6-8-19)22-12-11-21(30)13-24(22)31/h3-14,16,26,30-31H,15H2,1-2H3,(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 expressed in baculovirus/insect cell system |
J Med Chem 50: 5253-6 (2007)
Article DOI: 10.1021/jm0704604
BindingDB Entry DOI: 10.7270/Q2K0754D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50222914
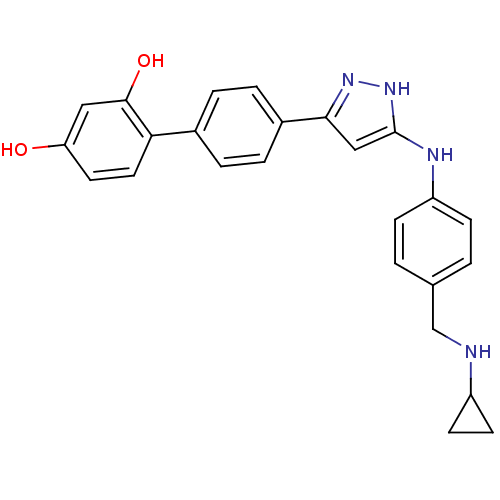
(4'-[5-(4-cyclopropylaminomethyl-phenylamino)-2H-py...)Show SMILES Oc1ccc(c(O)c1)-c1ccc(cc1)-c1cc(Nc2ccc(CNC3CC3)cc2)[nH]n1 Show InChI InChI=1S/C25H24N4O2/c30-21-11-12-22(24(31)13-21)17-3-5-18(6-4-17)23-14-25(29-28-23)27-20-7-1-16(2-8-20)15-26-19-9-10-19/h1-8,11-14,19,26,30-31H,9-10,15H2,(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 expressed in baculovirus/insect cell system |
J Med Chem 50: 5253-6 (2007)
Article DOI: 10.1021/jm0704604
BindingDB Entry DOI: 10.7270/Q2K0754D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50222921
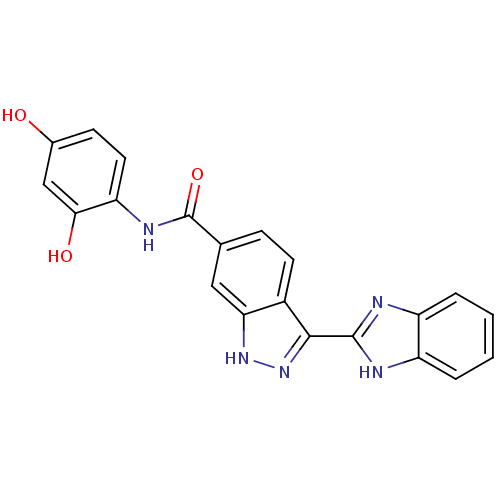
(3-(1H-benzo[d]imidazol-2-yl)-N-(2,4-dihydroxypheny...)Show SMILES Oc1ccc(NC(=O)c2ccc3c(n[nH]c3c2)-c2nc3ccccc3[nH]2)c(O)c1 Show InChI InChI=1S/C21H15N5O3/c27-12-6-8-16(18(28)10-12)24-21(29)11-5-7-13-17(9-11)25-26-19(13)20-22-14-3-1-2-4-15(14)23-20/h1-10,27-28H,(H,22,23)(H,24,29)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 expressed in baculovirus/insect cell system |
J Med Chem 50: 5253-6 (2007)
Article DOI: 10.1021/jm0704604
BindingDB Entry DOI: 10.7270/Q2K0754D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50222919
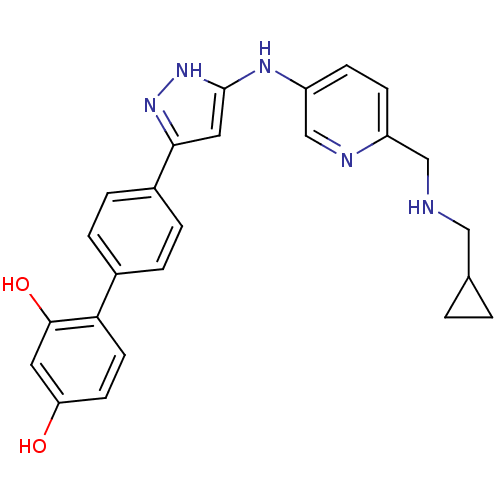
(4'-(5-{6-[(cyclopropylmethyl-amino)-methyl]-pyridi...)Show SMILES Oc1ccc(c(O)c1)-c1ccc(cc1)-c1cc(Nc2ccc(CNCC3CC3)nc2)[nH]n1 Show InChI InChI=1S/C25H25N5O2/c31-21-9-10-22(24(32)11-21)17-3-5-18(6-4-17)23-12-25(30-29-23)28-20-8-7-19(27-15-20)14-26-13-16-1-2-16/h3-12,15-16,26,31-32H,1-2,13-14H2,(H2,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 expressed in baculovirus/insect cell system |
J Med Chem 50: 5253-6 (2007)
Article DOI: 10.1021/jm0704604
BindingDB Entry DOI: 10.7270/Q2K0754D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50222918
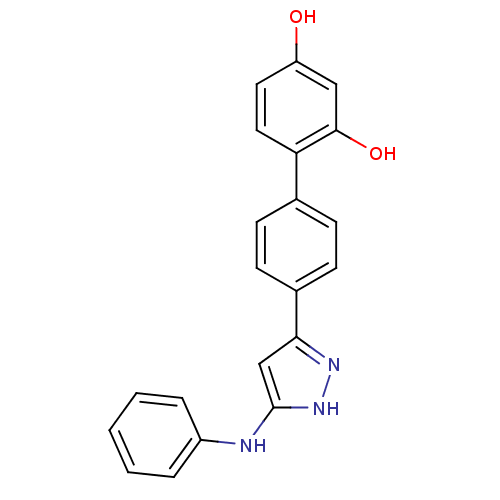
(4'-(5-phenylamino-2H-pyrazol-3-yl)-biphenyl-2,4-di...)Show SMILES Oc1ccc(c(O)c1)-c1ccc(cc1)-c1cc(Nc2ccccc2)[nH]n1 Show InChI InChI=1S/C21H17N3O2/c25-17-10-11-18(20(26)12-17)14-6-8-15(9-7-14)19-13-21(24-23-19)22-16-4-2-1-3-5-16/h1-13,25-26H,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 expressed in baculovirus/insect cell system |
J Med Chem 50: 5253-6 (2007)
Article DOI: 10.1021/jm0704604
BindingDB Entry DOI: 10.7270/Q2K0754D |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21737
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21751
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-33-105-35-106-34-46(79-56(93)40(20-13-25-72-68(70)71)74-50(89)31-73-55(92)42(30-51(90)91)76-61(98)47-21-14-26-84(47)65(102)43(77-59(45)96)29-39-17-9-8-10-18-39)60(97)83-54(38(5)88)64(101)75-41(19-11-12-24-69)57(94)78-44(32-87)58(95)82-53(37(4)7-2)67(104)86-28-16-23-49(86)66(103)85-27-15-22-48(85)62(99)81-52/h8-10,17-18,36-38,40-49,52-54,87-88H,6-7,11-16,19-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | -38.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50222922
(3-(1H-benzo[d]imidazol-2-yl)-N-(4-hydroxyphenyl)-1...)Show SMILES Oc1ccc(NC(=O)c2ccc3c(n[nH]c3c2)-c2nc3ccccc3[nH]2)cc1 Show InChI InChI=1S/C21H15N5O2/c27-14-8-6-13(7-9-14)22-21(28)12-5-10-15-18(11-12)25-26-19(15)20-23-16-3-1-2-4-17(16)24-20/h1-11,27H,(H,22,28)(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 expressed in baculovirus/insect cell system |
J Med Chem 50: 5253-6 (2007)
Article DOI: 10.1021/jm0704604
BindingDB Entry DOI: 10.7270/Q2K0754D |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21750
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31S,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-46-35-106-105-30-24-42(75-56(93)40(20-13-26-72-68(70)71)74-50(89)33-73-55(92)43(32-51(90)91)77-61(98)47-21-14-27-84(47)65(102)44(78-60(46)97)31-39-17-9-8-10-18-39)58(95)83-54(38(5)88)64(101)76-41(19-11-12-25-69)57(94)79-45(34-87)59(96)82-53(37(4)7-2)67(104)86-29-16-23-49(86)66(103)85-28-15-22-48(85)62(99)81-52/h8-10,17-18,36-38,40-49,52-54,87-88H,6-7,11-16,19-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,93)(H,76,101)(H,77,98)(H,78,97)(H,79,94)(H,80,100)(H,81,99)(H,82,96)(H,83,95)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | -36.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21746

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc3ccccc3c1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H106N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-44(18-10-11-25-72)60(97)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-22-52(89)69(106)88-28-14-21-51(88)65(102)84-55)82-59(96)43(19-12-26-75-71(73)74)77-53(92)33-76-58(95)45(32-54(93)94)79-64(101)50-20-13-27-87(50)68(105)46(80-62(48)99)31-40-23-24-41-16-8-9-17-42(41)30-40/h8-9,16-17,23-24,30,37-39,43-52,55-57,90-91H,6-7,10-15,18-22,25-29,31-36,72H2,1-5H3,(H,76,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,97)(H,82,96)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,75)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | -35.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21749
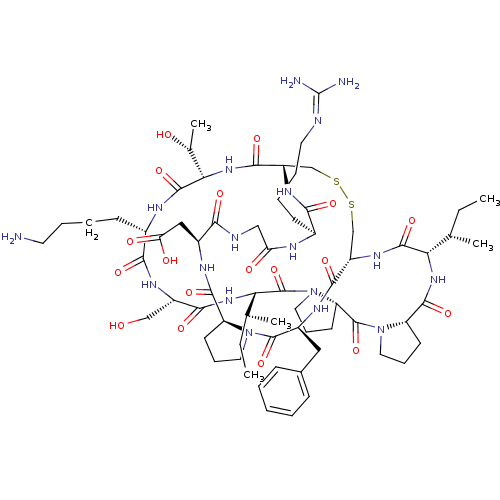
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-12-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-9-8-10-19-39)60(97)83-54(38(5)88)64(101)75-41(20-11-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8-10,18-19,36-38,40-49,52-54,87-88H,6-7,11-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 857 | -34.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21739
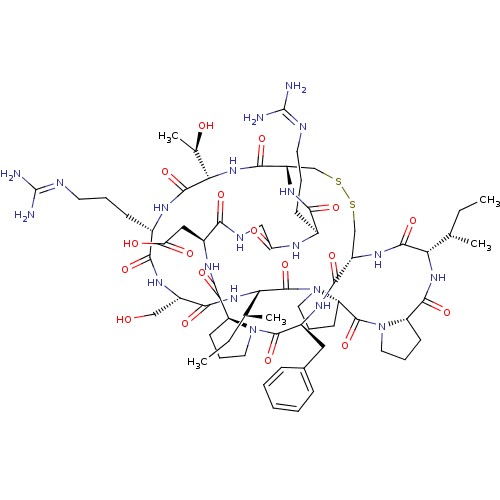
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N20O18S2/c1-6-34(3)50-61(101)81-43-32-106-107-33-44(80-54(94)38(18-11-23-72-66(68)69)75-48(90)30-74-53(93)40(29-49(91)92)77-59(99)45-20-13-25-85(45)63(103)41(78-57(43)97)28-37-16-9-8-10-17-37)58(98)84-52(36(5)89)62(102)76-39(19-12-24-73-67(70)71)55(95)79-42(31-88)56(96)83-51(35(4)7-2)65(105)87-27-15-22-47(87)64(104)86-26-14-21-46(86)60(100)82-50/h8-10,16-17,34-36,38-47,50-52,88-89H,6-7,11-15,18-33H2,1-5H3,(H,74,93)(H,75,90)(H,76,102)(H,77,99)(H,78,97)(H,79,95)(H,80,94)(H,81,101)(H,82,100)(H,83,96)(H,84,98)(H,91,92)(H4,68,69,72)(H4,70,71,73)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | -34.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21748
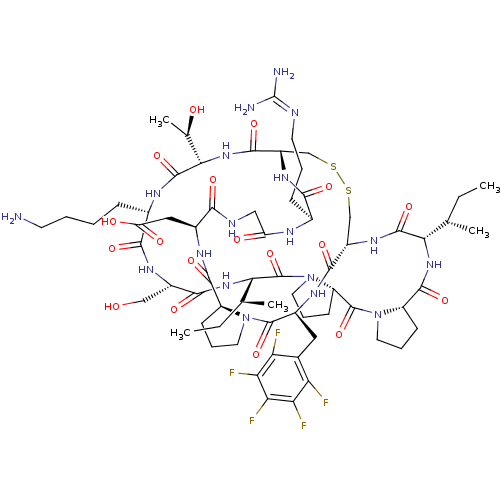
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1c(F)c(F)c(F)c(F)c1F)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H99F5N18O18S2/c1-6-30(3)51-62(104)84-39-28-109-110-29-40(59(101)87-53(32(5)92)63(105)79-35(14-8-9-19-73)56(98)82-38(27-91)57(99)86-52(31(4)7-2)66(108)90-23-13-18-43(90)65(107)89-22-12-17-42(89)61(103)85-51)83-55(97)34(15-10-20-76-67(74)75)78-44(93)26-77-54(96)36(25-45(94)95)80-60(102)41-16-11-21-88(41)64(106)37(81-58(39)100)24-33-46(68)48(70)50(72)49(71)47(33)69/h30-32,34-43,51-53,91-92H,6-29,73H2,1-5H3,(H,77,96)(H,78,93)(H,79,105)(H,80,102)(H,81,100)(H,82,98)(H,83,97)(H,84,104)(H,85,103)(H,86,99)(H,87,101)(H,94,95)(H4,74,75,76)/t30-,31-,32+,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 890 | -34.2 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21747

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C73H108N18O18S2/c1-6-39(3)57-68(105)85-50-37-110-111-38-51(65(102)88-59(41(5)93)69(106)80-46(19-11-12-28-74)62(99)83-49(36-92)63(100)87-58(40(4)7-2)72(109)91-32-16-23-54(91)71(108)90-31-15-22-53(90)67(104)86-57)84-61(98)45(20-13-29-77-73(75)76)79-55(94)35-78-60(97)47(34-56(95)96)81-66(103)52-21-14-30-89(52)70(107)48(82-64(50)101)33-42-24-26-44(27-25-42)43-17-9-8-10-18-43/h8-10,17-18,24-27,39-41,45-54,57-59,92-93H,6-7,11-16,19-23,28-38,74H2,1-5H3,(H,78,97)(H,79,94)(H,80,106)(H,81,103)(H,82,101)(H,83,99)(H,84,98)(H,85,105)(H,86,104)(H,87,100)(H,88,102)(H,95,96)(H4,75,76,77)/t39-,40-,41+,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-,58-,59-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | -33.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21745
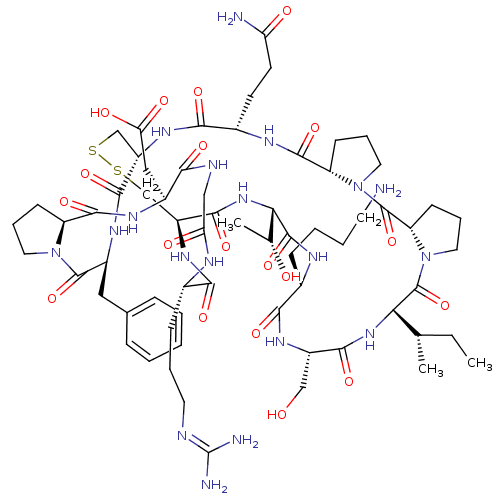
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8] |r| Show InChI InChI=1S/C66H101N19O19S2/c1-4-34(2)51-65(104)85-27-13-20-47(85)64(103)84-26-12-19-46(84)60(99)74-39(21-22-48(68)88)56(95)79-43-32-105-106-33-44(59(98)82-52(35(3)87)62(101)75-38(16-8-9-23-67)55(94)78-42(31-86)57(96)81-51)80-54(93)37(17-10-24-71-66(69)70)73-49(89)30-72-53(92)40(29-50(90)91)76-61(100)45-18-11-25-83(45)63(102)41(77-58(43)97)28-36-14-6-5-7-15-36/h5-7,14-15,34-35,37-47,51-52,86-87H,4,8-13,16-33,67H2,1-3H3,(H2,68,88)(H,72,92)(H,73,89)(H,74,99)(H,75,101)(H,76,100)(H,77,97)(H,78,94)(H,79,95)(H,80,93)(H,81,96)(H,82,98)(H,90,91)(H4,69,70,71)/t34-,35+,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,51-,52-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.33E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21739

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N20O18S2/c1-6-34(3)50-61(101)81-43-32-106-107-33-44(80-54(94)38(18-11-23-72-66(68)69)75-48(90)30-74-53(93)40(29-49(91)92)77-59(99)45-20-13-25-85(45)63(103)41(78-57(43)97)28-37-16-9-8-10-17-37)58(98)84-52(36(5)89)62(102)76-39(19-12-24-73-67(70)71)55(95)79-42(31-88)56(96)83-51(35(4)7-2)65(105)87-27-15-22-47(87)64(104)86-26-14-21-46(86)60(100)82-50/h8-10,16-17,34-36,38-47,50-52,88-89H,6-7,11-15,18-33H2,1-5H3,(H,74,93)(H,75,90)(H,76,102)(H,77,99)(H,78,97)(H,79,95)(H,80,94)(H,81,101)(H,82,100)(H,83,96)(H,84,98)(H,91,92)(H4,68,69,72)(H4,70,71,73)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.75E+3 | -30.1 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21737

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21749

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-12-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-9-8-10-19-39)60(97)83-54(38(5)88)64(101)75-41(20-11-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8-10,18-19,36-38,40-49,52-54,87-88H,6-7,11-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.63E+3 | -28.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21751

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-33-105-35-106-34-46(79-56(93)40(20-13-25-72-68(70)71)74-50(89)31-73-55(92)42(30-51(90)91)76-61(98)47-21-14-26-84(47)65(102)43(77-59(45)96)29-39-17-9-8-10-18-39)60(97)83-54(38(5)88)64(101)75-41(19-11-12-24-69)57(94)78-44(32-87)58(95)82-53(37(4)7-2)67(104)86-28-16-23-49(86)66(103)85-27-15-22-48(85)62(99)81-52/h8-10,17-18,36-38,40-49,52-54,87-88H,6-7,11-16,19-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21746

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc3ccccc3c1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H106N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-44(18-10-11-25-72)60(97)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-22-52(89)69(106)88-28-14-21-51(88)65(102)84-55)82-59(96)43(19-12-26-75-71(73)74)77-53(92)33-76-58(95)45(32-54(93)94)79-64(101)50-20-13-27-87(50)68(105)46(80-62(48)99)31-40-23-24-41-16-8-9-17-42(41)30-40/h8-9,16-17,23-24,30,37-39,43-52,55-57,90-91H,6-7,10-15,18-22,25-29,31-36,72H2,1-5H3,(H,76,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,97)(H,82,96)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,75)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.63E+4 | -27.1 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21742
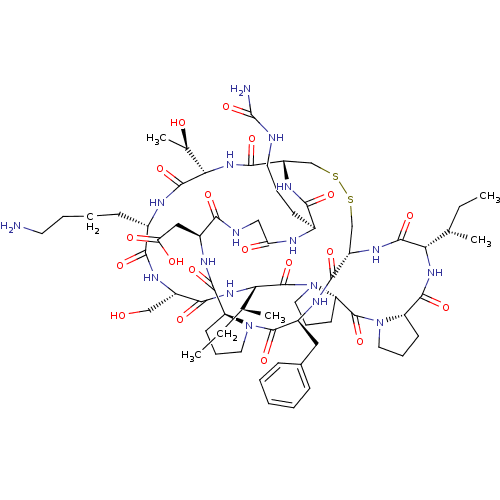
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=O)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C67H103N17O19S2/c1-6-35(3)51-62(98)78-44-33-104-105-34-45(77-55(91)39(20-13-25-70-67(69)103)72-49(87)31-71-54(90)41(30-50(88)89)74-60(96)46-21-14-26-82(46)64(100)42(75-58(44)94)29-38-17-9-8-10-18-38)59(95)81-53(37(5)86)63(99)73-40(19-11-12-24-68)56(92)76-43(32-85)57(93)80-52(36(4)7-2)66(102)84-28-16-23-48(84)65(101)83-27-15-22-47(83)61(97)79-51/h8-10,17-18,35-37,39-48,51-53,85-86H,6-7,11-16,19-34,68H2,1-5H3,(H,71,90)(H,72,87)(H,73,99)(H,74,96)(H,75,94)(H,76,92)(H,77,91)(H,78,98)(H,79,97)(H,80,93)(H,81,95)(H,88,89)(H3,69,70,103)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.25E+4 | -26.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21747

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C73H108N18O18S2/c1-6-39(3)57-68(105)85-50-37-110-111-38-51(65(102)88-59(41(5)93)69(106)80-46(19-11-12-28-74)62(99)83-49(36-92)63(100)87-58(40(4)7-2)72(109)91-32-16-23-54(91)71(108)90-31-15-22-53(90)67(104)86-57)84-61(98)45(20-13-29-77-73(75)76)79-55(94)35-78-60(97)47(34-56(95)96)81-66(103)52-21-14-30-89(52)70(107)48(82-64(50)101)33-42-24-26-44(27-25-42)43-17-9-8-10-18-43/h8-10,17-18,24-27,39-41,45-54,57-59,92-93H,6-7,11-16,19-23,28-38,74H2,1-5H3,(H,78,97)(H,79,94)(H,80,106)(H,81,103)(H,82,101)(H,83,99)(H,84,98)(H,85,105)(H,86,104)(H,87,100)(H,88,102)(H,95,96)(H4,75,76,77)/t39-,40-,41+,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.56E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21743
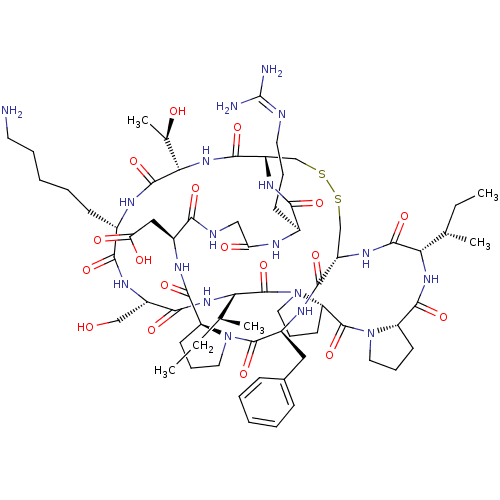
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-10-8-11-19-39)60(97)83-54(38(5)88)64(101)75-41(20-12-9-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8,10-11,18-19,36-38,40-49,52-54,87-88H,6-7,9,12-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | -25.7 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21748

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1c(F)c(F)c(F)c(F)c1F)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H99F5N18O18S2/c1-6-30(3)51-62(104)84-39-28-109-110-29-40(59(101)87-53(32(5)92)63(105)79-35(14-8-9-19-73)56(98)82-38(27-91)57(99)86-52(31(4)7-2)66(108)90-23-13-18-43(90)65(107)89-22-12-17-42(89)61(103)85-51)83-55(97)34(15-10-20-76-67(74)75)78-44(93)26-77-54(96)36(25-45(94)95)80-60(102)41-16-11-21-88(41)64(106)37(81-58(39)100)24-33-46(68)48(70)50(72)49(71)47(33)69/h30-32,34-43,51-53,91-92H,6-29,73H2,1-5H3,(H,77,96)(H,78,93)(H,79,105)(H,80,102)(H,81,100)(H,82,98)(H,83,97)(H,84,104)(H,85,103)(H,86,99)(H,87,101)(H,94,95)(H4,74,75,76)/t30-,31-,32+,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.36E+4 | -25.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21744
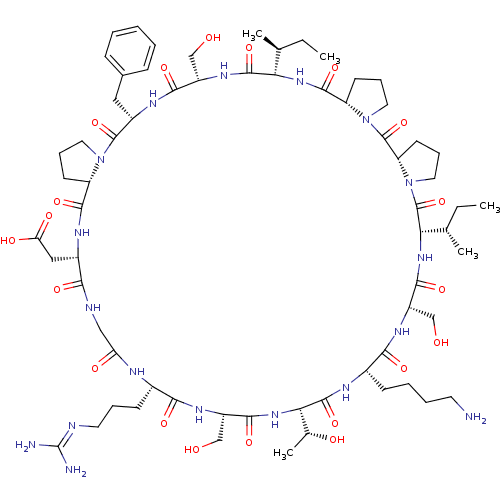
(2-[(1S,7S,10S,13S,16S,22S,25S,31S,34S,37S,40S,43S,...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6]-1=O)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H106N18O20/c1-6-35(3)51-62(101)79-43(32-86)57(96)76-42(29-38-17-9-8-10-18-38)64(103)83-26-14-21-46(83)60(99)75-41(30-50(91)92)54(93)72-31-49(90)73-39(20-13-25-71-67(69)70)55(94)77-45(34-88)59(98)82-53(37(5)89)63(102)74-40(19-11-12-24-68)56(95)78-44(33-87)58(97)81-52(36(4)7-2)66(105)85-28-16-23-48(85)65(104)84-27-15-22-47(84)61(100)80-51/h8-10,17-18,35-37,39-48,51-53,86-89H,6-7,11-16,19-34,68H2,1-5H3,(H,72,93)(H,73,90)(H,74,102)(H,75,99)(H,76,96)(H,77,94)(H,78,95)(H,79,101)(H,80,100)(H,81,97)(H,82,98)(H,91,92)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.04E+4 | -24.8 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21743

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-10-8-11-19-39)60(97)83-54(38(5)88)64(101)75-41(20-12-9-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8,10-11,18-19,36-38,40-49,52-54,87-88H,6-7,9,12-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.56E+4 | -24.5 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21741

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H104N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-43(18-11-12-26-72)59(96)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-21-52(89)69(106)88-28-14-20-51(88)65(102)84-55)82-60(97)44(30-41-22-24-42(25-23-41)76-71(73)74)77-53(92)33-75-58(95)45(32-54(93)94)79-64(101)50-19-13-27-87(50)68(105)46(80-62(48)99)31-40-16-9-8-10-17-40/h8-10,16-17,22-25,37-39,43-52,55-57,90-91H,6-7,11-15,18-21,26-36,72H2,1-5H3,(H,75,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,96)(H,82,97)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,76)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+4 | -22.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21744

(2-[(1S,7S,10S,13S,16S,22S,25S,31S,34S,37S,40S,43S,...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6]-1=O)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H106N18O20/c1-6-35(3)51-62(101)79-43(32-86)57(96)76-42(29-38-17-9-8-10-18-38)64(103)83-26-14-21-46(83)60(99)75-41(30-50(91)92)54(93)72-31-49(90)73-39(20-13-25-71-67(69)70)55(94)77-45(34-88)59(98)82-53(37(5)89)63(102)74-40(19-11-12-24-68)56(95)78-44(33-87)58(97)81-52(36(4)7-2)66(105)85-28-16-23-48(85)65(104)84-27-15-22-47(84)61(100)80-51/h8-10,17-18,35-37,39-48,51-53,86-89H,6-7,11-16,19-34,68H2,1-5H3,(H,72,93)(H,73,90)(H,74,102)(H,75,99)(H,76,96)(H,77,94)(H,78,95)(H,79,101)(H,80,100)(H,81,97)(H,82,98)(H,91,92)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+5 | -22.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21741

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H104N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-43(18-11-12-26-72)59(96)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-21-52(89)69(106)88-28-14-20-51(88)65(102)84-55)82-60(97)44(30-41-22-24-42(25-23-41)76-71(73)74)77-53(92)33-75-58(95)45(32-54(93)94)79-64(101)50-19-13-27-87(50)68(105)46(80-62(48)99)31-40-16-9-8-10-17-40/h8-10,16-17,22-25,37-39,43-52,55-57,90-91H,6-7,11-15,18-21,26-36,72H2,1-5H3,(H,75,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,96)(H,82,97)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,76)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+5 | -21.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21740
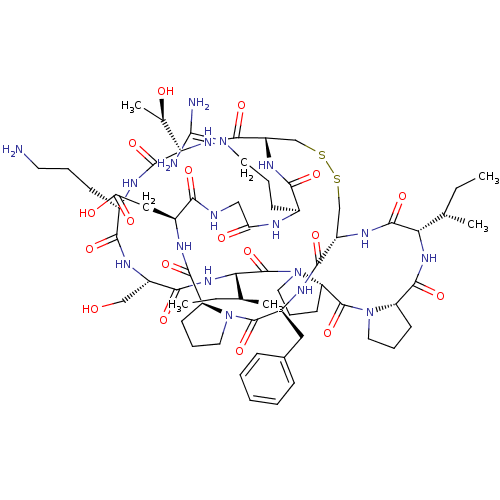
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C66H102N18O18S2/c1-6-34(3)50-61(98)78-43-32-103-104-33-44(77-54(91)38(19-12-24-70-66(68)69)72-48(87)30-71-53(90)40(29-49(88)89)74-59(96)45-20-13-25-82(45)63(100)41(75-57(43)94)28-37-16-9-8-10-17-37)58(95)81-52(36(5)86)62(99)73-39(18-11-23-67)55(92)76-42(31-85)56(93)80-51(35(4)7-2)65(102)84-27-15-22-47(84)64(101)83-26-14-21-46(83)60(97)79-50/h8-10,16-17,34-36,38-47,50-52,85-86H,6-7,11-15,18-33,67H2,1-5H3,(H,71,90)(H,72,87)(H,73,99)(H,74,96)(H,75,94)(H,76,92)(H,77,91)(H,78,98)(H,79,97)(H,80,93)(H,81,95)(H,88,89)(H4,68,69,70)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+5 | >-20.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21742

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=O)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C67H103N17O19S2/c1-6-35(3)51-62(98)78-44-33-104-105-34-45(77-55(91)39(20-13-25-70-67(69)103)72-49(87)31-71-54(90)41(30-50(88)89)74-60(96)46-21-14-26-82(46)64(100)42(75-58(44)94)29-38-17-9-8-10-18-38)59(95)81-53(37(5)86)63(99)73-40(19-11-12-24-68)56(92)76-43(32-85)57(93)80-52(36(4)7-2)66(102)84-28-16-23-48(84)65(101)83-27-15-22-47(83)61(97)79-51/h8-10,17-18,35-37,39-48,51-53,85-86H,6-7,11-16,19-34,68H2,1-5H3,(H,71,90)(H,72,87)(H,73,99)(H,74,96)(H,75,94)(H,76,92)(H,77,91)(H,78,98)(H,79,97)(H,80,93)(H,81,95)(H,88,89)(H3,69,70,103)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.27E+5 | -17.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21745

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8] |r| Show InChI InChI=1S/C66H101N19O19S2/c1-4-34(2)51-65(104)85-27-13-20-47(85)64(103)84-26-12-19-46(84)60(99)74-39(21-22-48(68)88)56(95)79-43-32-105-106-33-44(59(98)82-52(35(3)87)62(101)75-38(16-8-9-23-67)55(94)78-42(31-86)57(96)81-51)80-54(93)37(17-10-24-71-66(69)70)73-49(89)30-72-53(92)40(29-50(90)91)76-61(100)45-18-11-25-83(45)63(102)41(77-58(43)97)28-36-14-6-5-7-15-36/h5-7,14-15,34-35,37-47,51-52,86-87H,4,8-13,16-33,67H2,1-3H3,(H2,68,88)(H,72,92)(H,73,89)(H,74,99)(H,75,101)(H,76,100)(H,77,97)(H,78,94)(H,79,95)(H,80,93)(H,81,96)(H,82,98)(H,90,91)(H4,69,70,71)/t34-,35+,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+6 | >-14.7 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Rattus norvegicus) | BDBM50122102
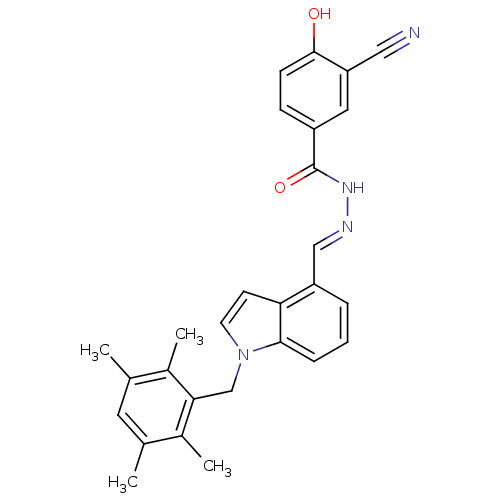
(3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetrame...)Show SMILES Cc1cc(C)c(C)c(Cn2ccc3c(\C=N\NC(=O)c4ccc(O)c(c4)C#N)cccc23)c1C Show InChI InChI=1S/C28H26N4O2/c1-17-12-18(2)20(4)25(19(17)3)16-32-11-10-24-22(6-5-7-26(24)32)15-30-31-28(34)21-8-9-27(33)23(13-21)14-29/h5-13,15,33H,16H2,1-4H3,(H,31,34)/b30-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound towards rat glucagon receptor |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122102

(3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetrame...)Show SMILES Cc1cc(C)c(C)c(Cn2ccc3c(\C=N\NC(=O)c4ccc(O)c(c4)C#N)cccc23)c1C Show InChI InChI=1S/C28H26N4O2/c1-17-12-18(2)20(4)25(19(17)3)16-32-11-10-24-22(6-5-7-26(24)32)15-30-31-28(34)21-8-9-27(33)23(13-21)14-29/h5-13,15,33H,16H2,1-4H3,(H,31,34)/b30-15+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122159
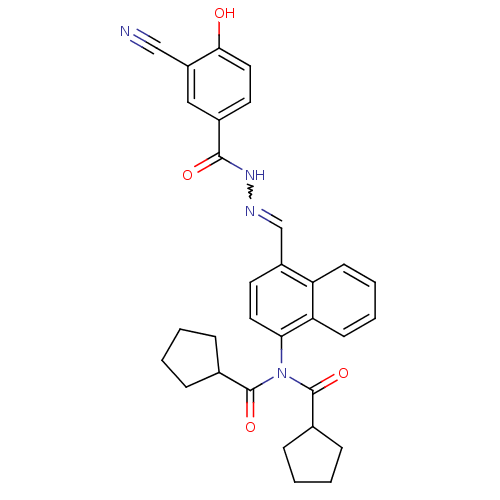
(CHEMBL152477 | Cyclopentanecarboxylic acid {4-[(3-...)Show SMILES Oc1ccc(cc1C#N)C(=O)NN=Cc1ccc(N(C(=O)C2CCCC2)C(=O)C2CCCC2)c2ccccc12 |w:12.12| Show InChI InChI=1S/C31H30N4O4/c32-18-24-17-22(14-16-28(24)36)29(37)34-33-19-23-13-15-27(26-12-6-5-11-25(23)26)35(30(38)20-7-1-2-8-20)31(39)21-9-3-4-10-21/h5-6,11-17,19-21,36H,1-4,7-10H2,(H,34,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122153
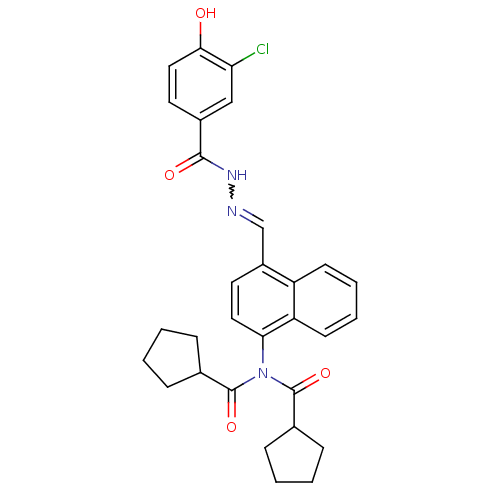
(CHEMBL156724 | Cyclopentanecarboxylic acid {4-[(3-...)Show SMILES Oc1ccc(cc1Cl)C(=O)NN=Cc1ccc(N(C(=O)C2CCCC2)C(=O)C2CCCC2)c2ccccc12 |w:11.11| Show InChI InChI=1S/C30H30ClN3O4/c31-25-17-21(14-16-27(25)35)28(36)33-32-18-22-13-15-26(24-12-6-5-11-23(22)24)34(29(37)19-7-1-2-8-19)30(38)20-9-3-4-10-20/h5-6,11-20,35H,1-4,7-10H2,(H,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50110074

(3-Chloro-4-hydroxy-benzoic acid [1-[4-(4-isopropyl...)Show SMILES CC(C)c1ccc(COc2ccc(\C=N\NC(=O)c3ccc(O)c(Cl)c3)c3ccccc23)cc1 Show InChI InChI=1S/C28H25ClN2O3/c1-18(2)20-9-7-19(8-10-20)17-34-27-14-12-22(23-5-3-4-6-24(23)27)16-30-31-28(33)21-11-13-26(32)25(29)15-21/h3-16,18,32H,17H2,1-2H3,(H,31,33)/b30-16+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122116
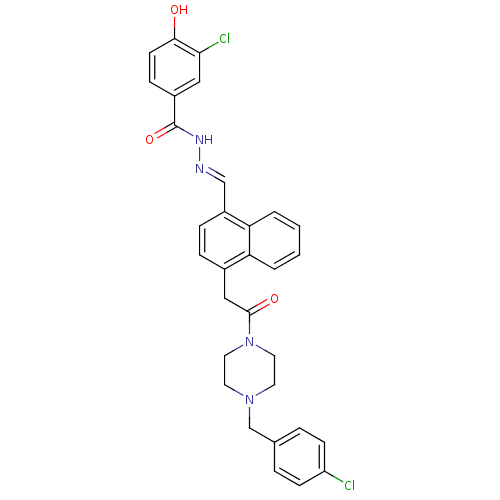
(3-Chloro-4-hydroxy-benzoic acid (4-{2-[4-(4-chloro...)Show SMILES Oc1ccc(cc1Cl)C(=O)N\N=C\c1ccc(CC(=O)N2CCN(Cc3ccc(Cl)cc3)CC2)c2ccccc12 Show InChI InChI=1S/C31H28Cl2N4O3/c32-25-10-5-21(6-11-25)20-36-13-15-37(16-14-36)30(39)18-22-7-8-24(27-4-2-1-3-26(22)27)19-34-35-31(40)23-9-12-29(38)28(33)17-23/h1-12,17,19,38H,13-16,18,20H2,(H,35,40)/b34-19+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122160

(3-Cyano-4-hydroxy-benzoic acid {4-[4-(4-chloro-ben...)Show SMILES Oc1ccc(cc1C#N)C(=O)N\N=C\c1ccc(C(=O)N2CCN(Cc3ccc(Cl)cc3)CC2)c2ccccc12 Show InChI InChI=1S/C31H26ClN5O3/c32-25-9-5-21(6-10-25)20-36-13-15-37(16-14-36)31(40)28-11-7-23(26-3-1-2-4-27(26)28)19-34-35-30(39)22-8-12-29(38)24(17-22)18-33/h1-12,17,19,38H,13-16,20H2,(H,35,39)/b34-19+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122135

(3-Chloro-4-hydroxy-benzoic acid [1-(2,3,5,6-tetram...)Show SMILES Cc1cc(C)c(C)c(Cn2ccc3c(\C=N\NC(=O)c4ccc(O)c(Cl)c4)cccc23)c1C Show InChI InChI=1S/C27H26ClN3O2/c1-16-12-17(2)19(4)23(18(16)3)15-31-11-10-22-21(6-5-7-25(22)31)14-29-30-27(33)20-8-9-26(32)24(28)13-20/h5-14,32H,15H2,1-4H3,(H,30,33)/b29-14+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122142

(3-Chloro-4-hydroxy-benzoic acid [1-(2,4,6-trimethy...)Show SMILES Cc1cc(C)c(Cn2ccc3c(\C=N\NC(=O)c4ccc(O)c(Cl)c4)cccc23)c(C)c1 Show InChI InChI=1S/C26H24ClN3O2/c1-16-11-17(2)22(18(3)12-16)15-30-10-9-21-20(5-4-6-24(21)30)14-28-29-26(32)19-7-8-25(31)23(27)13-19/h4-14,31H,15H2,1-3H3,(H,29,32)/b28-14+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122152
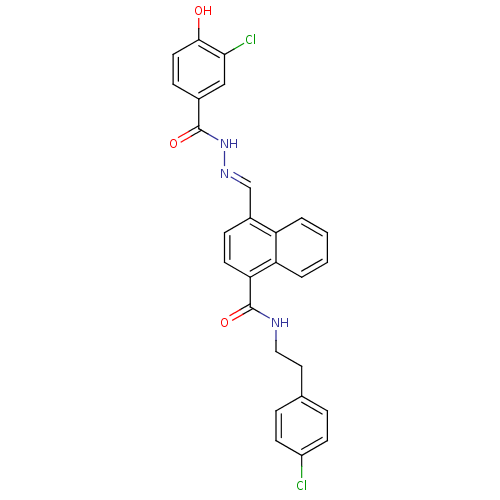
(4-[(3-Chloro-4-hydroxy-benzoyl)-hydrazonomethyl]-n...)Show SMILES Oc1ccc(cc1Cl)C(=O)N\N=C\c1ccc(C(=O)NCCc2ccc(Cl)cc2)c2ccccc12 Show InChI InChI=1S/C27H21Cl2N3O3/c28-20-9-5-17(6-10-20)13-14-30-27(35)23-11-7-19(21-3-1-2-4-22(21)23)16-31-32-26(34)18-8-12-25(33)24(29)15-18/h1-12,15-16,33H,13-14H2,(H,30,35)(H,32,34)/b31-16+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122158

(3-Cyano-4-hydroxy-benzoic acid (4-{2-[4-(4-bromo-p...)Show SMILES Oc1ccc(cc1C#N)C(=O)N\N=C\c1ccc(OCC(=O)N2CCC(=CC2)c2ccc(Br)cc2)c2ccccc12 |c:26| Show InChI InChI=1S/C32H25BrN4O4/c33-26-9-5-21(6-10-26)22-13-15-37(16-14-22)31(39)20-41-30-12-8-24(27-3-1-2-4-28(27)30)19-35-36-32(40)23-7-11-29(38)25(17-23)18-34/h1-13,17,19,38H,14-16,20H2,(H,36,40)/b35-19+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122125
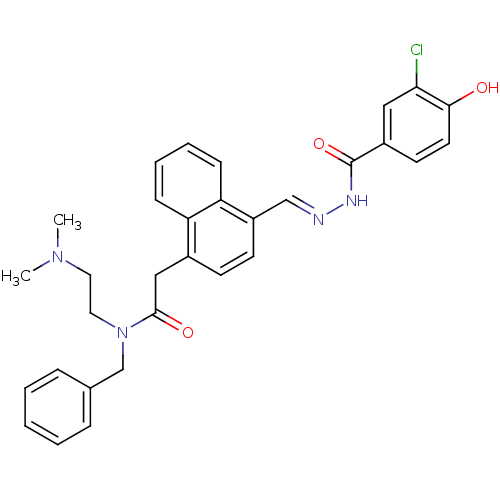
(CHEMBL421809 | N-Benzyl-2-{4-[(3-chloro-4-hydroxy-...)Show SMILES CN(C)CCN(Cc1ccccc1)C(=O)Cc1ccc(\C=N\NC(=O)c2ccc(O)c(Cl)c2)c2ccccc12 Show InChI InChI=1S/C31H31ClN4O3/c1-35(2)16-17-36(21-22-8-4-3-5-9-22)30(38)19-23-12-13-25(27-11-7-6-10-26(23)27)20-33-34-31(39)24-14-15-29(37)28(32)18-24/h3-15,18,20,37H,16-17,19,21H2,1-2H3,(H,34,39)/b33-20+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50409681
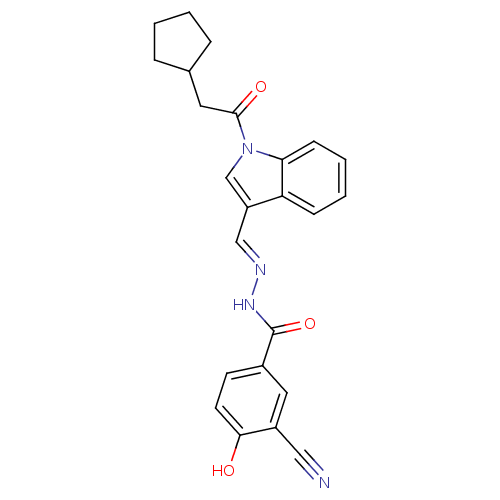
(CHEMBL2112908)Show SMILES Oc1ccc(cc1C#N)C(=O)N\N=C\c1cn(C(=O)CC2CCCC2)c2ccccc12 Show InChI InChI=1S/C24H22N4O3/c25-13-18-12-17(9-10-22(18)29)24(31)27-26-14-19-15-28(21-8-4-3-7-20(19)21)23(30)11-16-5-1-2-6-16/h3-4,7-10,12,14-16,29H,1-2,5-6,11H2,(H,27,31)/b26-14+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50122124

(3-Chloro-4-hydroxy-benzoic acid (4-{2-[4-(4-bromo-...)Show SMILES Oc1ccc(cc1Cl)C(=O)N\N=C\c1ccc(OCC(=O)N2CCC(=CC2)c2ccc(Br)cc2)c2ccccc12 |c:25| Show InChI InChI=1S/C31H25BrClN3O4/c32-24-9-5-20(6-10-24)21-13-15-36(16-14-21)30(38)19-40-29-12-8-23(25-3-1-2-4-26(25)29)18-34-35-31(39)22-7-11-28(37)27(33)17-22/h1-13,17-18,37H,14-16,19H2,(H,35,39)/b34-18+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































