 Found 1301 hits with Last Name = 'kong' and Initial = 'j'
Found 1301 hits with Last Name = 'kong' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50505279

(CHEMBL4436207)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(cc1)B(O)O |r| Show InChI InChI=1S/C27H37BN2O9S/c1-18(2)15-30(40(35,36)21-10-8-20(9-11-21)28(33)34)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)39-25-17-38-26-22(25)12-13-37-26/h3-11,18,22-26,31,33-34H,12-17H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50304826
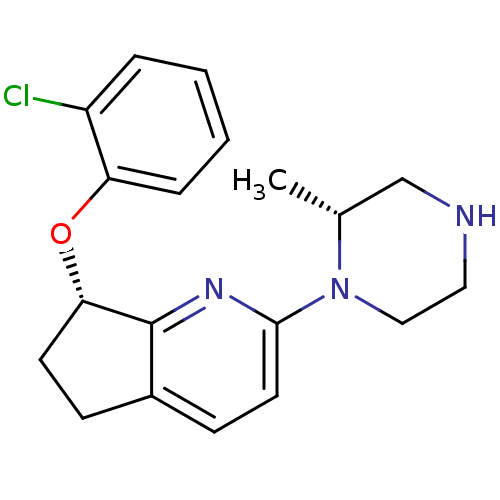
((S)-7-(2-chlorophenoxy)-2-((R)-2-methylpiperazin-1...)Show SMILES C[C@@H]1CNCCN1c1ccc2CC[C@H](Oc3ccccc3Cl)c2n1 |r| Show InChI InChI=1S/C19H22ClN3O/c1-13-12-21-10-11-23(13)18-9-7-14-6-8-17(19(14)22-18)24-16-5-3-2-4-15(16)20/h2-5,7,9,13,17,21H,6,8,10-12H2,1H3/t13-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2C receptor expressed in mouse 3T3 cells by scintillation counting |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50304805
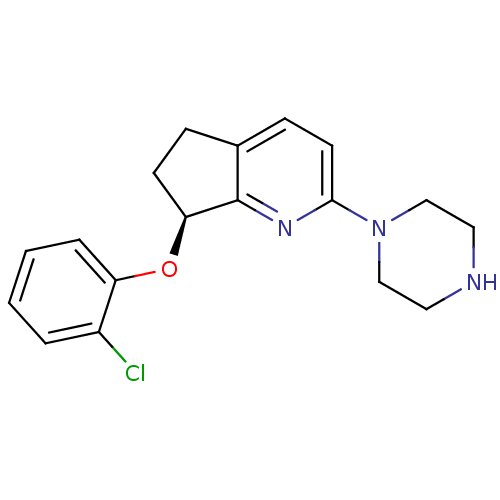
((S)-7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dih...)Show InChI InChI=1S/C18H20ClN3O/c19-14-3-1-2-4-15(14)23-16-7-5-13-6-8-17(21-18(13)16)22-11-9-20-10-12-22/h1-4,6,8,16,20H,5,7,9-12H2/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50304802
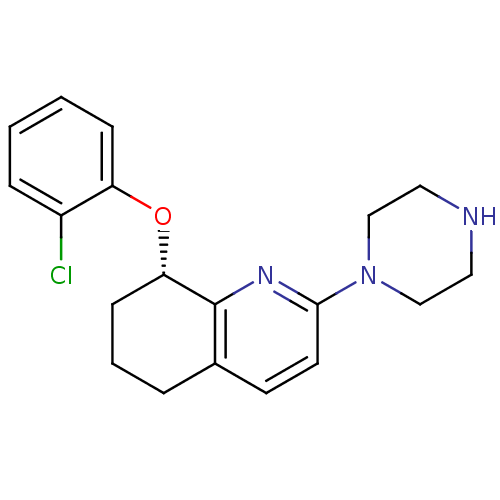
((S)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...)Show SMILES Clc1ccccc1O[C@H]1CCCc2ccc(nc12)N1CCNCC1 |r| Show InChI InChI=1S/C19H22ClN3O/c20-15-5-1-2-6-16(15)24-17-7-3-4-14-8-9-18(22-19(14)17)23-12-10-21-11-13-23/h1-2,5-6,8-9,17,21H,3-4,7,10-13H2/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50304804
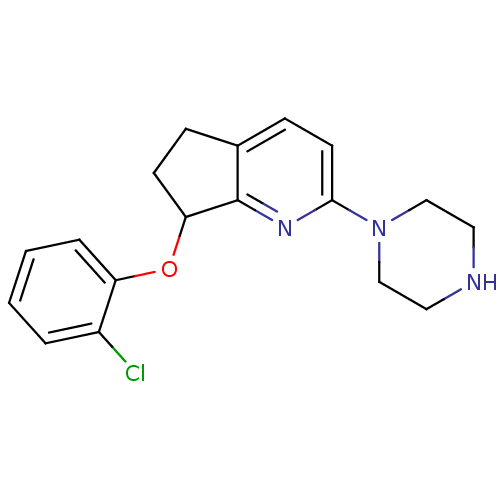
(7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dihydro...)Show InChI InChI=1S/C18H20ClN3O/c19-14-3-1-2-4-15(14)23-16-7-5-13-6-8-17(21-18(13)16)22-11-9-20-10-12-22/h1-4,6,8,16,20H,5,7,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50314613
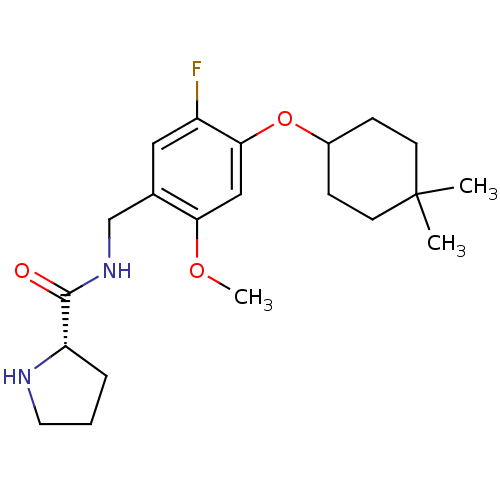
((S)-N-(4-(4,4-dimethylcyclohexyloxy)-5-fluoro-2-me...)Show SMILES COc1cc(OC2CCC(C)(C)CC2)c(F)cc1CNC(=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C21H31FN2O3/c1-21(2)8-6-15(7-9-21)27-19-12-18(26-3)14(11-16(19)22)13-24-20(25)17-5-4-10-23-17/h11-12,15,17,23H,4-10,13H2,1-3H3,(H,24,25)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2C receptor |
Bioorg Med Chem Lett 20: 2365-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.107
BindingDB Entry DOI: 10.7270/Q24M94QJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
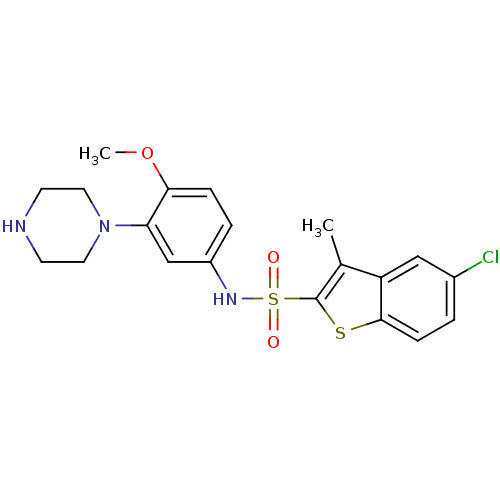
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50304805

((S)-7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dih...)Show InChI InChI=1S/C18H20ClN3O/c19-14-3-1-2-4-15(14)23-16-7-5-13-6-8-17(21-18(13)16)22-11-9-20-10-12-22/h1-4,6,8,16,20H,5,7,9-12H2/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50228649
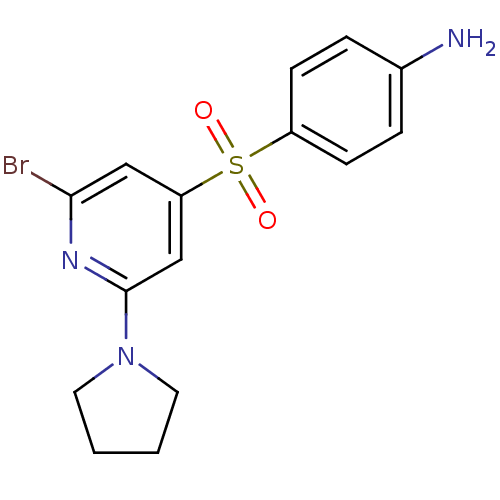
(4-(2-Bromo-6-pyrrolidin-1-yl-pyridine-4-sulfonyl)-...)Show InChI InChI=1S/C15H16BrN3O2S/c16-14-9-13(10-15(18-14)19-7-1-2-8-19)22(20,21)12-5-3-11(17)4-6-12/h3-6,9-10H,1-2,7-8,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50304808
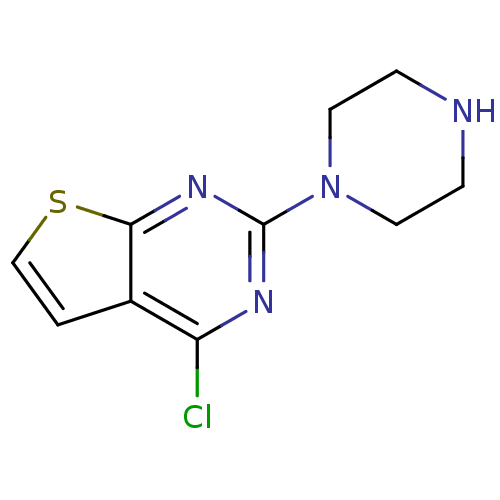
(4-chloro-2-(piperazin-1-yl)thieno[2,3-d]pyrimidine...)Show InChI InChI=1S/C10H11ClN4S/c11-8-7-1-6-16-9(7)14-10(13-8)15-4-2-12-3-5-15/h1,6,12H,2-5H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130264
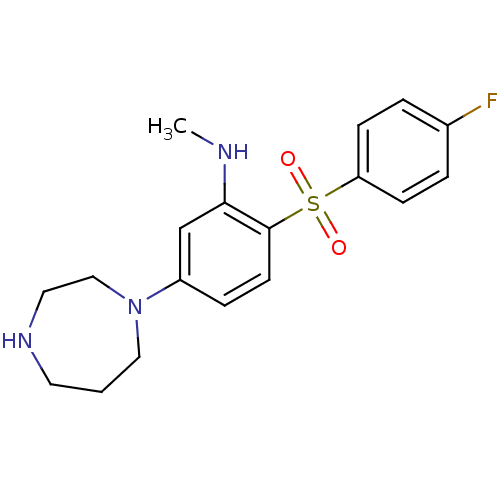
(5-(1,4-diazepan-1-yl)-2-(4-fluorophenylsulfonyl)-N...)Show InChI InChI=1S/C18H22FN3O2S/c1-20-17-13-15(22-11-2-9-21-10-12-22)5-8-18(17)25(23,24)16-6-3-14(19)4-7-16/h3-8,13,20-21H,2,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50304807
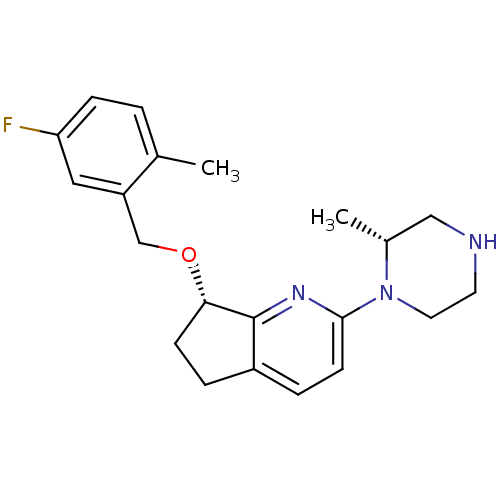
((S)-7-(5-fluoro-2-methylbenzyloxy)-2-((R)-2-methyl...)Show SMILES C[C@@H]1CNCCN1c1ccc2CC[C@H](OCc3cc(F)ccc3C)c2n1 |r| Show InChI InChI=1S/C21H26FN3O/c1-14-3-6-18(22)11-17(14)13-26-19-7-4-16-5-8-20(24-21(16)19)25-10-9-23-12-15(25)2/h3,5-6,8,11,15,19,23H,4,7,9-10,12-13H2,1-2H3/t15-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50304802

((S)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...)Show SMILES Clc1ccccc1O[C@H]1CCCc2ccc(nc12)N1CCNCC1 |r| Show InChI InChI=1S/C19H22ClN3O/c20-15-5-1-2-6-16(15)24-17-7-3-4-14-8-9-18(22-19(14)17)23-12-10-21-11-13-23/h1-2,5-6,8-9,17,21H,3-4,7,10-13H2/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122928

(5-{6-[5-Chloro-2-(3-methyl-isoxazol-5-ylmethoxy)-b...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)ncnc12 Show InChI InChI=1S/C23H24ClN7O6/c1-11-5-14(37-30-11)8-35-15-4-3-13(24)6-12(15)7-26-20-16-21(28-9-27-20)31(10-29-16)23-18(33)17(32)19(36-23)22(34)25-2/h3-6,9-10,17-19,23,32-33H,7-8H2,1-2H3,(H,25,34)(H,26,27,28)/t17-,18+,19-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737
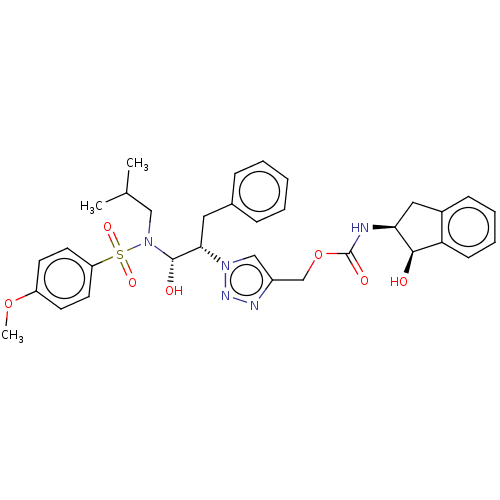
(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50314616
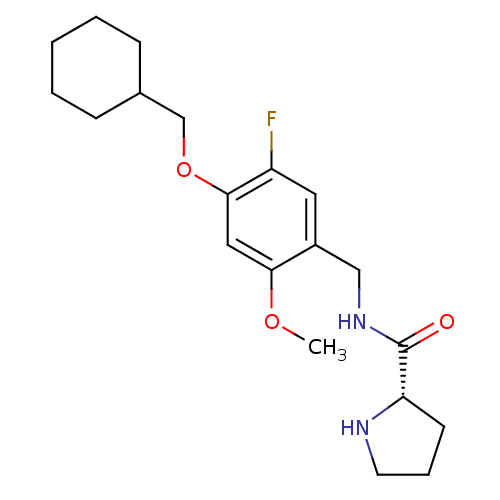
((S)-N-(4-(cyclohexylmethoxy)-5-fluoro-2-methoxyben...)Show SMILES COc1cc(OCC2CCCCC2)c(F)cc1CNC(=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C20H29FN2O3/c1-25-18-11-19(26-13-14-6-3-2-4-7-14)16(21)10-15(18)12-23-20(24)17-8-5-9-22-17/h10-11,14,17,22H,2-9,12-13H2,1H3,(H,23,24)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2C receptor |
Bioorg Med Chem Lett 20: 2365-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.107
BindingDB Entry DOI: 10.7270/Q24M94QJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50314614
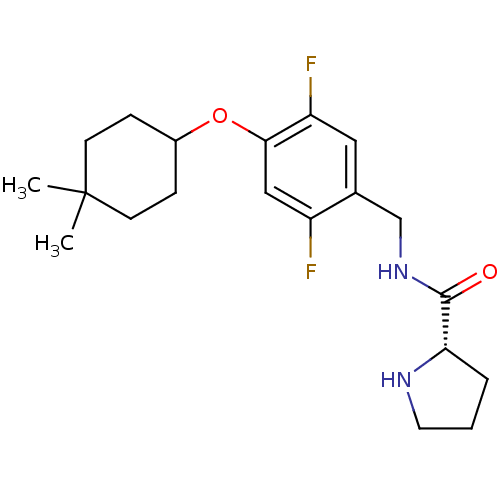
((S)-N-(4-(4,4-dimethylcyclohexyloxy)-2,5-difluorob...)Show SMILES CC1(C)CCC(CC1)Oc1cc(F)c(CNC(=O)[C@@H]2CCCN2)cc1F |r| Show InChI InChI=1S/C20H28F2N2O2/c1-20(2)7-5-14(6-8-20)26-18-11-15(21)13(10-16(18)22)12-24-19(25)17-4-3-9-23-17/h10-11,14,17,23H,3-9,12H2,1-2H3,(H,24,25)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2C receptor |
Bioorg Med Chem Lett 20: 2365-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.107
BindingDB Entry DOI: 10.7270/Q24M94QJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50304803
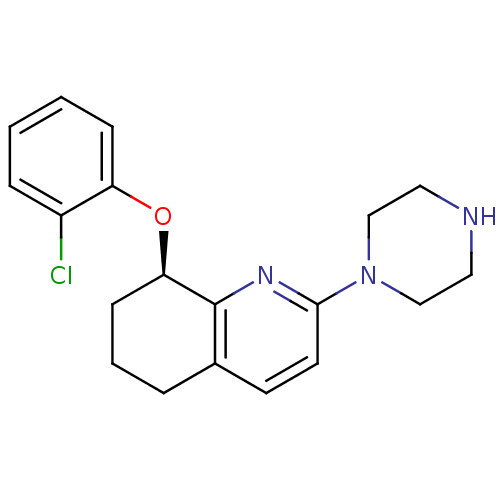
((R)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...)Show SMILES Clc1ccccc1O[C@@H]1CCCc2ccc(nc12)N1CCNCC1 |r| Show InChI InChI=1S/C19H22ClN3O/c20-15-5-1-2-6-16(15)24-17-7-3-4-14-8-9-18(22-19(14)17)23-12-10-21-11-13-23/h1-2,5-6,8-9,17,21H,3-4,7,10-13H2/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50077095

(CHEMBL281945 | Trans-7-Methyl-4-trifluoromethyl-6-...)Show SMILES C[C@H]1CNc2cc3[nH]c(=O)cc(c3cc2C1CCC(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H16F6N2O/c1-8-7-24-13-6-14-11(12(17(21,22)23)5-15(26)25-14)4-10(13)9(8)2-3-16(18,19)20/h4-6,8-9,24H,2-3,7H2,1H3,(H,25,26)/t8-,9?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to the human androgen receptor (hAR), using [3H]DHT as radioligand in a competitive binding assay |
Bioorg Med Chem Lett 9: 1335-40 (1999)
BindingDB Entry DOI: 10.7270/Q2JM2B4G |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50077104
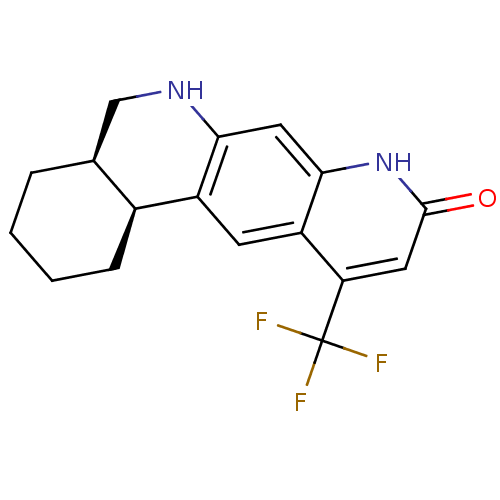
(CHEMBL431493 | Cis-(4aR,12bS)-11-Trifluoromethyl-2...)Show SMILES FC(F)(F)c1cc(=O)[nH]c2cc3NC[C@@H]4CCCC[C@@H]4c3cc12 Show InChI InChI=1S/C17H17F3N2O/c18-17(19,20)13-6-16(23)22-15-7-14-11(5-12(13)15)10-4-2-1-3-9(10)8-21-14/h5-7,9-10,21H,1-4,8H2,(H,22,23)/t9-,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to the human androgen receptor (hAR), using [3H]DHT as radioligand in a competitive binding assay |
Bioorg Med Chem Lett 9: 1335-40 (1999)
BindingDB Entry DOI: 10.7270/Q2JM2B4G |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity to the human androgen receptor (hAR) in CV-1 cells |
Bioorg Med Chem Lett 9: 1335-40 (1999)
BindingDB Entry DOI: 10.7270/Q2JM2B4G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM34141
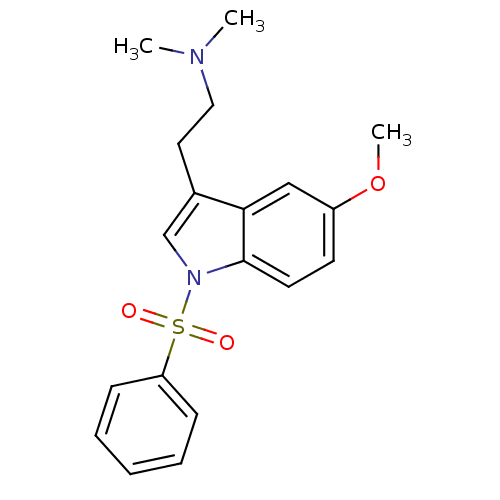
(CHEMBL76237 | MS-245)Show SMILES COc1ccc2n(cc(CCN(C)C)c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H22N2O3S/c1-20(2)12-11-15-14-21(19-10-9-16(24-3)13-18(15)19)25(22,23)17-7-5-4-6-8-17/h4-10,13-14H,11-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182312
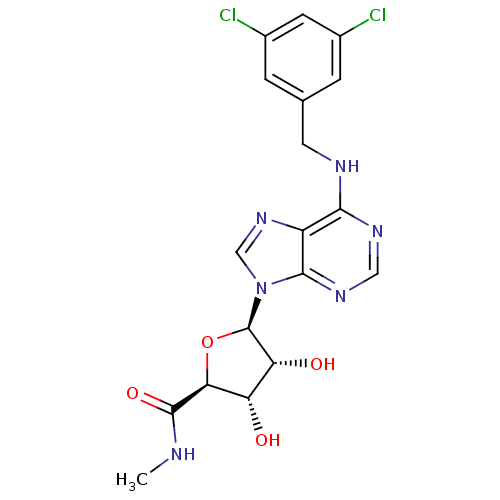
((2S,3S,4R,5R)-5-(6-(3,5-dichlorobenzylamino)-9H-pu...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cc(Cl)cc(Cl)c3)ncnc12 Show InChI InChI=1S/C18H18Cl2N6O4/c1-21-17(29)14-12(27)13(28)18(30-14)26-7-25-11-15(23-6-24-16(11)26)22-5-8-2-9(19)4-10(20)3-8/h2-4,6-7,12-14,18,27-28H,5H2,1H3,(H,21,29)(H,22,23,24)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50112797

(1-{4-[3-(3,4-Dimethoxy-phenyl)-isoxazol-5-yl]-buty...)Show SMILES CCOc1ccccc1N1CCN(CCCCc2cc(no2)-c2ccc(OC)c(OC)c2)CC1 Show InChI InChI=1S/C27H35N3O4/c1-4-33-25-11-6-5-10-24(25)30-17-15-29(16-18-30)14-8-7-9-22-20-23(28-34-22)21-12-13-26(31-2)27(19-21)32-3/h5-6,10-13,19-20H,4,7-9,14-18H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of the radioligand [3H]YM-09151-2 from cloned human Dopamine receptor D3 expressed in CHO cells |
Bioorg Med Chem Lett 12: 1327-30 (2002)
BindingDB Entry DOI: 10.7270/Q2C53MC8 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50304827
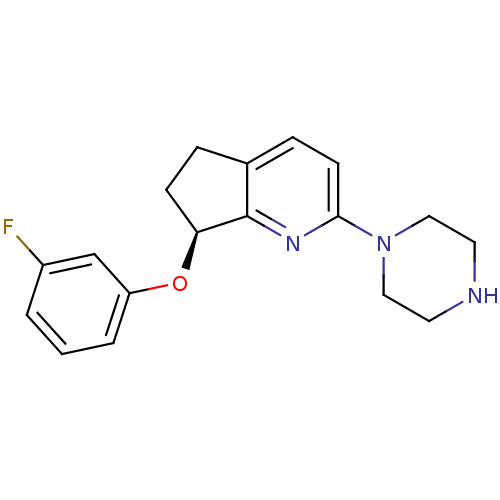
((S)-7-(3-fluorophenoxy)-2-(piperazin-1-yl)-6,7-dih...)Show SMILES Fc1cccc(O[C@H]2CCc3ccc(nc23)N2CCNCC2)c1 |r| Show InChI InChI=1S/C18H20FN3O/c19-14-2-1-3-15(12-14)23-16-6-4-13-5-7-17(21-18(13)16)22-10-8-20-9-11-22/h1-3,5,7,12,16,20H,4,6,8-11H2/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2C receptor expressed in mouse 3T3 cells by scintillation counting |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130268
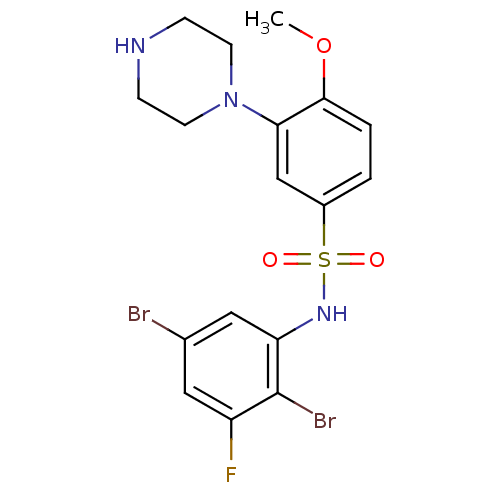
(CHEMBL329383 | N-(2,5-Dibromo-3-fluoro-phenyl)-4-m...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)Nc1cc(Br)cc(F)c1Br Show InChI InChI=1S/C17H18Br2FN3O3S/c1-26-16-3-2-12(10-15(16)23-6-4-21-5-7-23)27(24,25)22-14-9-11(18)8-13(20)17(14)19/h2-3,8-10,21-22H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440252
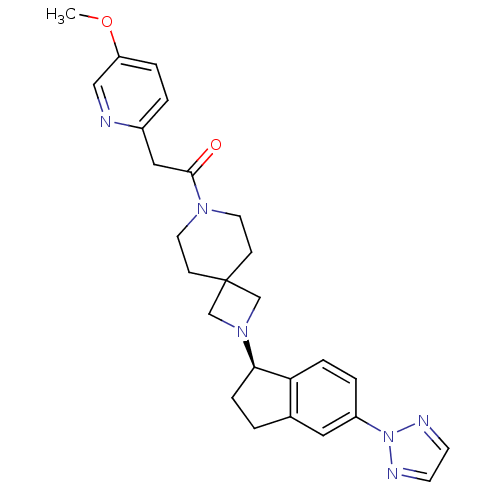
(CHEMBL2426686)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-n3nccn3)CC2)nc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-34-22-5-3-20(27-16-22)15-25(33)30-12-8-26(9-13-30)17-31(18-26)24-7-2-19-14-21(4-6-23(19)24)32-28-10-11-29-32/h3-6,10-11,14,16,24H,2,7-9,12-13,15,17-18H2,1H3/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting |
Bioorg Med Chem Lett 23: 5410-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.044
BindingDB Entry DOI: 10.7270/Q25H7HP5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50304804

(7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dihydro...)Show InChI InChI=1S/C18H20ClN3O/c19-14-3-1-2-4-15(14)23-16-7-5-13-6-8-17(21-18(13)16)22-11-9-20-10-12-22/h1-4,6,8,16,20H,5,7,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182322
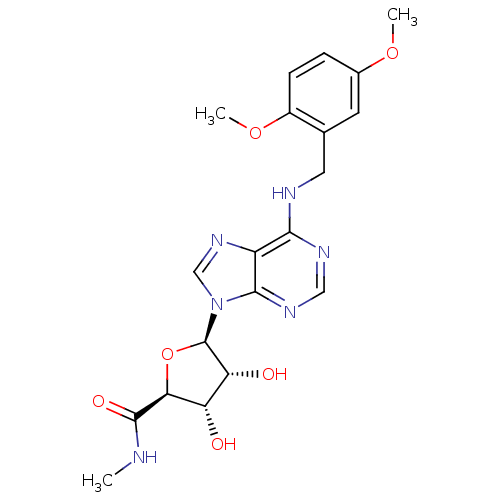
((2S,3S,4R,5R)-5-(6-(2,5-dimethoxybenzylamino)-9H-p...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cc(OC)ccc3OC)ncnc12 Show InChI InChI=1S/C20H24N6O6/c1-21-19(29)16-14(27)15(28)20(32-16)26-9-25-13-17(23-8-24-18(13)26)22-7-10-6-11(30-2)4-5-12(10)31-3/h4-6,8-9,14-16,20,27-28H,7H2,1-3H3,(H,21,29)(H,22,23,24)/t14-,15+,16-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50112787
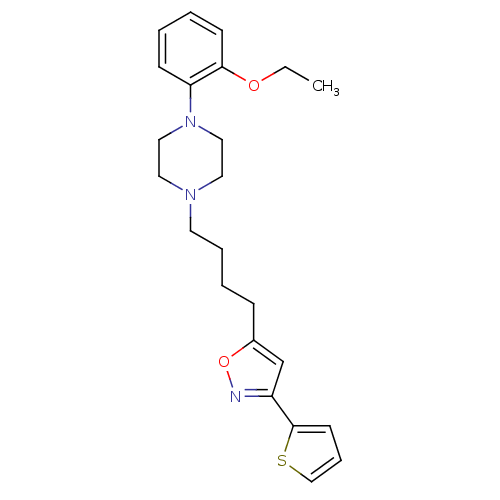
(1-(2-Ethoxy-phenyl)-4-[4-(3-thiophen-2-yl-isoxazol...)Show InChI InChI=1S/C23H29N3O2S/c1-2-27-22-10-4-3-9-21(22)26-15-13-25(14-16-26)12-6-5-8-19-18-20(24-28-19)23-11-7-17-29-23/h3-4,7,9-11,17-18H,2,5-6,8,12-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of the radioligand [3H]YM-09151-2 from cloned human Dopamine receptor D3 expressed in CHO cells |
Bioorg Med Chem Lett 12: 1327-30 (2002)
BindingDB Entry DOI: 10.7270/Q2C53MC8 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750
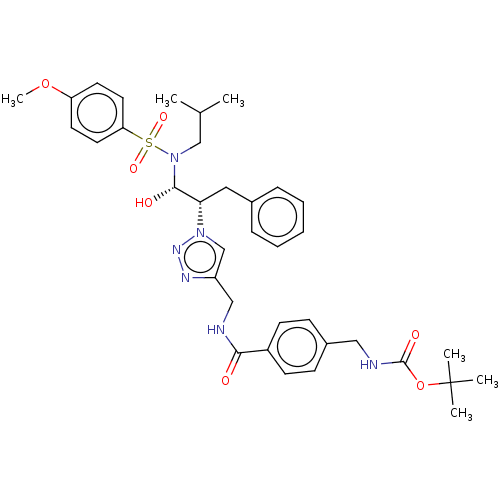
(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50314618
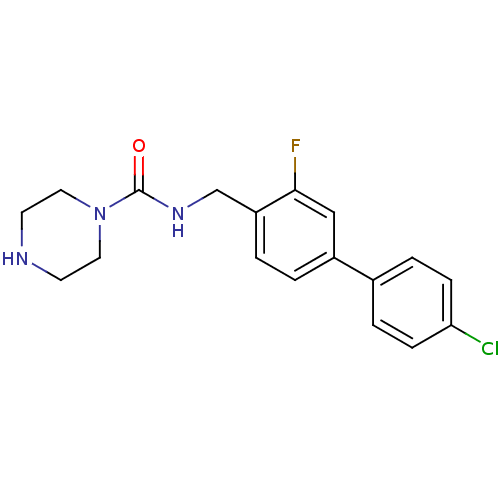
(CHEMBL1090397 | N-((4'-chloro-3-fluorobiphenyl-4-y...)Show InChI InChI=1S/C18H19ClFN3O/c19-16-5-3-13(4-6-16)14-1-2-15(17(20)11-14)12-22-18(24)23-9-7-21-8-10-23/h1-6,11,21H,7-10,12H2,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2C receptor |
Bioorg Med Chem Lett 20: 2365-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.107
BindingDB Entry DOI: 10.7270/Q24M94QJ |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440255
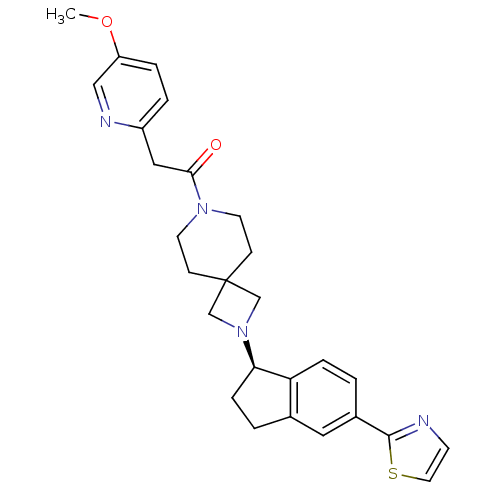
(CHEMBL2426683)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3nccs3)CC2)nc1 |r| Show InChI InChI=1S/C27H30N4O2S/c1-33-22-5-4-21(29-16-22)15-25(32)30-11-8-27(9-12-30)17-31(18-27)24-7-3-19-14-20(2-6-23(19)24)26-28-10-13-34-26/h2,4-6,10,13-14,16,24H,3,7-9,11-12,15,17-18H2,1H3/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting |
Bioorg Med Chem Lett 23: 5410-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.044
BindingDB Entry DOI: 10.7270/Q25H7HP5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50304809
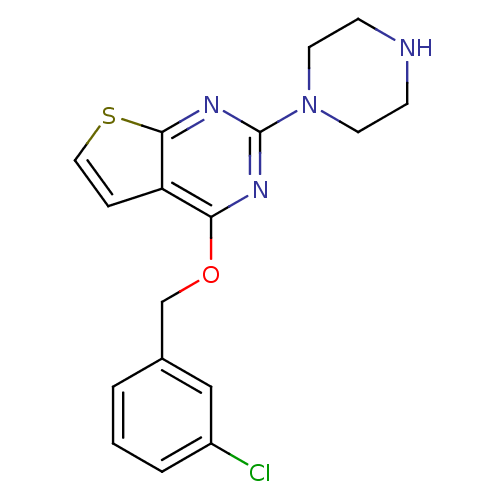
(4-(3-chlorobenzyloxy)-2-(piperazin-1-yl)thieno[2,3...)Show InChI InChI=1S/C17H17ClN4OS/c18-13-3-1-2-12(10-13)11-23-15-14-4-9-24-16(14)21-17(20-15)22-7-5-19-6-8-22/h1-4,9-10,19H,5-8,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50314623
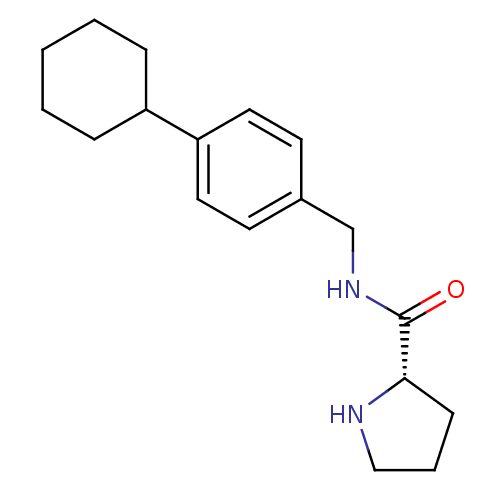
((S)-N-(4-cyclohexylbenzyl)pyrrolidine-2-carboxamid...)Show InChI InChI=1S/C18H26N2O/c21-18(17-7-4-12-19-17)20-13-14-8-10-16(11-9-14)15-5-2-1-3-6-15/h8-11,15,17,19H,1-7,12-13H2,(H,20,21)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2C receptor |
Bioorg Med Chem Lett 20: 2365-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.107
BindingDB Entry DOI: 10.7270/Q24M94QJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50304807

((S)-7-(5-fluoro-2-methylbenzyloxy)-2-((R)-2-methyl...)Show SMILES C[C@@H]1CNCCN1c1ccc2CC[C@H](OCc3cc(F)ccc3C)c2n1 |r| Show InChI InChI=1S/C21H26FN3O/c1-14-3-6-18(22)11-17(14)13-26-19-7-4-16-5-8-20(24-21(16)19)25-10-9-23-12-15(25)2/h3,5-6,8,11,15,19,23H,4,7,9-10,12-13H2,1-2H3/t15-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells |
Bioorg Med Chem Lett 20: 266-71 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.112
BindingDB Entry DOI: 10.7270/Q2154H5M |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019921
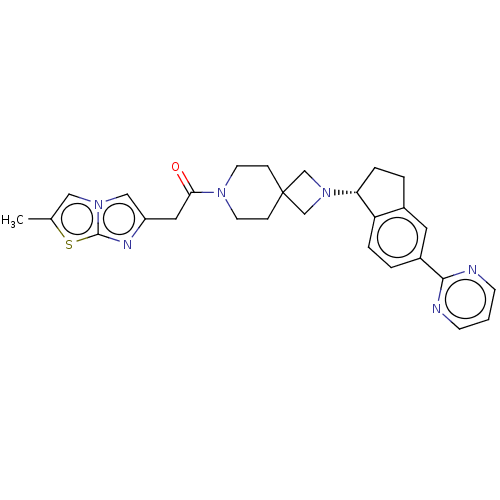
(CHEMBL3287213)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(C4)[C@@H]4CCc5cc(ccc45)-c4ncccn4)CC3)nc2s1 |r| Show InChI InChI=1S/C28H30N6OS/c1-19-15-33-16-22(31-27(33)36-19)14-25(35)32-11-7-28(8-12-32)17-34(18-28)24-6-4-20-13-21(3-5-23(20)24)26-29-9-2-10-30-26/h2-3,5,9-10,13,15-16,24H,4,6-8,11-12,14,17-18H2,1H3/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50112806
(1-(2-Methoxy-phenyl)-4-[4-(3-thiophen-2-yl-isoxazo...)Show InChI InChI=1S/C22H27N3O2S/c1-26-21-9-3-2-8-20(21)25-14-12-24(13-15-25)11-5-4-7-18-17-19(23-27-18)22-10-6-16-28-22/h2-3,6,8-10,16-17H,4-5,7,11-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of the radioligand [3H]YM-09151-2 from cloned human Dopamine receptor D3 expressed in CHO cells |
Bioorg Med Chem Lett 12: 1327-30 (2002)
BindingDB Entry DOI: 10.7270/Q2C53MC8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122927
((2S,3S,4R,5R)-3,4-dihydroxy-N-methyl-5-(6-(methyla...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-13-9-5-10(16-3-15-9)18(4-17-5)12-7(20)6(19)8(22-12)11(21)14-2/h3-4,6-8,12,19-20H,1-2H3,(H,14,21)(H,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122927

((2S,3S,4R,5R)-3,4-dihydroxy-N-methyl-5-(6-(methyla...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-13-9-5-10(16-3-15-9)18(4-17-5)12-7(20)6(19)8(22-12)11(21)14-2/h3-4,6-8,12,19-20H,1-2H3,(H,14,21)(H,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50086525

(2,2-Dimethyl-6-trifluoromethyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C15H15F3N2/c1-14(2)5-3-9-7-10-11(15(16,17)18)4-6-19-13(10)8-12(9)20-14/h4,6-8,20H,3,5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human androgen receptor transfected into mammalian COS-1 cells |
Bioorg Med Chem Lett 10: 411-4 (2000)
BindingDB Entry DOI: 10.7270/Q2K073HH |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50112792
(1-{4-[3-(3,4-Dimethoxy-phenyl)-isoxazol-5-yl]-buty...)Show SMILES COc1ccc(cc1OC)-c1cc(CCCCN2CCN(CC2)c2ccccc2OC)on1 Show InChI InChI=1S/C26H33N3O4/c1-30-24-10-5-4-9-23(24)29-16-14-28(15-17-29)13-7-6-8-21-19-22(27-33-21)20-11-12-25(31-2)26(18-20)32-3/h4-5,9-12,18-19H,6-8,13-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of the radioligand [3H]YM-09151-2 from cloned human Dopamine receptor D3 expressed in CHO cells |
Bioorg Med Chem Lett 12: 1327-30 (2002)
BindingDB Entry DOI: 10.7270/Q2C53MC8 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50386955
(CHEMBL2048820)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(Cc5ccc(cc5Cl)-n5nccn5)C4)CC3)nc2s1 Show InChI InChI=1S/C24H26ClN7OS/c1-17-12-31-14-19(28-23(31)34-17)10-22(33)30-8-4-24(5-9-30)15-29(16-24)13-18-2-3-20(11-21(18)25)32-26-6-7-27-32/h2-3,6-7,11-12,14H,4-5,8-10,13,15-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting |
Bioorg Med Chem Lett 23: 5410-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.044
BindingDB Entry DOI: 10.7270/Q25H7HP5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122926
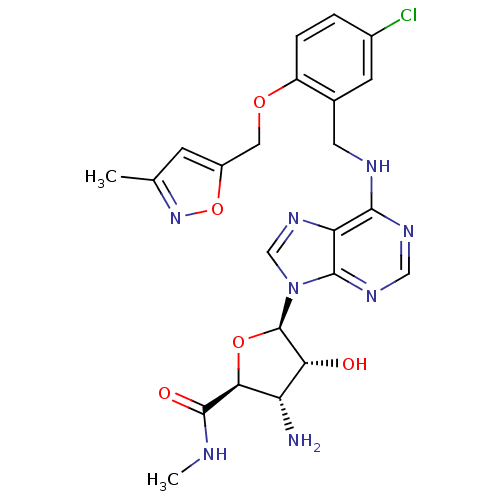
(3-Amino-5-{6-[5-chloro-2-(3-methyl-isoxazol-5-ylme...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)ncnc12 Show InChI InChI=1S/C23H25ClN8O5/c1-11-5-14(37-31-11)8-35-15-4-3-13(24)6-12(15)7-27-20-17-21(29-9-28-20)32(10-30-17)23-18(33)16(25)19(36-23)22(34)26-2/h3-6,9-10,16,18-19,23,33H,7-8,25H2,1-2H3,(H,26,34)(H,27,28,29)/t16-,18+,19-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50112813

(1-(2-Methoxy-phenyl)-4-{4-[3-(3-nitro-phenyl)-isox...)Show SMILES COc1ccccc1N1CCN(CCCCc2cc([n-][o+]2)-c2cccc(c2)[N+]([O-])=O)CC1 Show InChI InChI=1S/C24H28N4O4/c1-31-24-11-3-2-10-23(24)27-15-13-26(14-16-27)12-5-4-9-21-18-22(25-32-21)19-7-6-8-20(17-19)28(29)30/h2-3,6-8,10-11,17-18H,4-5,9,12-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of the radioligand [3H]YM-09151-2 from cloned human Dopamine receptor D3 expressed in CHO cells |
Bioorg Med Chem Lett 12: 1327-30 (2002)
BindingDB Entry DOI: 10.7270/Q2C53MC8 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440261
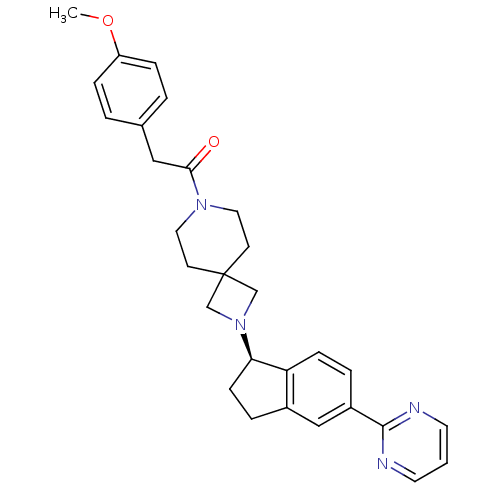
(CHEMBL2426677)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3ncccn3)CC2)cc1 |r| Show InChI InChI=1S/C29H32N4O2/c1-35-24-7-3-21(4-8-24)17-27(34)32-15-11-29(12-16-32)19-33(20-29)26-10-6-22-18-23(5-9-25(22)26)28-30-13-2-14-31-28/h2-5,7-9,13-14,18,26H,6,10-12,15-17,19-20H2,1H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting |
Bioorg Med Chem Lett 23: 5410-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.044
BindingDB Entry DOI: 10.7270/Q25H7HP5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data