 Found 263 hits with Last Name = 'liao' and Initial = 'cc'
Found 263 hits with Last Name = 'liao' and Initial = 'cc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322822
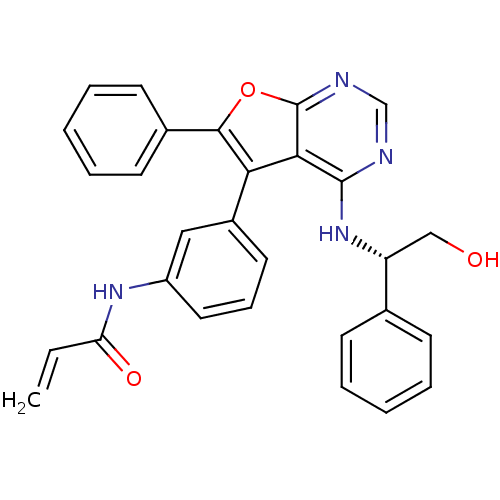
((S)-N-(3-(4-(2-hydroxy-1-phenylethylamino)-6-pheny...)Show SMILES OC[C@@H](Nc1ncnc2oc(c(-c3cccc(NC(=O)C=C)c3)c12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C29H24N4O3/c1-2-24(35)32-22-15-9-14-21(16-22)25-26-28(33-23(17-34)19-10-5-3-6-11-19)30-18-31-29(26)36-27(25)20-12-7-4-8-13-20/h2-16,18,23,34H,1,17H2,(H,32,35)(H,30,31,33)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged EGFR expressed in Escherichia coli |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330230
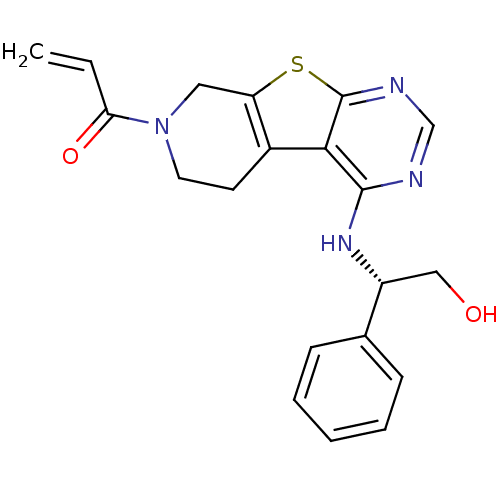
(1-(4-(((1S)-2-Hydroxy-1-phenylethyl)amino)-5,6,7,8...)Show SMILES OC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C20H20N4O2S/c1-2-17(26)24-9-8-14-16(10-24)27-20-18(14)19(21-12-22-20)23-15(11-25)13-6-4-3-5-7-13/h2-7,12,15,25H,1,8-11H2,(H,21,22,23)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330238

((E)-4-(Diethylamino)-1-(4-(((1R)-2-hydroxy-1-pheny...)Show SMILES CCN(CC)C\C=C\C(=O)N1CCc2c(C1)sc1ncnc(N[C@H](CO)c3ccccc3)c21 |r| Show InChI InChI=1S/C25H31N5O2S/c1-3-29(4-2)13-8-11-22(32)30-14-12-19-21(15-30)33-25-23(19)24(26-17-27-25)28-20(16-31)18-9-6-5-7-10-18/h5-11,17,20,31H,3-4,12-16H2,1-2H3,(H,26,27,28)/b11-8+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823
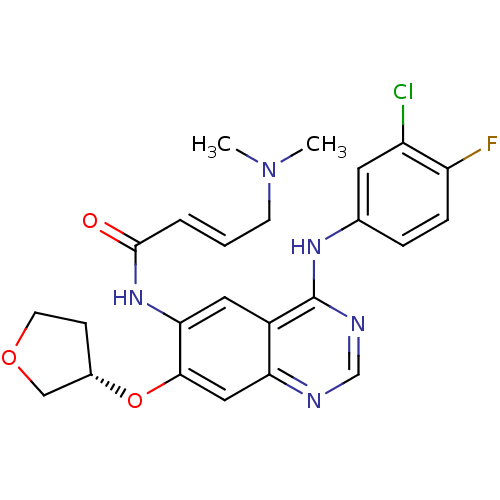
((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged EGFR expressed in Escherichia coli |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330241
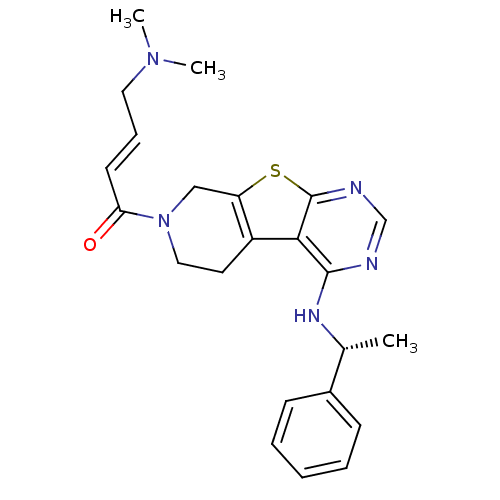
((E)-4-(Dimethylamino)-1-(4-[(1R)-1-phenylethyl]ami...)Show SMILES C[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)\C=C\CN(C)C)c1ccccc1 |r| Show InChI InChI=1S/C23H27N5OS/c1-16(17-8-5-4-6-9-17)26-22-21-18-11-13-28(20(29)10-7-12-27(2)3)14-19(18)30-23(21)25-15-24-22/h4-10,15-16H,11-14H2,1-3H3,(H,24,25,26)/b10-7+/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330242
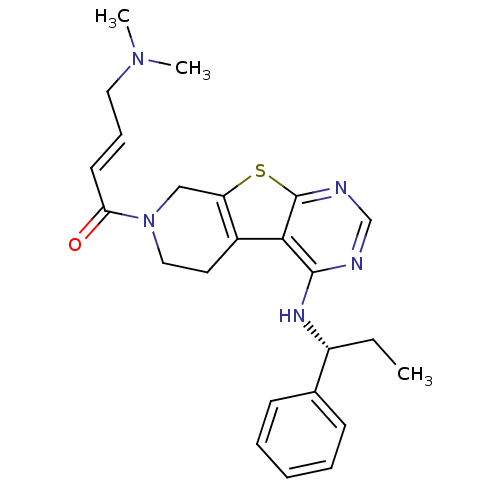
((E)-4-(Dimethylamino)-1-(4-[(1S)-1-phenylpropyl]am...)Show SMILES CC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)\C=C\CN(C)C)c1ccccc1 |r| Show InChI InChI=1S/C24H29N5OS/c1-4-19(17-9-6-5-7-10-17)27-23-22-18-12-14-29(21(30)11-8-13-28(2)3)15-20(18)31-24(22)26-16-25-23/h5-11,16,19H,4,12-15H2,1-3H3,(H,25,26,27)/b11-8+/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330234

(1-(4-[(1R)-1-Phenylethyl]amino-5,6,7,8-tetrahydrop...)Show SMILES C[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C20H20N4OS/c1-3-17(25)24-10-9-15-16(11-24)26-20-18(15)19(21-12-22-20)23-13(2)14-7-5-4-6-8-14/h3-8,12-13H,1,9-11H2,2H3,(H,21,22,23)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447
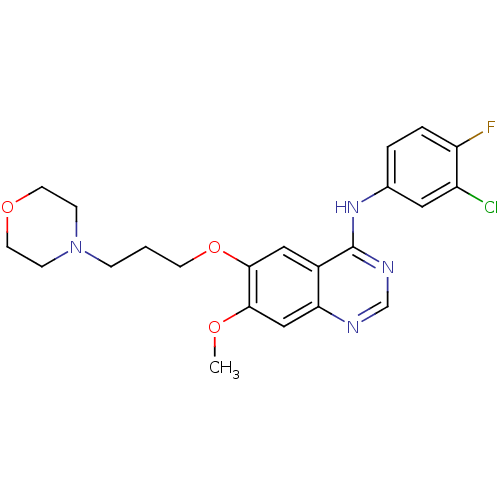
(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged EGFR expressed in Escherichia coli |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330239
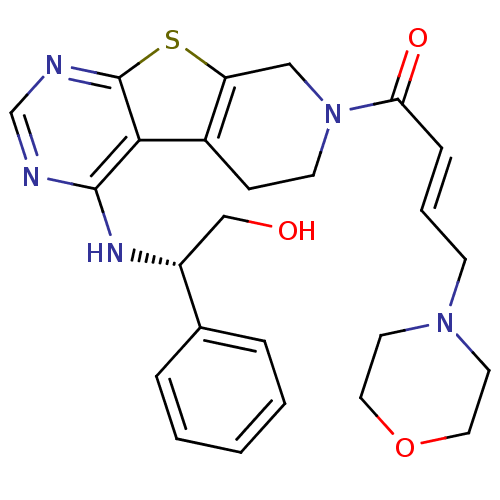
((E)-1-(4-[(1R)-2-Hydroxy-1-phenylethyl]amino-5,6,7...)Show SMILES OC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)\C=C\CN1CCOCC1)c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O3S/c31-16-20(18-5-2-1-3-6-18)28-24-23-19-8-10-30(15-21(19)34-25(23)27-17-26-24)22(32)7-4-9-29-11-13-33-14-12-29/h1-7,17,20,31H,8-16H2,(H,26,27,28)/b7-4+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50185944
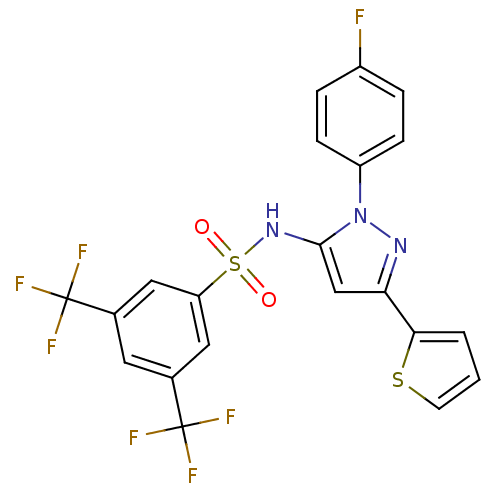
(CHEMBL208472 | N-(1-(4-fluorophenyl)-3-(thiophen-2...)Show SMILES Fc1ccc(cc1)-n1nc(cc1NS(=O)(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)-c1cccs1 Show InChI InChI=1S/C21H12F7N3O2S2/c22-14-3-5-15(6-4-14)31-19(11-17(29-31)18-2-1-7-34-18)30-35(32,33)16-9-12(20(23,24)25)8-13(10-16)21(26,27)28/h1-11,30H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from PPARgamma by SPA |
J Med Chem 49: 2703-12 (2006)
Article DOI: 10.1021/jm051129s
BindingDB Entry DOI: 10.7270/Q2736QHH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50322816
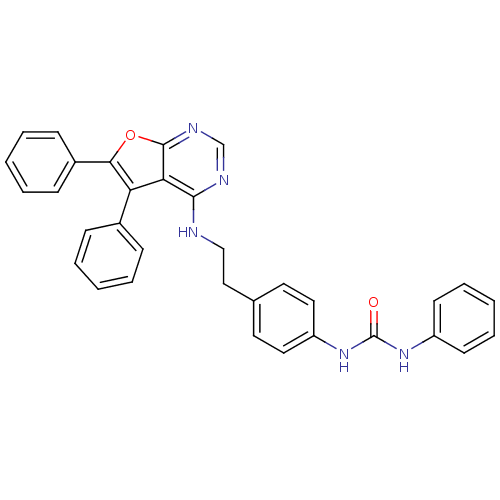
(1-{4-[2-(5,6-Diphenylfuro[2,3-d]pyrimidin-4-ylamin...)Show SMILES O=C(Nc1ccccc1)Nc1ccc(CCNc2ncnc3oc(c(-c4ccccc4)c23)-c2ccccc2)cc1 Show InChI InChI=1S/C33H27N5O2/c39-33(37-26-14-8-3-9-15-26)38-27-18-16-23(17-19-27)20-21-34-31-29-28(24-10-4-1-5-11-24)30(25-12-6-2-7-13-25)40-32(29)36-22-35-31/h1-19,22H,20-21H2,(H,34,35,36)(H2,37,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330235
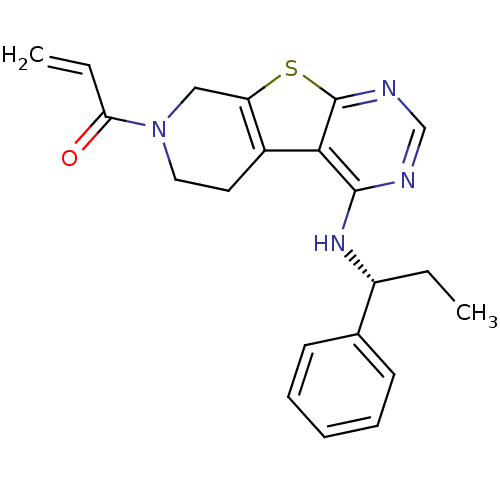
(1-(4-[(1R)-1-Phenylpropyl]amino-5,6,7,8-tetrahydro...)Show SMILES CC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C21H22N4OS/c1-3-16(14-8-6-5-7-9-14)24-20-19-15-10-11-25(18(26)4-2)12-17(15)27-21(19)23-13-22-20/h4-9,13,16H,2-3,10-12H2,1H3,(H,22,23,24)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50181911
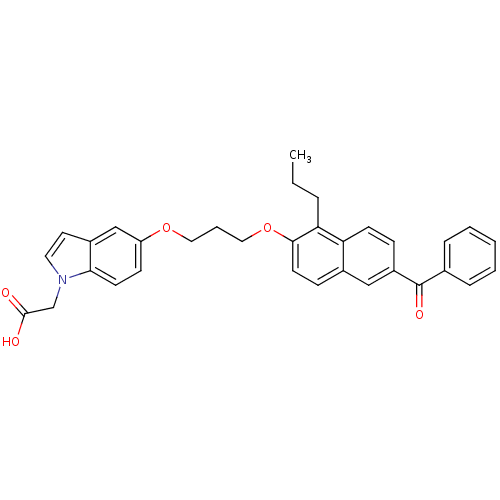
((5-{3-[(6-BENZOYL-1-PROPYL-2-NAPHTHYL)OXY]PROPOXY}...)Show SMILES CCCc1c(OCCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2cc(ccc12)C(=O)c1ccccc1 Show InChI InChI=1S/C33H31NO5/c1-2-7-29-28-13-10-26(33(37)23-8-4-3-5-9-23)20-24(28)11-15-31(29)39-19-6-18-38-27-12-14-30-25(21-27)16-17-34(30)22-32(35)36/h3-5,8-17,20-21H,2,6-7,18-19,22H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330237
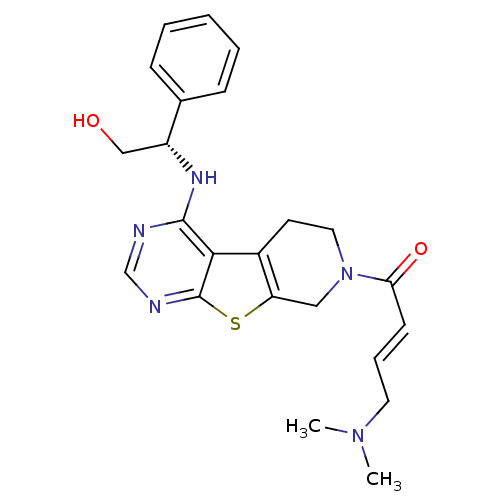
((E)-4-(Dimethylamino)-1-(4-[(1S)-2-hydroxy-1-pheny...)Show SMILES CN(C)C\C=C\C(=O)N1CCc2c(C1)sc1ncnc(N[C@H](CO)c3ccccc3)c21 |r| Show InChI InChI=1S/C23H27N5O2S/c1-27(2)11-6-9-20(30)28-12-10-17-19(13-28)31-23-21(17)22(24-15-25-23)26-18(14-29)16-7-4-3-5-8-16/h3-9,15,18,29H,10-14H2,1-2H3,(H,24,25,26)/b9-6+/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322821
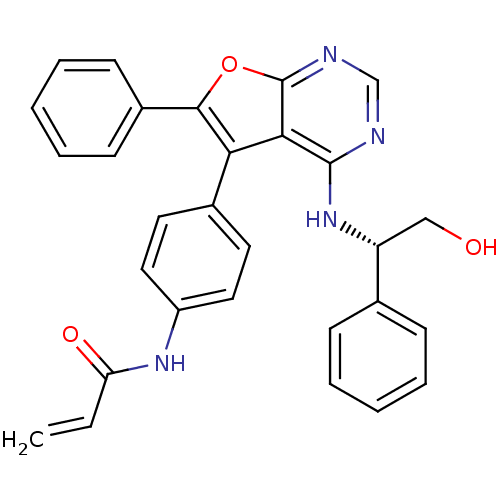
((S)-N-(4-(4-(2-hydroxy-1-phenylethylamino)-6-pheny...)Show SMILES OC[C@@H](Nc1ncnc2oc(c(-c3ccc(NC(=O)C=C)cc3)c12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C29H24N4O3/c1-2-24(35)32-22-15-13-20(14-16-22)25-26-28(33-23(17-34)19-9-5-3-6-10-19)30-18-31-29(26)36-27(25)21-11-7-4-8-12-21/h2-16,18,23,34H,1,17H2,(H,32,35)(H,30,31,33)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged EGFR expressed in Escherichia coli |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330240
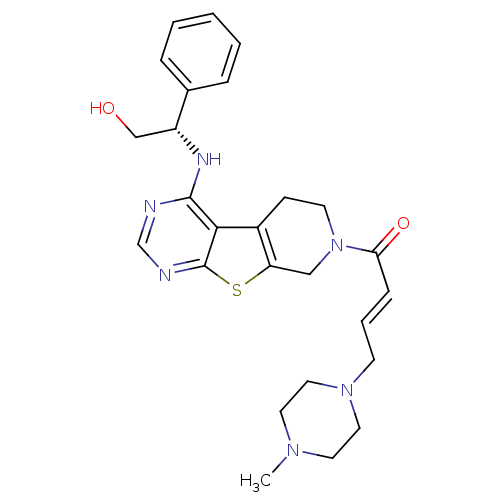
((E)-1-(4-[(1S)-2-Hydroxy-1-phenylethyl]amino-5,6,7...)Show SMILES CN1CCN(C\C=C\C(=O)N2CCc3c(C2)sc2ncnc(N[C@H](CO)c4ccccc4)c32)CC1 |r| Show InChI InChI=1S/C26H32N6O2S/c1-30-12-14-31(15-13-30)10-5-8-23(34)32-11-9-20-22(16-32)35-26-24(20)25(27-18-28-26)29-21(17-33)19-6-3-2-4-7-19/h2-8,18,21,33H,9-17H2,1H3,(H,27,28,29)/b8-5+/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330232
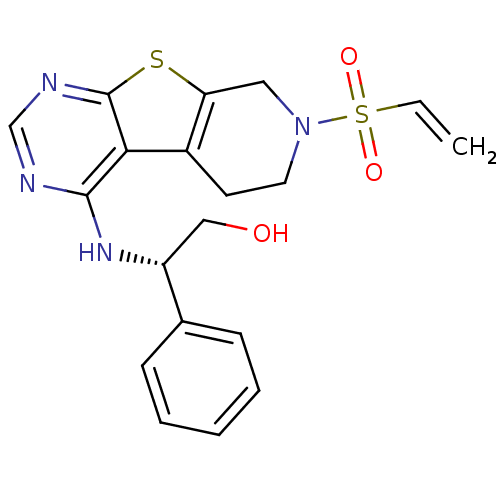
((2S)-2-Phenyl-2-[7-(vinylsulfonyl)-5,6,7,8-tetrahy...)Show SMILES OC[C@@H](Nc1ncnc2sc3CN(CCc3c12)S(=O)(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C19H20N4O3S2/c1-2-28(25,26)23-9-8-14-16(10-23)27-19-17(14)18(20-12-21-19)22-15(11-24)13-6-4-3-5-7-13/h2-7,12,15,24H,1,8-11H2,(H,20,21,22)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681
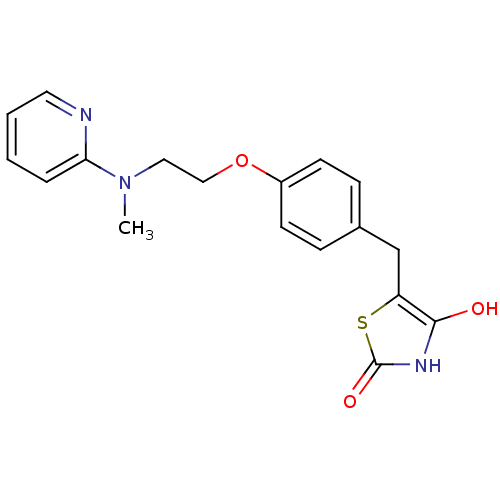
(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 48: 8194-208 (2005)
Article DOI: 10.1021/jm0506930
BindingDB Entry DOI: 10.7270/Q2R78DS5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50179236
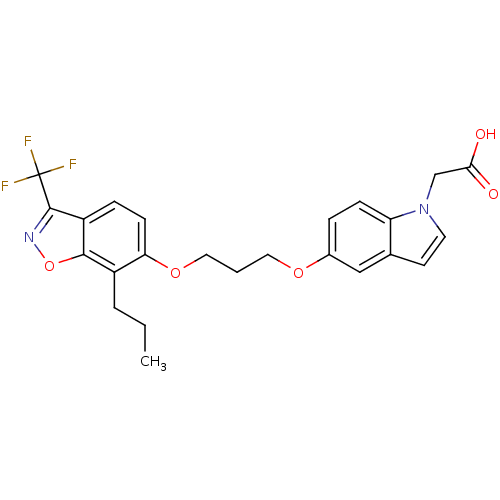
(2-(5-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C24H23F3N2O5/c1-2-4-17-20(8-6-18-22(17)34-28-23(18)24(25,26)27)33-12-3-11-32-16-5-7-19-15(13-16)9-10-29(19)14-21(30)31/h5-10,13H,2-4,11-12,14H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]L-783,483 from human PPAR delta by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50179235
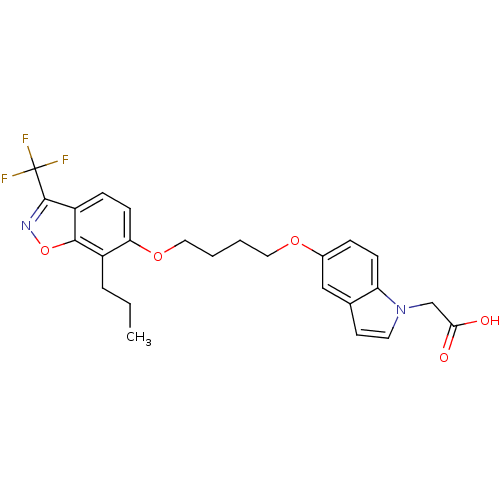
(2-{5-[4-(7-propyl-3-trifluoromethylbenzo[d]isoxazo...)Show SMILES CCCc1c(OCCCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C25H25F3N2O5/c1-2-5-18-21(9-7-19-23(18)35-29-24(19)25(26,27)28)34-13-4-3-12-33-17-6-8-20-16(14-17)10-11-30(20)15-22(31)32/h6-11,14H,2-5,12-13,15H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 48: 8194-208 (2005)
Article DOI: 10.1021/jm0506930
BindingDB Entry DOI: 10.7270/Q2R78DS5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50181911

((5-{3-[(6-BENZOYL-1-PROPYL-2-NAPHTHYL)OXY]PROPOXY}...)Show SMILES CCCc1c(OCCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2cc(ccc12)C(=O)c1ccccc1 Show InChI InChI=1S/C33H31NO5/c1-2-7-29-28-13-10-26(33(37)23-8-4-3-5-9-23)20-24(28)11-15-31(29)39-19-6-18-38-27-12-14-30-25(21-27)16-17-34(30)22-32(35)36/h3-5,8-17,20-21H,2,6-7,18-19,22H2,1H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50181902
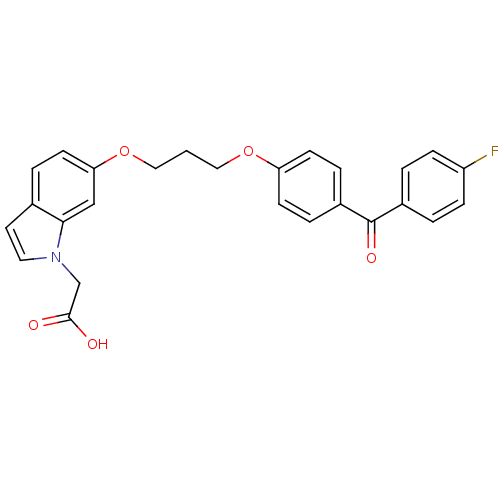
(2-(6-(3-(4-(4-fluorobenzoyl)phenoxy)propoxy)-1H-in...)Show SMILES OC(=O)Cn1ccc2ccc(OCCCOc3ccc(cc3)C(=O)c3ccc(F)cc3)cc12 Show InChI InChI=1S/C26H22FNO5/c27-21-7-2-19(3-8-21)26(31)20-5-9-22(10-6-20)32-14-1-15-33-23-11-4-18-12-13-28(17-25(29)30)24(18)16-23/h2-13,16H,1,14-15,17H2,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50179229
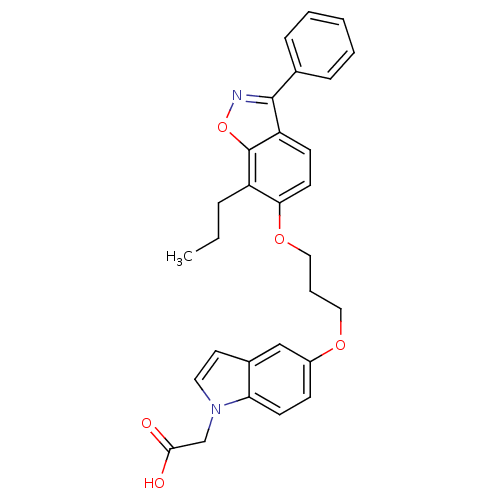
(2-{5-[3-(3-phenyl-7-propylbenzo[d]isoxazol-6-yloxy...)Show SMILES CCCc1c(OCCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2c(noc12)-c1ccccc1 Show InChI InChI=1S/C29H28N2O5/c1-2-7-23-26(13-11-24-28(30-36-29(23)24)20-8-4-3-5-9-20)35-17-6-16-34-22-10-12-25-21(18-22)14-15-31(25)19-27(32)33/h3-5,8-15,18H,2,6-7,16-17,19H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 48: 8194-208 (2005)
Article DOI: 10.1021/jm0506930
BindingDB Entry DOI: 10.7270/Q2R78DS5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50179225
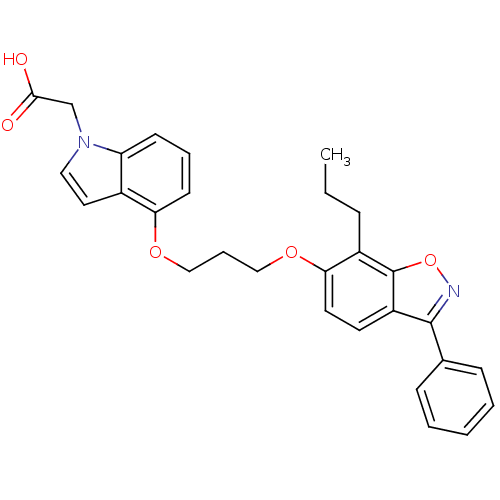
(2-{4-[3-(3-phenyl-7-propylbenzo[d]isoxazol-6-yloxy...)Show SMILES CCCc1c(OCCCOc2cccc3n(CC(O)=O)ccc23)ccc2c(noc12)-c1ccccc1 Show InChI InChI=1S/C29H28N2O5/c1-2-8-22-26(14-13-23-28(30-36-29(22)23)20-9-4-3-5-10-20)35-18-7-17-34-25-12-6-11-24-21(25)15-16-31(24)19-27(32)33/h3-6,9-16H,2,7-8,17-19H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 48: 8194-208 (2005)
Article DOI: 10.1021/jm0506930
BindingDB Entry DOI: 10.7270/Q2R78DS5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50181903
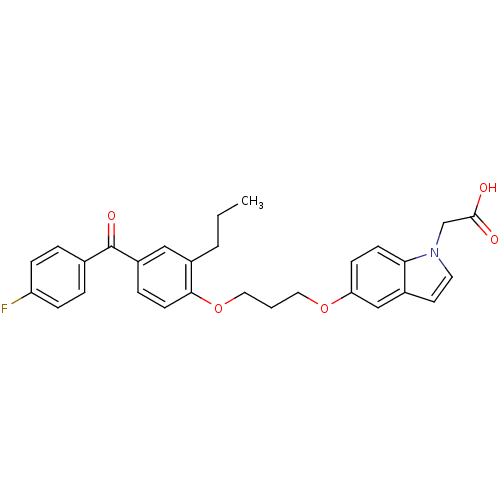
(2-(5-(3-(4-(4-fluorobenzoyl)-2-propylphenoxy)propo...)Show SMILES CCCc1cc(ccc1OCCCOc1ccc2n(CC(O)=O)ccc2c1)C(=O)c1ccc(F)cc1 Show InChI InChI=1S/C29H28FNO5/c1-2-4-22-17-23(29(34)20-5-8-24(30)9-6-20)7-12-27(22)36-16-3-15-35-25-10-11-26-21(18-25)13-14-31(26)19-28(32)33/h5-14,17-18H,2-4,15-16,19H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330243
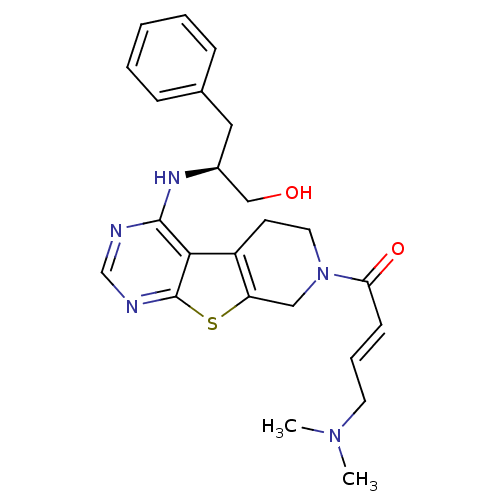
((E)-1-(4-[(1R)-1-Benzyl-2-hydroxyethyl]amino-5,6,7...)Show SMILES CN(C)C\C=C\C(=O)N1CCc2c(C1)sc1ncnc(N[C@H](CO)Cc3ccccc3)c21 |r| Show InChI InChI=1S/C24H29N5O2S/c1-28(2)11-6-9-21(31)29-12-10-19-20(14-29)32-24-22(19)23(25-16-26-24)27-18(15-30)13-17-7-4-3-5-8-17/h3-9,16,18,30H,10-15H2,1-2H3,(H,25,26,27)/b9-6+/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50179236

(2-(5-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C24H23F3N2O5/c1-2-4-17-20(8-6-18-22(17)34-28-23(18)24(25,26)27)33-12-3-11-32-16-5-7-19-15(13-16)9-10-29(19)14-21(30)31/h5-10,13H,2-4,11-12,14H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 48: 8194-208 (2005)
Article DOI: 10.1021/jm0506930
BindingDB Entry DOI: 10.7270/Q2R78DS5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50179236

(2-(5-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C24H23F3N2O5/c1-2-4-17-20(8-6-18-22(17)34-28-23(18)24(25,26)27)33-12-3-11-32-16-5-7-19-15(13-16)9-10-29(19)14-21(30)31/h5-10,13H,2-4,11-12,14H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50185942
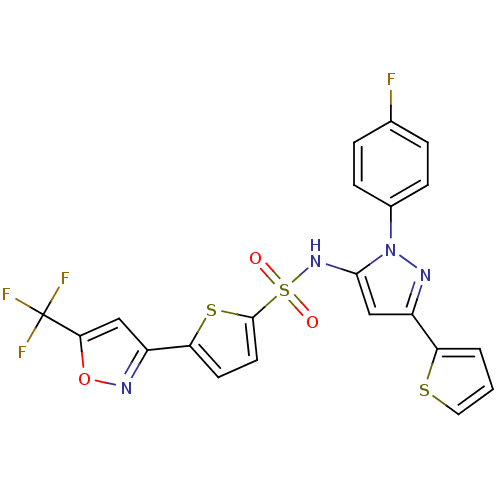
((N-[1-(4-fluorophenyl)-3-(2-thienyl)-1H-pyrazol-5-...)Show SMILES Fc1ccc(cc1)-n1nc(cc1NS(=O)(=O)c1ccc(s1)-c1cc(on1)C(F)(F)F)-c1cccs1 Show InChI InChI=1S/C21H12F4N4O3S3/c22-12-3-5-13(6-4-12)29-19(11-14(26-29)16-2-1-9-33-16)28-35(30,31)20-8-7-17(34-20)15-10-18(32-27-15)21(23,24)25/h1-11,28H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from PPARgamma by SPA |
J Med Chem 49: 2703-12 (2006)
Article DOI: 10.1021/jm051129s
BindingDB Entry DOI: 10.7270/Q2736QHH |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50181913
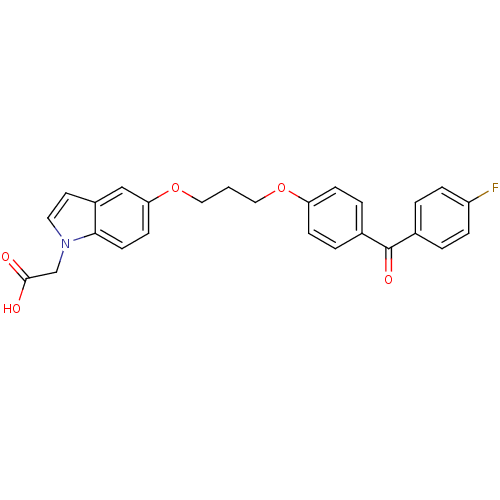
(2-(5-(3-(4-(4-fluorobenzoyl)phenoxy)propoxy)-1H-in...)Show SMILES OC(=O)Cn1ccc2cc(OCCCOc3ccc(cc3)C(=O)c3ccc(F)cc3)ccc12 Show InChI InChI=1S/C26H22FNO5/c27-21-6-2-18(3-7-21)26(31)19-4-8-22(9-5-19)32-14-1-15-33-23-10-11-24-20(16-23)12-13-28(24)17-25(29)30/h2-13,16H,1,14-15,17H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50181911

((5-{3-[(6-BENZOYL-1-PROPYL-2-NAPHTHYL)OXY]PROPOXY}...)Show SMILES CCCc1c(OCCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2cc(ccc12)C(=O)c1ccccc1 Show InChI InChI=1S/C33H31NO5/c1-2-7-29-28-13-10-26(33(37)23-8-4-3-5-9-23)20-24(28)11-15-31(29)39-19-6-18-38-27-12-14-30-25(21-27)16-17-34(30)22-32(35)36/h3-5,8-17,20-21H,2,6-7,18-19,22H2,1H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]L-783,483 from human PPAR delta by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322820
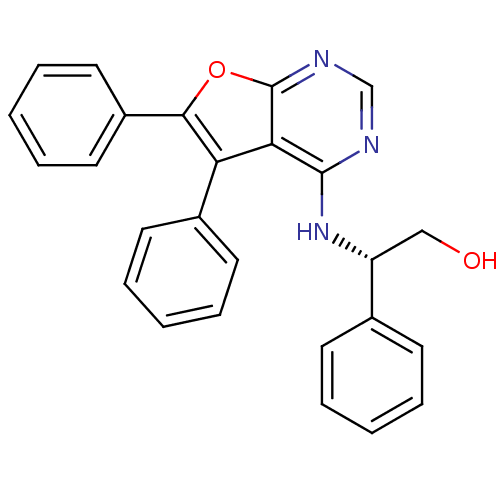
(CHEMBL1172781 | S-2-(5,6-Diphenylfuro[2,3-d]pyrimi...)Show SMILES OC[C@@H](Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H21N3O2/c30-16-21(18-10-4-1-5-11-18)29-25-23-22(19-12-6-2-7-13-19)24(20-14-8-3-9-15-20)31-26(23)28-17-27-25/h1-15,17,21,30H,16H2,(H,27,28,29)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322820

(CHEMBL1172781 | S-2-(5,6-Diphenylfuro[2,3-d]pyrimi...)Show SMILES OC[C@@H](Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H21N3O2/c30-16-21(18-10-4-1-5-11-18)29-25-23-22(19-12-6-2-7-13-19)24(20-14-8-3-9-15-20)31-26(23)28-17-27-25/h1-15,17,21,30H,16H2,(H,27,28,29)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged EGFR expressed in Escherichia coli |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50322805
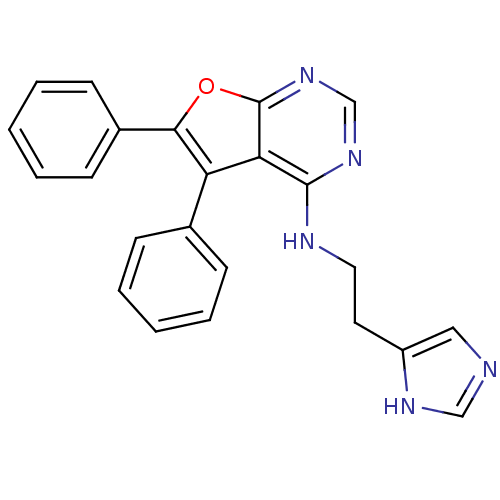
((5,6-Diphenylfuro[2,3-d]pyrimidin-4-yl)-[2-(1H-imi...)Show SMILES C(Cc1cnc[nH]1)Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccccc1 Show InChI InChI=1S/C23H19N5O/c1-3-7-16(8-4-1)19-20-22(25-12-11-18-13-24-14-26-18)27-15-28-23(20)29-21(19)17-9-5-2-6-10-17/h1-10,13-15H,11-12H2,(H,24,26)(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50322817

(CHEMBL1173801 | N-{4-[2-(5,6-Diphenylfuro[2,3-d]py...)Show SMILES O=C(Nc1ccc(CCNc2ncnc3oc(c(-c4ccccc4)c23)-c2ccccc2)cc1)c1ccccc1 Show InChI InChI=1S/C33H26N4O2/c38-32(26-14-8-3-9-15-26)37-27-18-16-23(17-19-27)20-21-34-31-29-28(24-10-4-1-5-11-24)30(25-12-6-2-7-13-25)39-33(29)36-22-35-31/h1-19,22H,20-21H2,(H,37,38)(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM14800
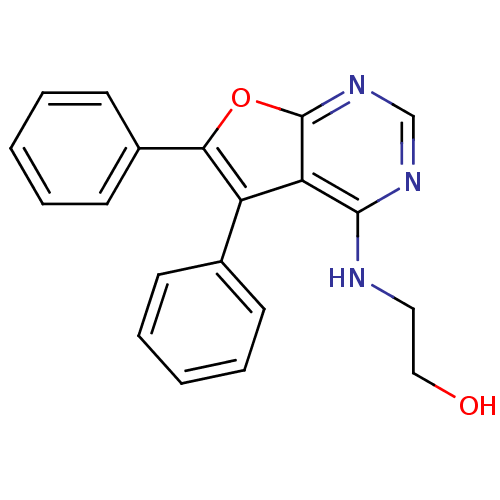
(2-(5,6-Diphenyl-furo[2,3-d]pyrimidin-4-ylamino)-et...)Show InChI InChI=1S/C20H17N3O2/c24-12-11-21-19-17-16(14-7-3-1-4-8-14)18(15-9-5-2-6-10-15)25-20(17)23-13-22-19/h1-10,13,24H,11-12H2,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 309 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50322812

(4-(3,5-Dimethylpiperazin-1-yl)-5,6-diphenylfuro[2,...)Show SMILES CC1CN(CC(C)N1)c1ncnc2oc(c(-c3ccccc3)c12)-c1ccccc1 Show InChI InChI=1S/C24H24N4O/c1-16-13-28(14-17(2)27-16)23-21-20(18-9-5-3-6-10-18)22(19-11-7-4-8-12-19)29-24(21)26-15-25-23/h3-12,15-17,27H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50181907

(2-(4-(3-(4-(4-fluorobenzoyl)-2-propylphenoxy)propo...)Show SMILES CCCc1cc(ccc1OCCCOc1cccc2n(CC(O)=O)ccc12)C(=O)c1ccc(F)cc1 Show InChI InChI=1S/C29H28FNO5/c1-2-5-21-18-22(29(34)20-8-11-23(30)12-9-20)10-13-26(21)35-16-4-17-36-27-7-3-6-25-24(27)14-15-31(25)19-28(32)33/h3,6-15,18H,2,4-5,16-17,19H2,1H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 414 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50179227
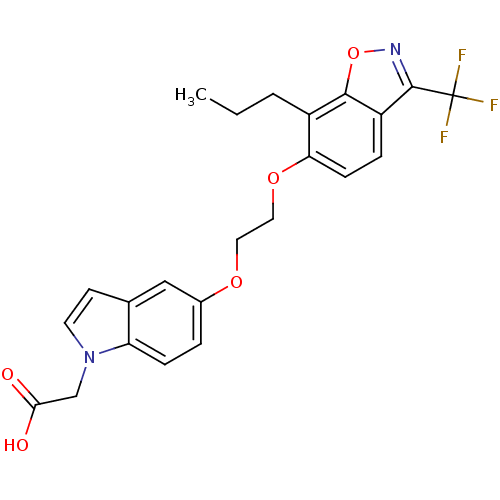
(2-(5-(2-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C23H21F3N2O5/c1-2-3-16-19(7-5-17-21(16)33-27-22(17)23(24,25)26)32-11-10-31-15-4-6-18-14(12-15)8-9-28(18)13-20(29)30/h4-9,12H,2-3,10-11,13H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 48: 8194-208 (2005)
Article DOI: 10.1021/jm0506930
BindingDB Entry DOI: 10.7270/Q2R78DS5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50181903

(2-(5-(3-(4-(4-fluorobenzoyl)-2-propylphenoxy)propo...)Show SMILES CCCc1cc(ccc1OCCCOc1ccc2n(CC(O)=O)ccc2c1)C(=O)c1ccc(F)cc1 Show InChI InChI=1S/C29H28FNO5/c1-2-4-22-17-23(29(34)20-5-8-24(30)9-6-20)7-12-27(22)36-16-3-15-35-25-10-11-26-21(18-25)13-14-31(26)19-28(32)33/h5-14,17-18H,2-4,15-16,19H2,1H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50181904
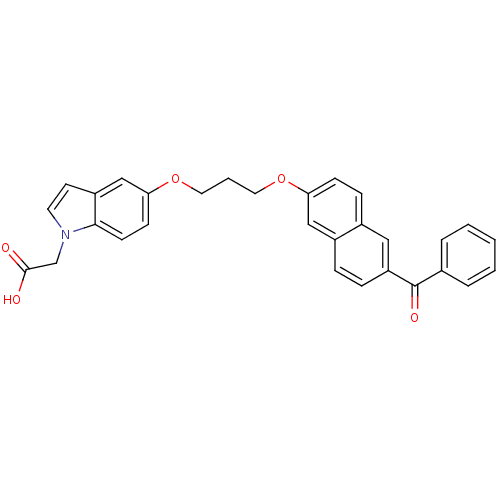
(2-(5-(3-(6-benzoylnaphthalen-2-yloxy)propoxy)-1H-i...)Show SMILES OC(=O)Cn1ccc2cc(OCCCOc3ccc4cc(ccc4c3)C(=O)c3ccccc3)ccc12 Show InChI InChI=1S/C30H25NO5/c32-29(33)20-31-14-13-24-19-27(11-12-28(24)31)36-16-4-15-35-26-10-9-22-17-25(8-7-23(22)18-26)30(34)21-5-2-1-3-6-21/h1-3,5-14,17-19H,4,15-16,20H2,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50179236

(2-(5-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C24H23F3N2O5/c1-2-4-17-20(8-6-18-22(17)34-28-23(18)24(25,26)27)33-12-3-11-32-16-5-7-19-15(13-16)9-10-29(19)14-21(30)31/h5-10,13H,2-4,11-12,14H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50185943

(3-FLUORO-N-[1-(4-FLUOROPHENYL)-3-(2-THIENYL)-1H-PY...)Show SMILES Fc1ccc(cc1)-n1nc(cc1NS(=O)(=O)c1cccc(F)c1)-c1cccs1 Show InChI InChI=1S/C19H13F2N3O2S2/c20-13-6-8-15(9-7-13)24-19(12-17(22-24)18-5-2-10-27-18)23-28(25,26)16-4-1-3-14(21)11-16/h1-12,23H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 512 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from PPARgamma by SPA |
J Med Chem 49: 2703-12 (2006)
Article DOI: 10.1021/jm051129s
BindingDB Entry DOI: 10.7270/Q2736QHH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50322810
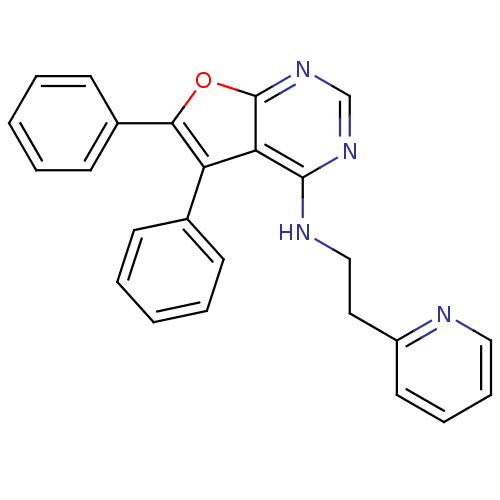
((5,6-Diphenylfuro[2,3-d]pyrimidin-4-yl)-(2-pyridin...)Show SMILES C(Cc1ccccn1)Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccccc1 Show InChI InChI=1S/C25H20N4O/c1-3-9-18(10-4-1)21-22-24(27-16-14-20-13-7-8-15-26-20)28-17-29-25(22)30-23(21)19-11-5-2-6-12-19/h1-13,15,17H,14,16H2,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 535 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 4980-8 (2010)
Checked by Author
Article DOI: 10.1021/jm1000198
BindingDB Entry DOI: 10.7270/Q2Z60P87 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50181905
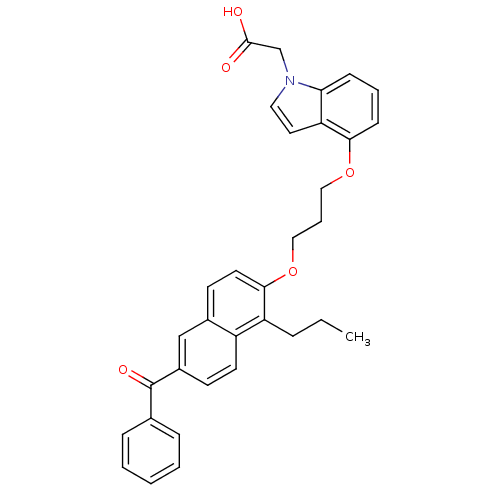
(2-(4-(3-(6-benzoyl-1-propylnaphthalen-2-yloxy)prop...)Show SMILES CCCc1c(OCCCOc2cccc3n(CC(O)=O)ccc23)ccc2cc(ccc12)C(=O)c1ccccc1 Show InChI InChI=1S/C33H31NO5/c1-2-8-27-26-15-13-25(33(37)23-9-4-3-5-10-23)21-24(26)14-16-31(27)39-20-7-19-38-30-12-6-11-29-28(30)17-18-34(29)22-32(35)36/h3-6,9-18,21H,2,7-8,19-20,22H2,1H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 578 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50179240
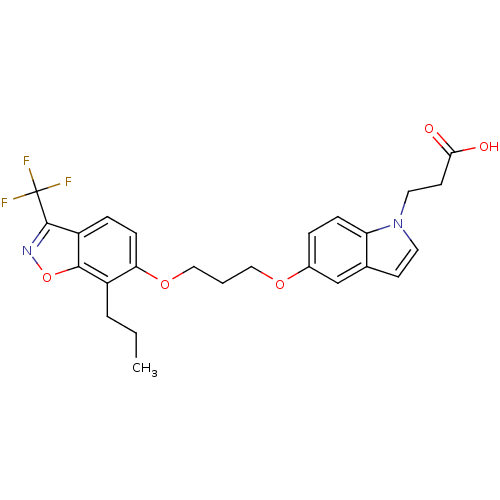
(3-{5-[3-(7-propyl-3-trifluoromethylbenzo[d]isoxazo...)Show SMILES CCCc1c(OCCCOc2ccc3n(CCC(O)=O)ccc3c2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C25H25F3N2O5/c1-2-4-18-21(8-6-19-23(18)35-29-24(19)25(26,27)28)34-14-3-13-33-17-5-7-20-16(15-17)9-11-30(20)12-10-22(31)32/h5-9,11,15H,2-4,10,12-14H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 584 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 48: 8194-208 (2005)
Article DOI: 10.1021/jm0506930
BindingDB Entry DOI: 10.7270/Q2R78DS5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50181904

(2-(5-(3-(6-benzoylnaphthalen-2-yloxy)propoxy)-1H-i...)Show SMILES OC(=O)Cn1ccc2cc(OCCCOc3ccc4cc(ccc4c3)C(=O)c3ccccc3)ccc12 Show InChI InChI=1S/C30H25NO5/c32-29(33)20-31-14-13-24-19-27(11-12-28(24)31)36-16-4-15-35-26-10-9-22-17-25(8-7-23(22)18-26)30(34)21-5-2-1-3-6-21/h1-3,5-14,17-19H,4,15-16,20H2,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 629 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay |
J Med Chem 49: 1212-6 (2006)
Article DOI: 10.1021/jm0510373
BindingDB Entry DOI: 10.7270/Q2QV3M3P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data