

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50232355 ((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361235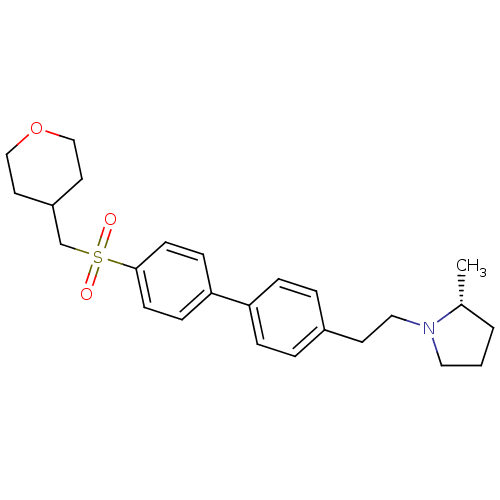 (CHEMBL1934525) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352357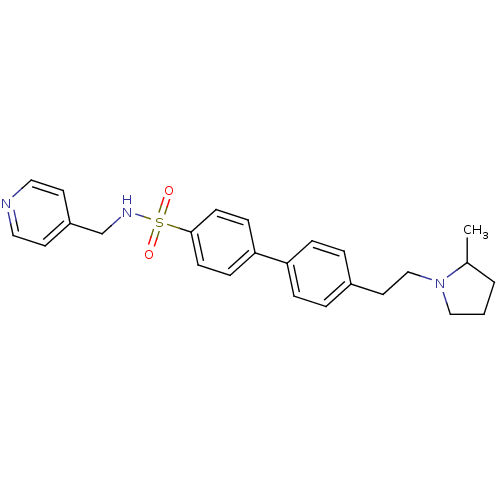 (CHEMBL558655) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361237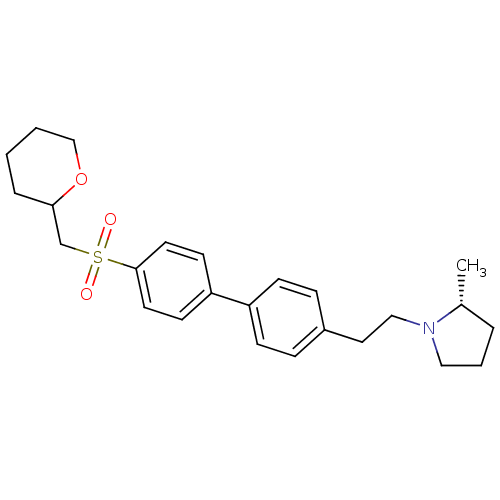 (CHEMBL1934527) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352358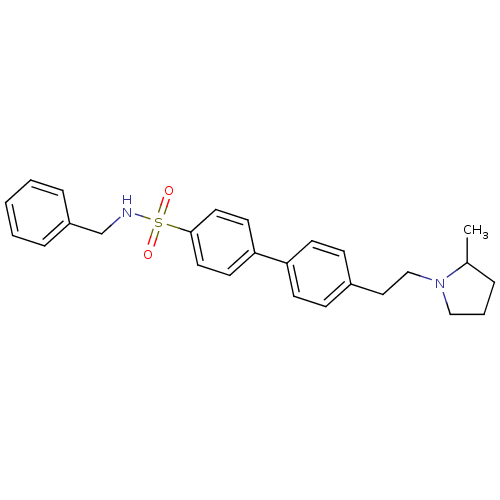 (CHEMBL558456) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243638 (1-((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243122 (2-methyl-1-((3aR,6aR)-6-(4-(2-((R)-2-methylpyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243123 (CHEMBL488464 | cyclopentyl((3aR,6aR)-6-(4-(2-((R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50414743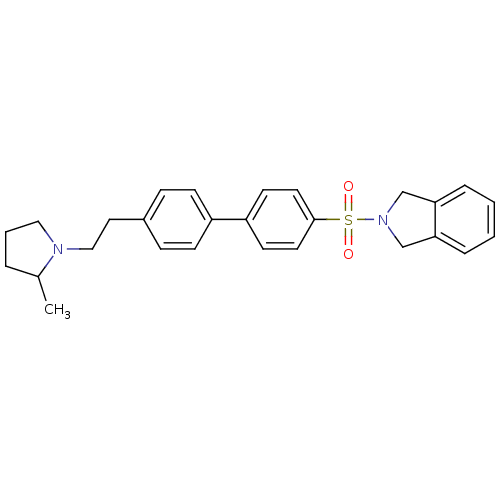 (CHEMBL564803) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361226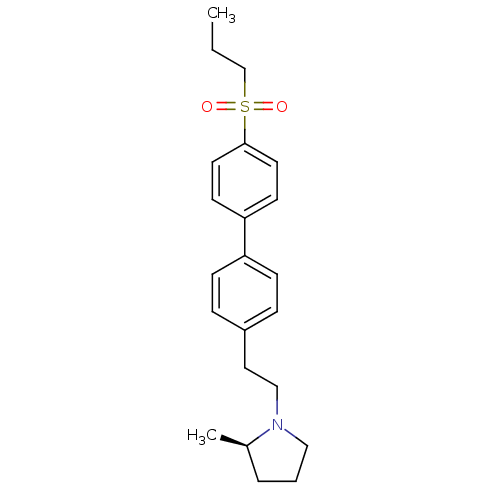 (CHEMBL1934356) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361228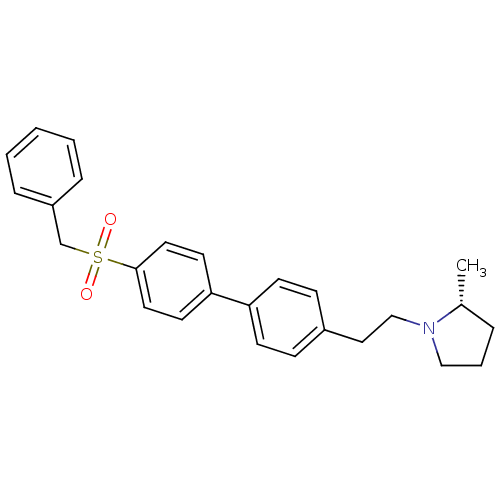 (CHEMBL1934358) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352354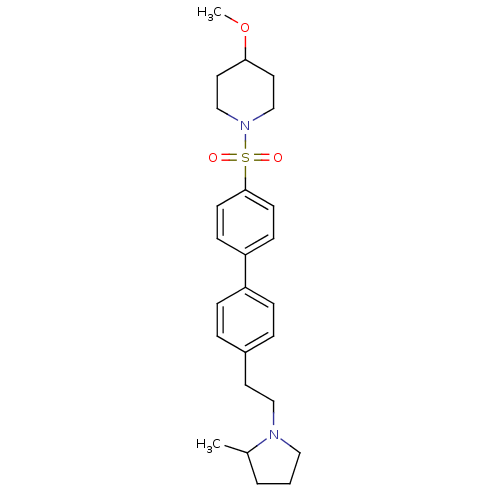 (CHEMBL554506) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361233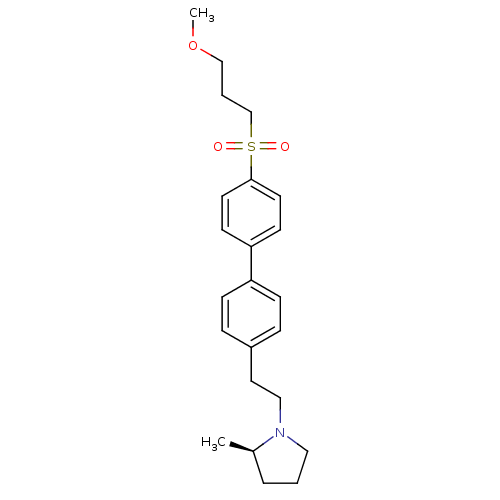 (CHEMBL1934523) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243674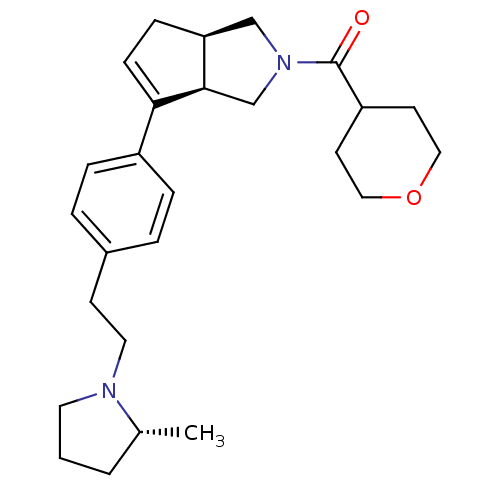 (((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361234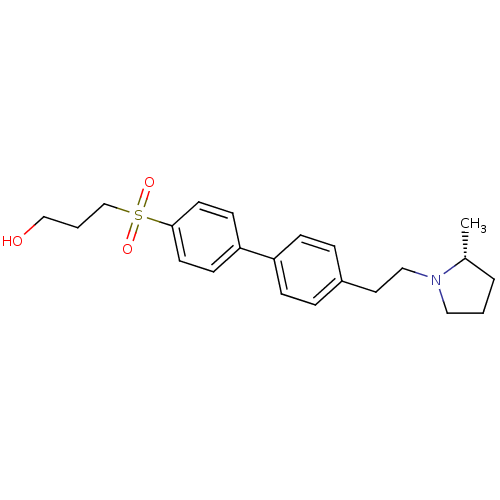 (CHEMBL1934524) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361225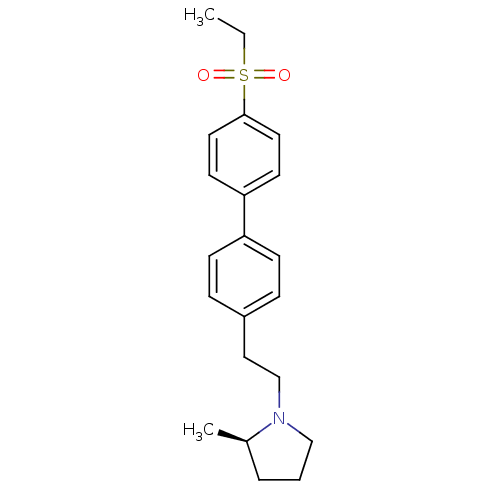 (CHEMBL1934355) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50232355 ((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHOK1 cells by [3H]R(-)-alpha-methylhistamine displacement assay | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352355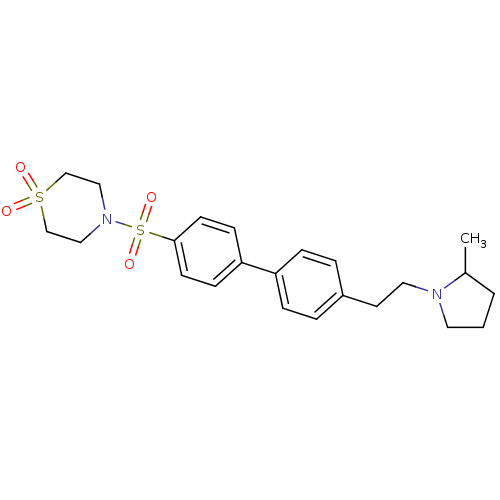 (CHEMBL560140) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50297366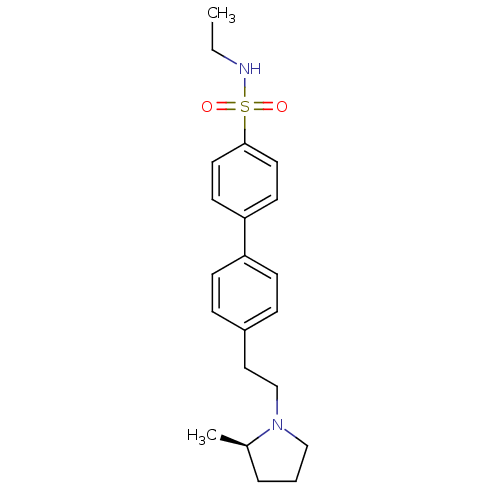 (4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity against human histamine H3 receptor by [35S]GTPgamma binding assay | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50297367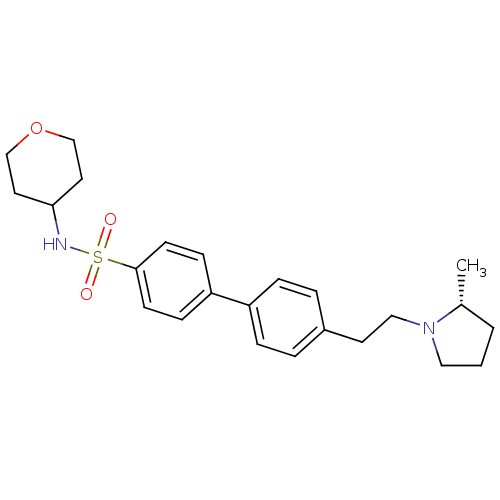 (4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity against human histamine H3 receptor by [35S]GTPgamma binding assay | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50232355 ((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity at human H3 receptor expressed in CHOK1 cells by GTPgammaS binding assay | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361229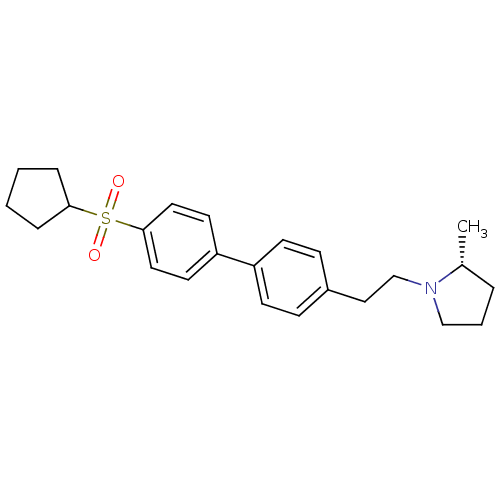 (CHEMBL1934519) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361232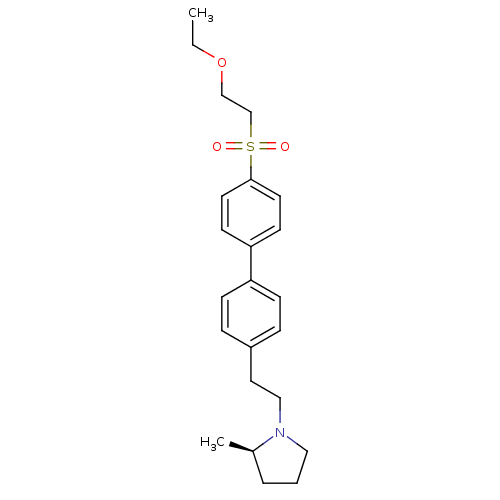 (CHEMBL1934522) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50297368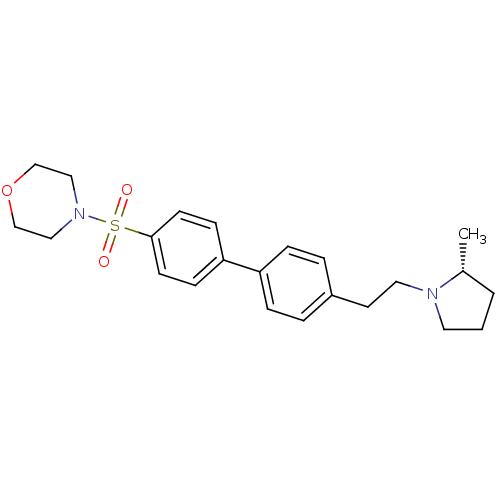 (4-{4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity against human histamine H3 receptor by [35S]GTPgamma binding assay | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243639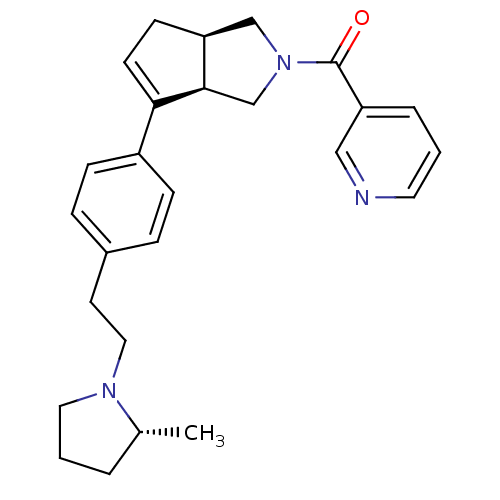 (((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361236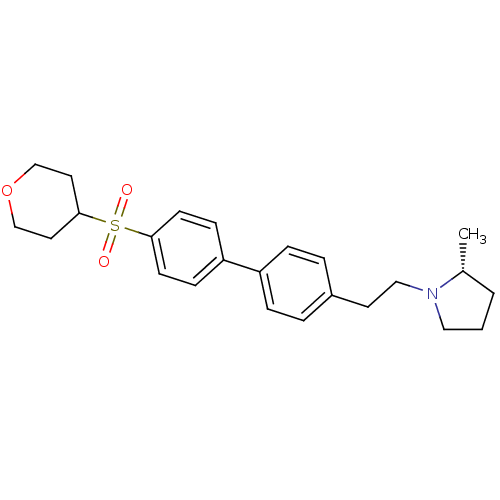 (CHEMBL1934526) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361231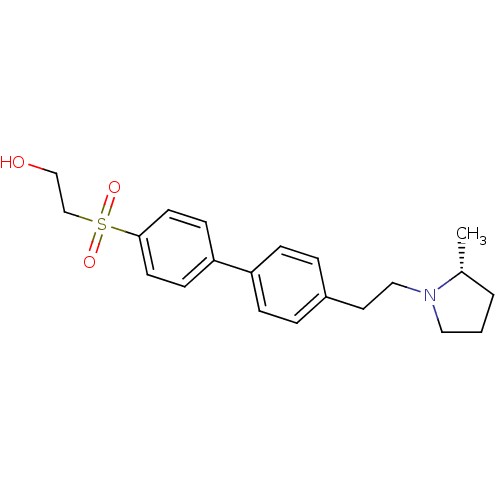 (CHEMBL1934521) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361224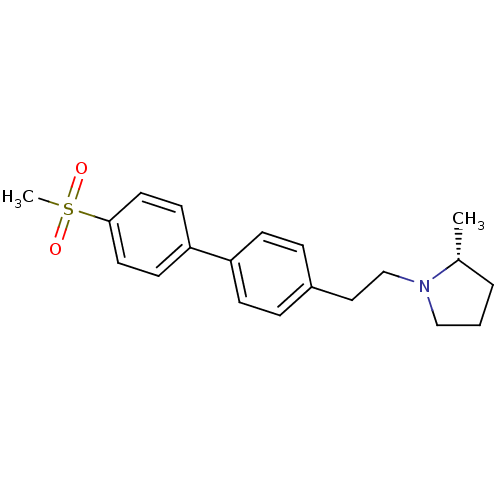 (CHEMBL1934354) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361230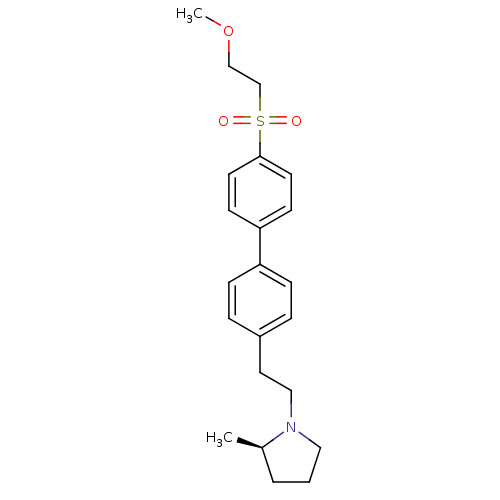 (CHEMBL1934520) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50414742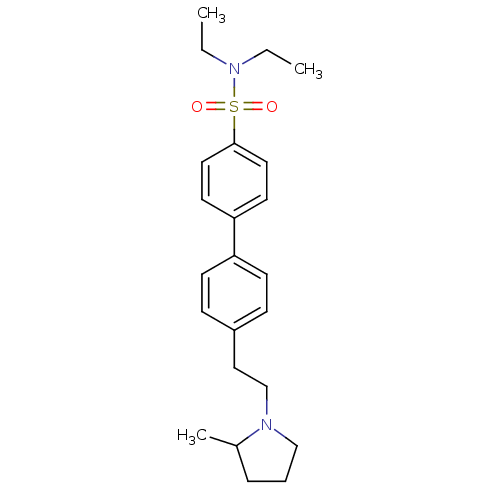 (CHEMBL560799) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50297368 (4-{4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352364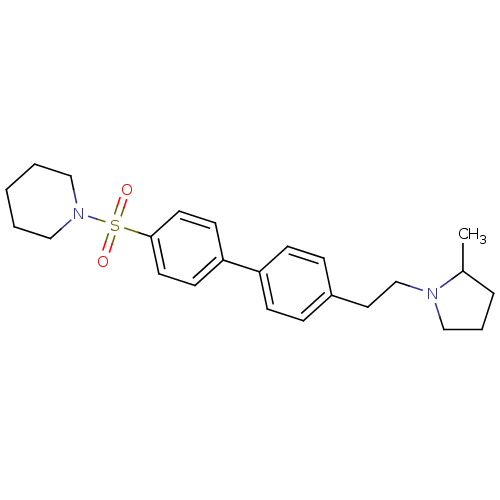 (CHEMBL562629) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50297367 (4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352359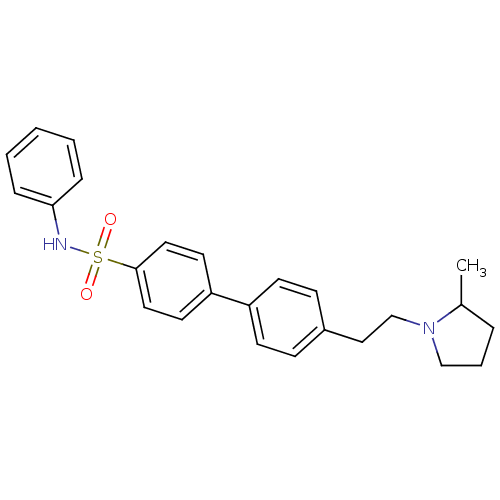 (CHEMBL552279) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50243674 (((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHOK1 cells by [3H]R(-)-alpha-methylhistamine displacement assay | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352360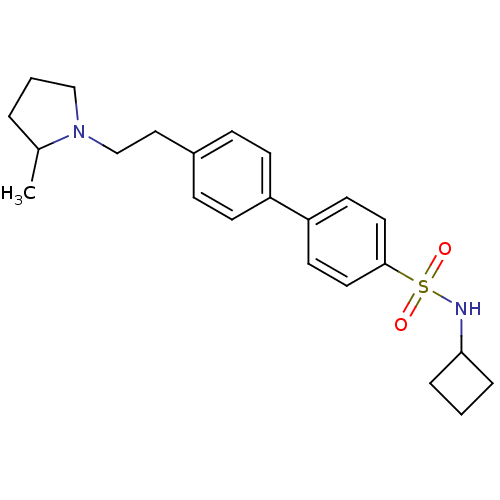 (CHEMBL563118) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361238 (CHEMBL1934528) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352353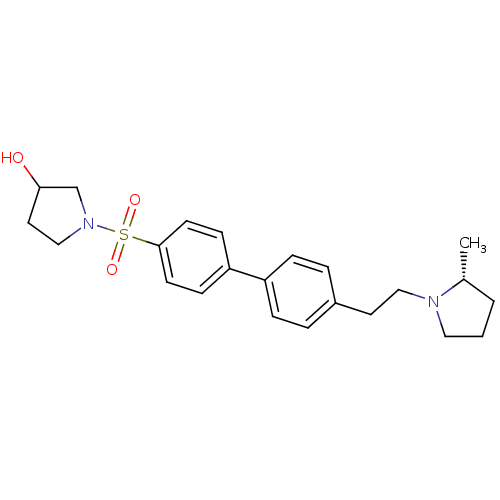 (CHEMBL1823053) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352361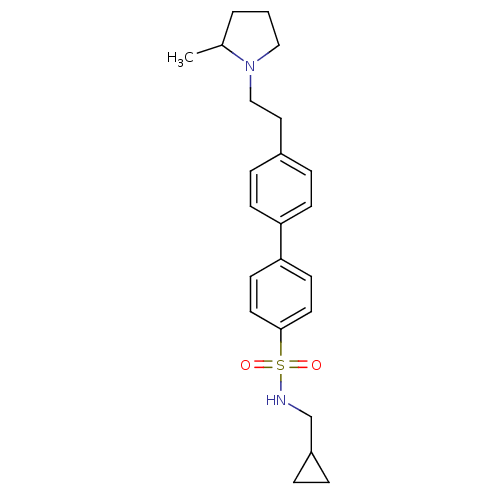 (CHEMBL558260) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361227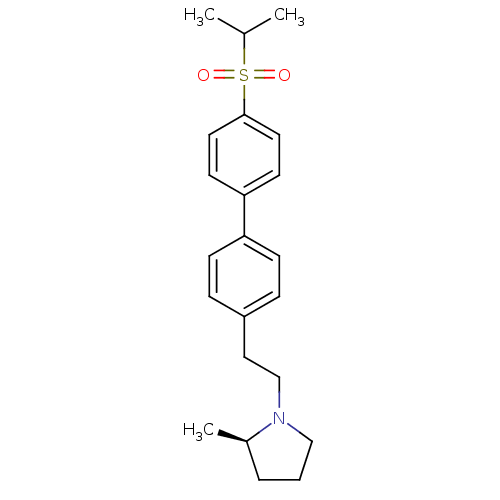 (CHEMBL1934357) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50297368 (4-{4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243120 (CHEMBL520719 | cyclopentyl((3aR,6aR)-6-(4-(2-(pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243175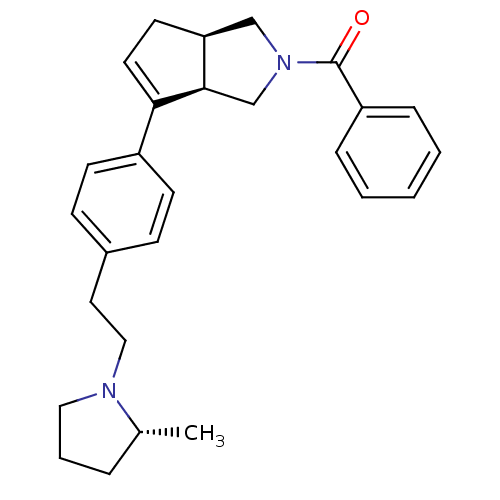 (((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50297369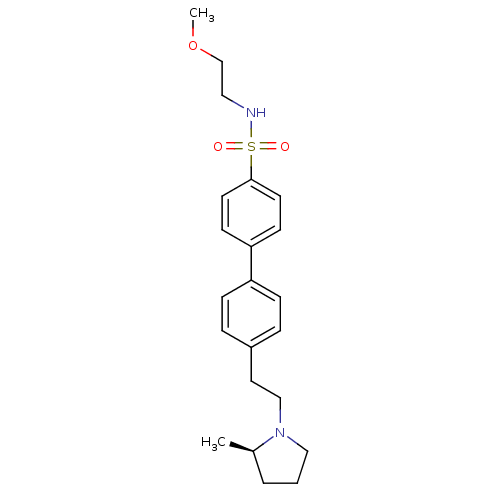 (4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243676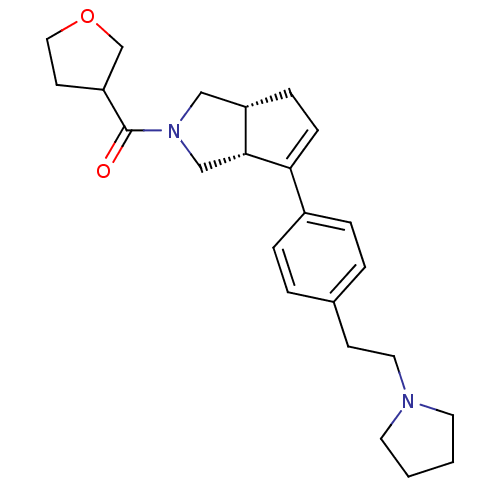 (((3aR,6aR)-6-(4-(2-(pyrrolidin-1-yl)ethyl)phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50243674 (((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity at human H3 receptor expressed in CHOK1 cells by GTPgammaS binding assay | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50361233 (CHEMBL1934523) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human cloned histamine H3 receptor | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50414741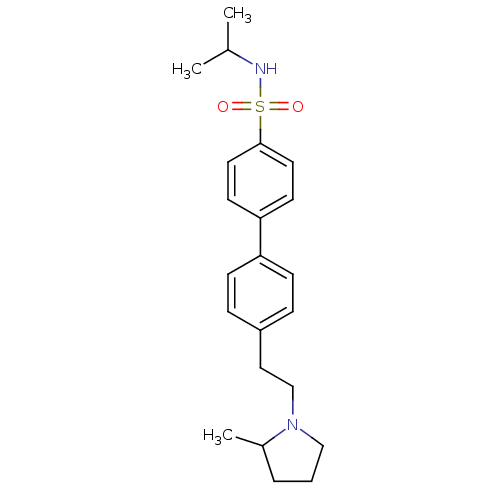 (CHEMBL550198) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352363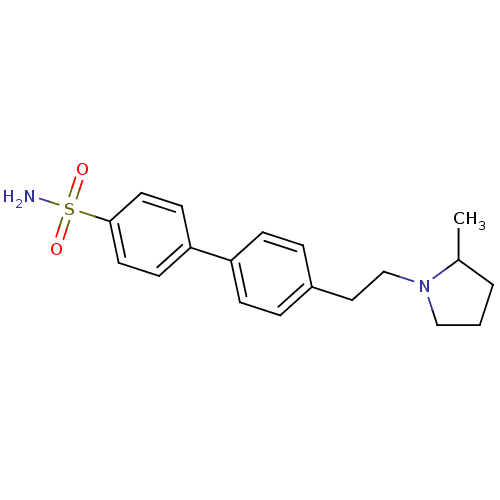 (CHEMBL558923) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50297366 (4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 84 total ) | Next | Last >> |