

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM132084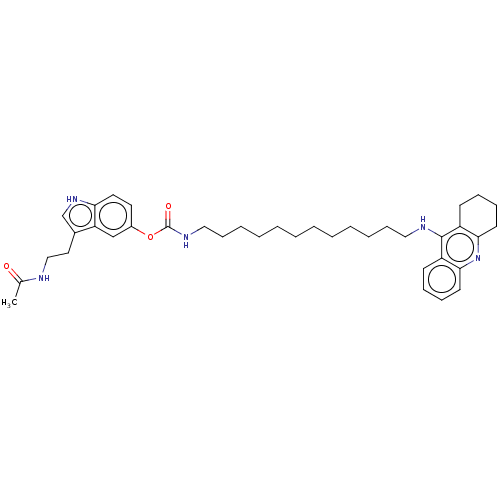 (US8841453, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132080 (US8841453, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132071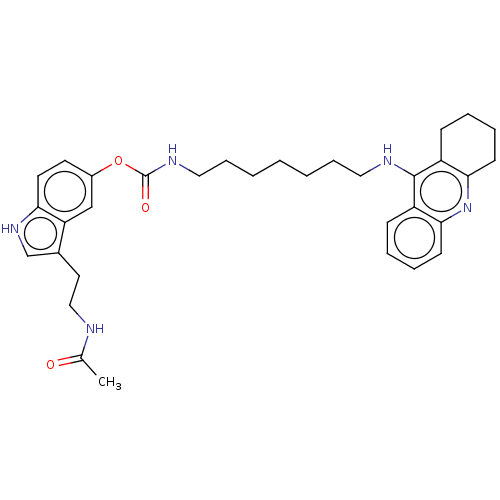 (US8841453, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132083 (US8841453, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132074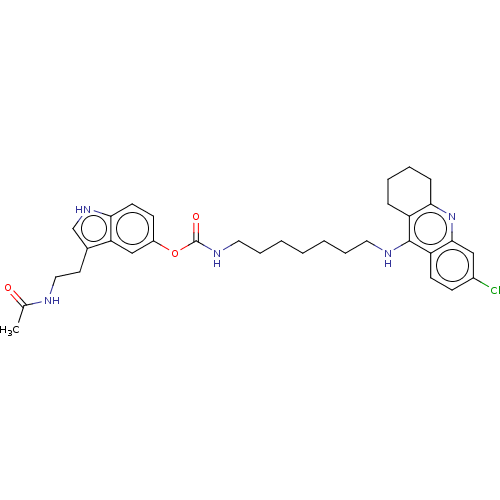 (US8841453, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132088 (US8841453, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132081 (US8841453, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132083 (US8841453, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132086 (US8841453, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132087 (US8841453, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132085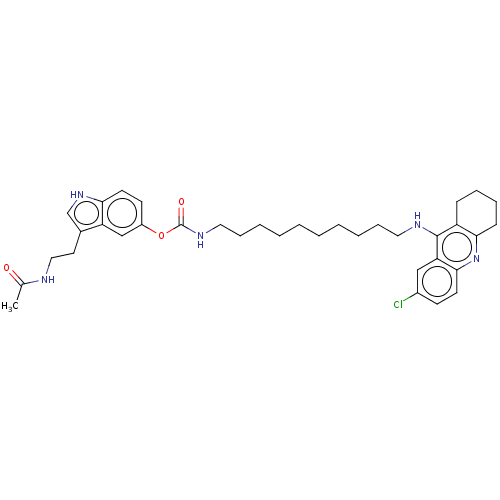 (US8841453, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132085 (US8841453, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132073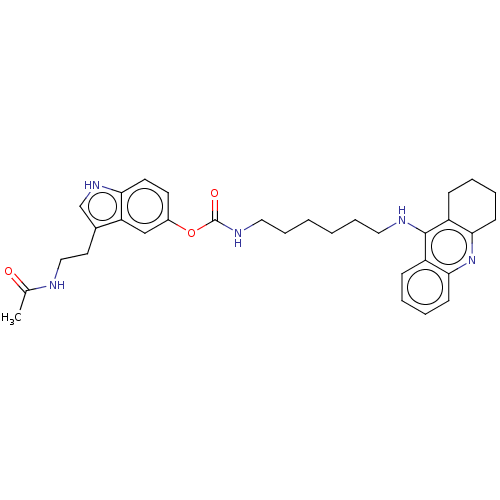 (US8841453, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132079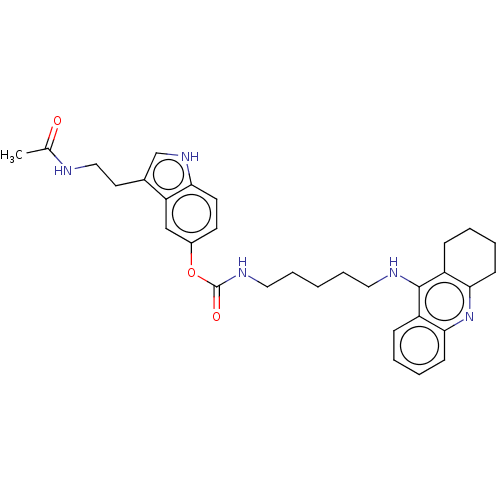 (US8841453, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.07 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132076 (US8841453, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132084 (US8841453, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439218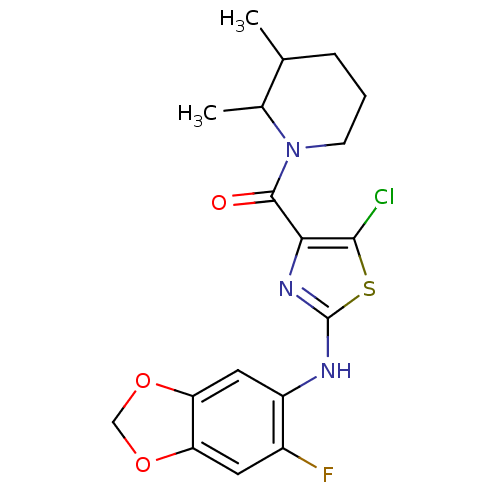 (CHEMBL2418809) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132072 (US8841453, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.28 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132080 (US8841453, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132088 (US8841453, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132074 (US8841453, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132086 (US8841453, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132082 (US8841453, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132078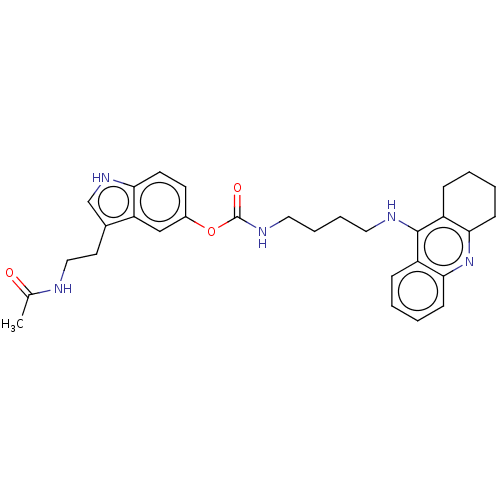 (US8841453, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439220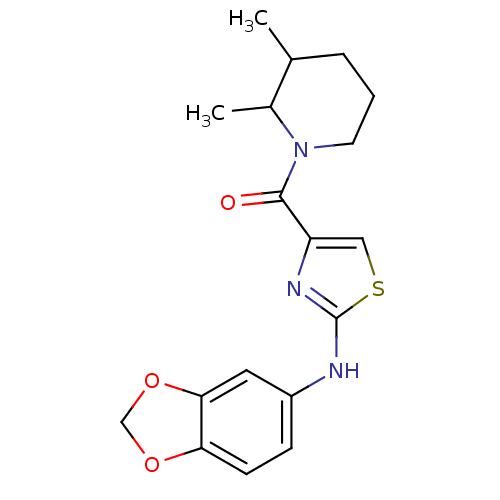 (CHEMBL2418807) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439217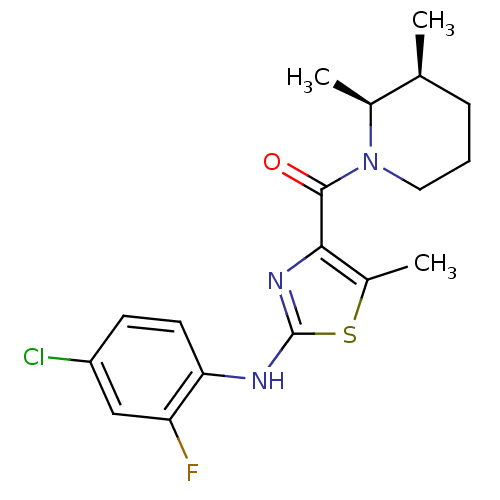 (CHEMBL2418811) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132075 (US8841453, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439219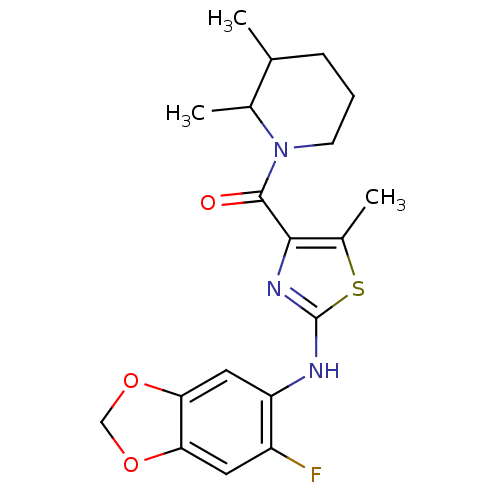 (CHEMBL2418808) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132087 (US8841453, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 3 (Homo sapiens (Human)) | BDBM50439218 (CHEMBL2418809) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC3 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439213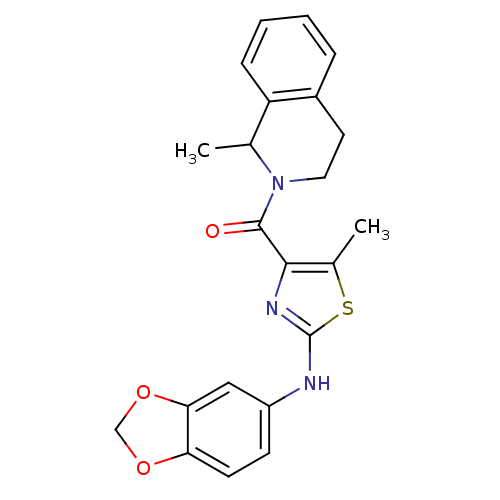 (CHEMBL2418815) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132071 (US8841453, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132075 (US8841453, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132076 (US8841453, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132081 (US8841453, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439216 (CHEMBL2418812) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132077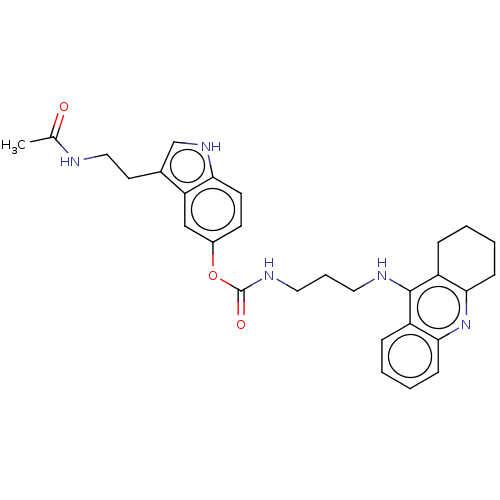 (US8841453, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 58.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439214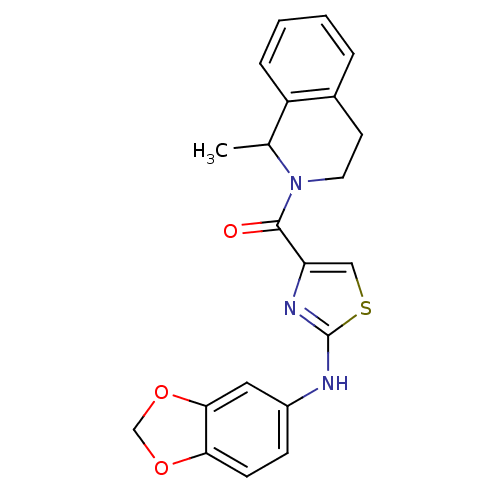 (CHEMBL2418814) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439221 (CHEMBL2418806) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 3 (Homo sapiens (Human)) | BDBM50439220 (CHEMBL2418807) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC3 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 3 (Homo sapiens (Human)) | BDBM50439217 (CHEMBL2418811) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC3 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 3 (Homo sapiens (Human)) | BDBM50439219 (CHEMBL2418808) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC3 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439227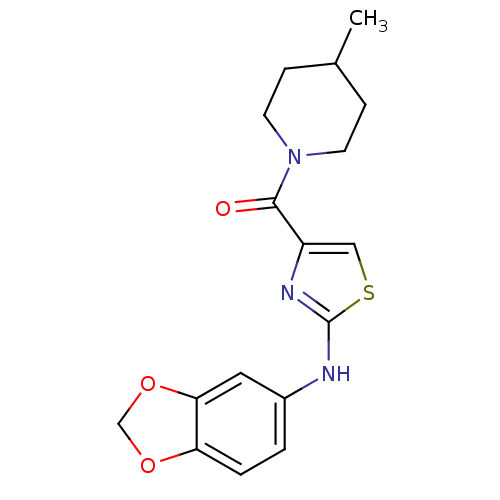 (CHEMBL2418823) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439215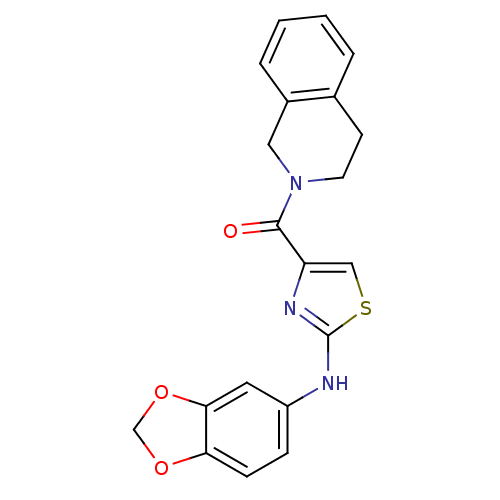 (CHEMBL2418813) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132073 (US8841453, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132079 (US8841453, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 3 (Homo sapiens (Human)) | BDBM50439221 (CHEMBL2418806) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC3 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 3 (Homo sapiens (Human)) | BDBM50439216 (CHEMBL2418812) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC3 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132077 (US8841453, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 3 (Homo sapiens (Human)) | BDBM50439214 (CHEMBL2418814) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC3 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |