

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007522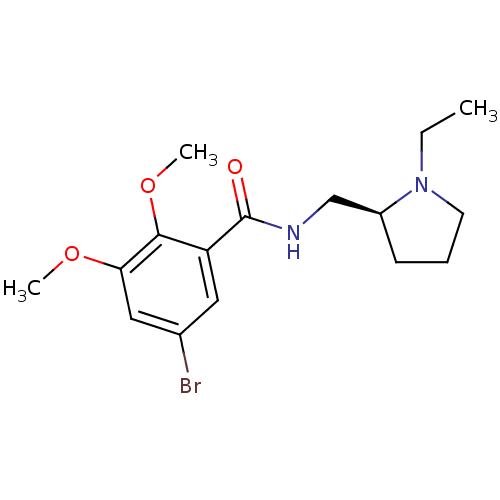 (5-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,3-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007508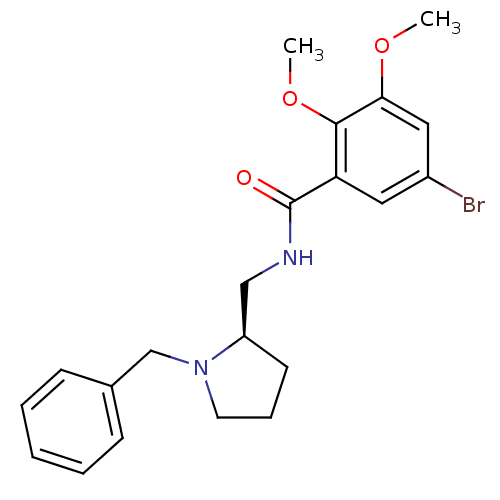 ((R) N-(1-Benzyl-pyrrolidin-2-ylmethyl)-5-bromo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50155307 (CHEMBL3781796) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Displacement of [125I]tyr11-SRIF from human sst2 receptor after 60 mins by liquid scintillation counting method | Eur J Med Chem 114: 59-64 (2016) Article DOI: 10.1016/j.ejmech.2016.02.046 BindingDB Entry DOI: 10.7270/Q2WH2RWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451823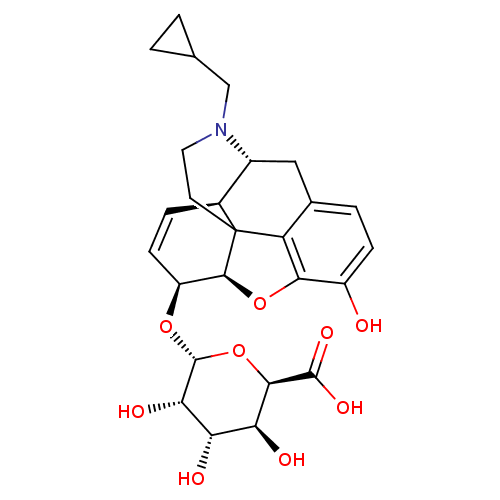 (CHEMBL2112840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007517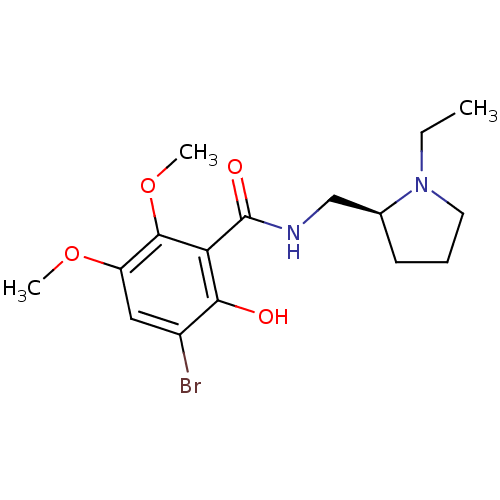 ((S)-3-bromo-N-((1-ethylpyrrolidin-2-yl)methyl)-2-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283248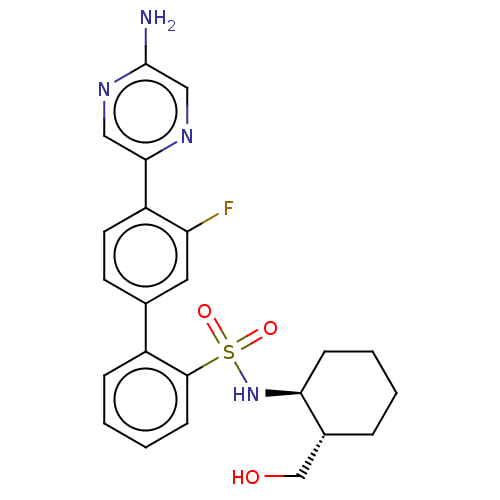 ((trans)-4'-(5-Aminopyrazin-2-yl)-3'-fluoro-N-[2- (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283288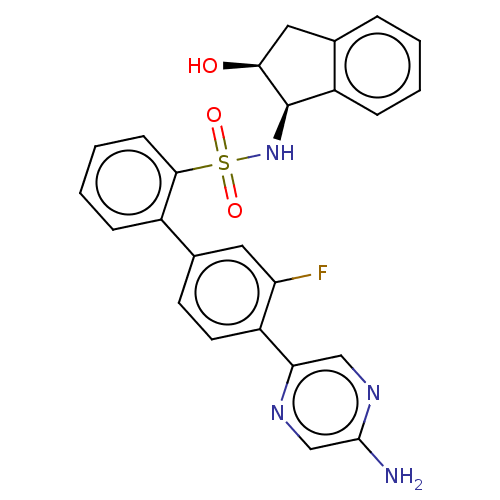 (4'-(5-aminopyrazin-2-yl)-3'-fluoro-N-((1R,2S)- 2-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283245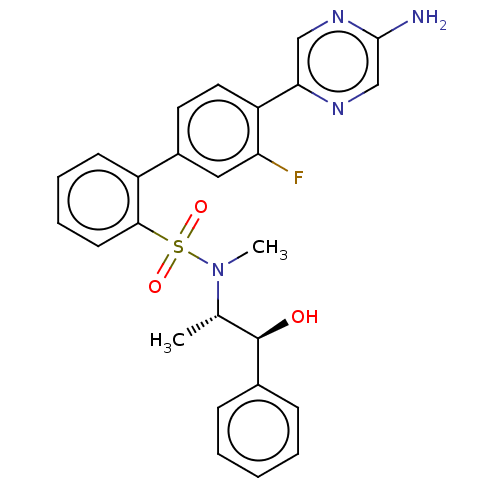 (4'-(5-aminopyrazin-2-yl)-3'-fluoro-N-((1S,2S)- 1-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283287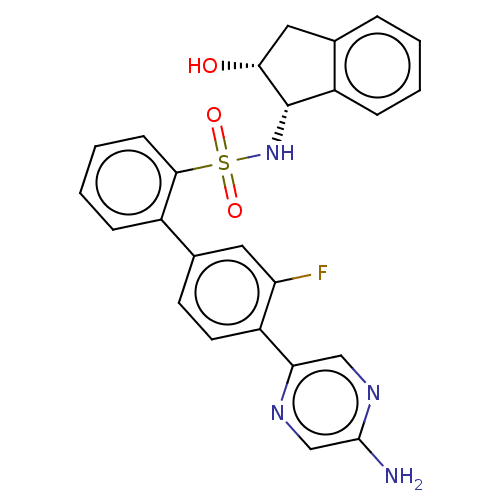 (4'-(5-aminopyrazin-2-yl)-3'-fluoro-N-((1S,2R)- 2-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50223890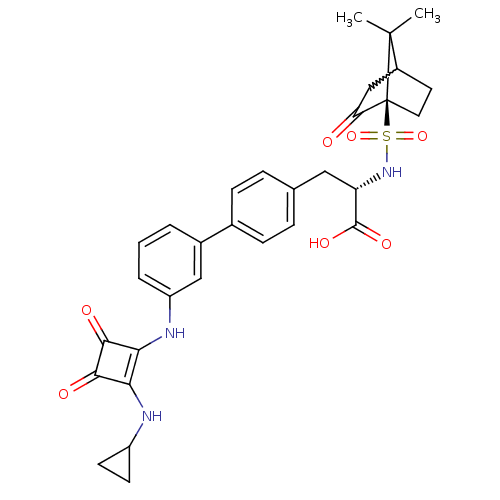 ((S)-3-[3'-(2-cyclopropylamino-3,4-dioxo-cyclobut-1...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Healthcare AG Curated by ChEMBL | Assay Description Binding affinity to integrin alphavbeta3 receptor | Bioorg Med Chem Lett 17: 6151-4 (2007) Article DOI: 10.1016/j.bmcl.2007.09.039 BindingDB Entry DOI: 10.7270/Q2KS6R8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283262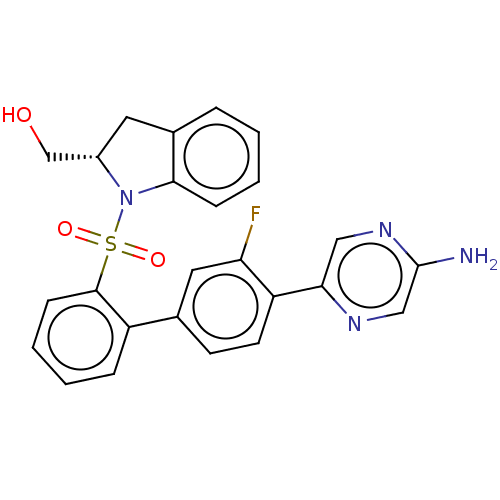 ((S)-(1-{[4'-(5-Aminopyrazin-2-yl)-3'- fluorobiphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947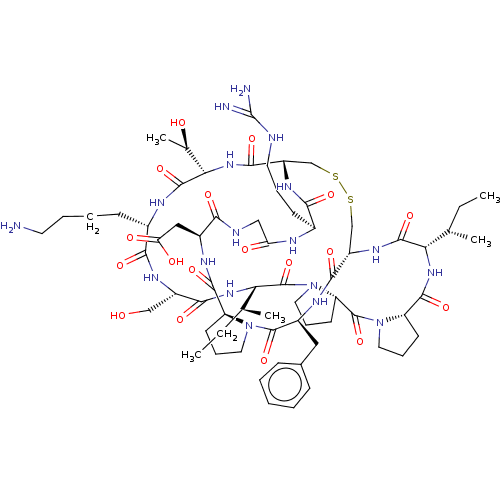 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin type-3 using L-BAPNA as substrate preincubated for 5 mins followed by substrate addition measured over 60 mins | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283508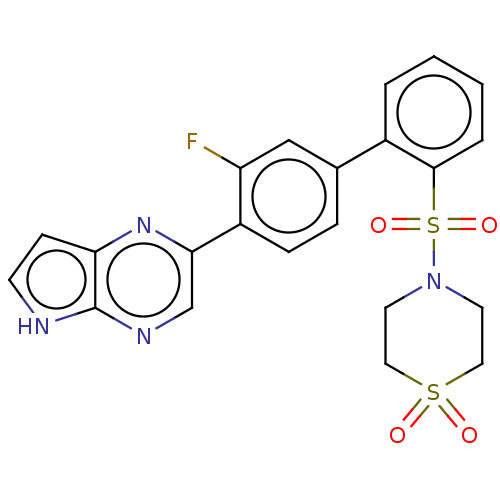 (2-{2'-[(1,1-Dioxidothiomorpholin-4-yl)sulfonyl]- 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283516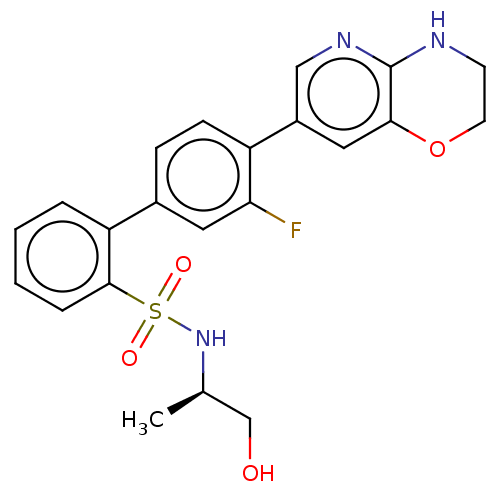 (4'-(3,4-Dihydro-2H-pyrido[3,2-b][1,4]oxazin-7- yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283335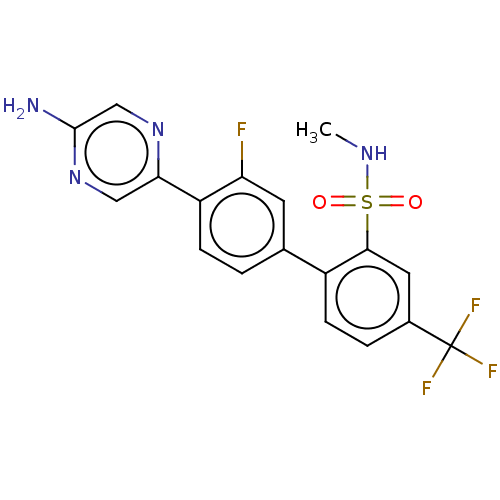 (4'-(5-Aminopyrazxin-2-yl)-3'-fluoro-N-methyl-4- (t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283337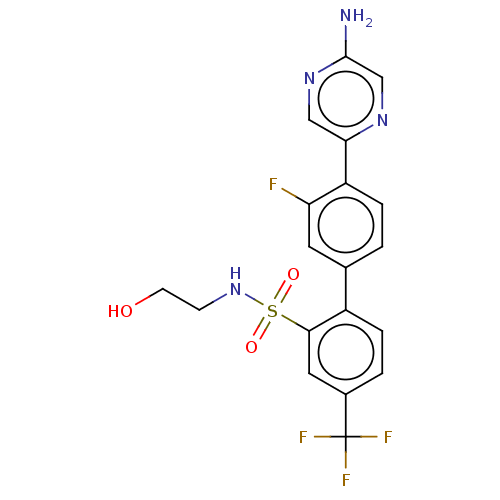 (4'-(5-aminopyrazin-2-yl)-3'-fluoro-N-(2- hydroxyet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283303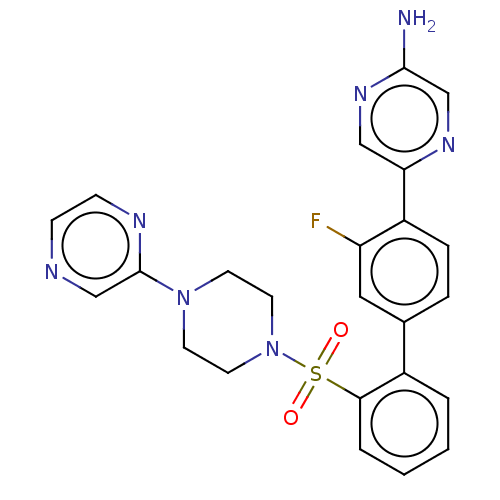 (5-{3-Fluoro-2'-[(4-pyrazin-2-ylpiperazin-1- yl)sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283677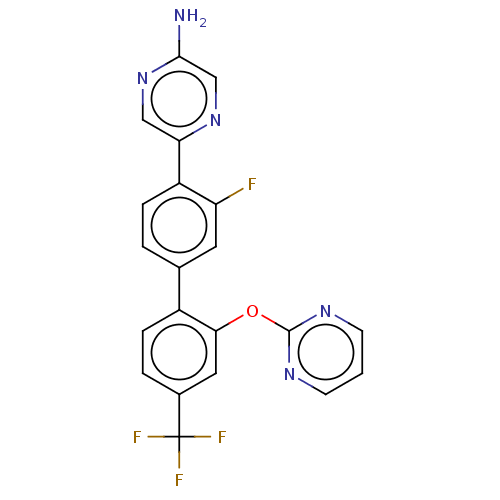 (5-[3-Fluoro-2'-(pyrimidin-2-yloxy)-4'- (trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283502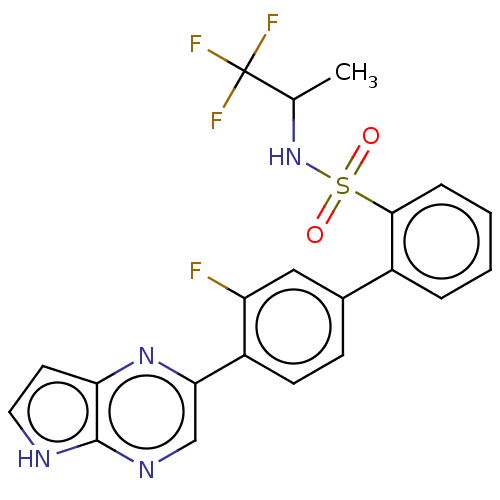 (3'-Fluoro-4'-(5H-pyrrolo[2,3-b]pyrazin-2-yl)N-- N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283229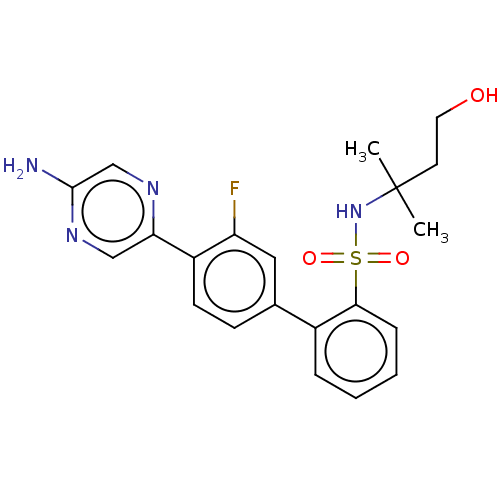 (4'-(5-Aminopyrazin-2-yl)-3'-fluoro-N-(3- hydroxy-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283304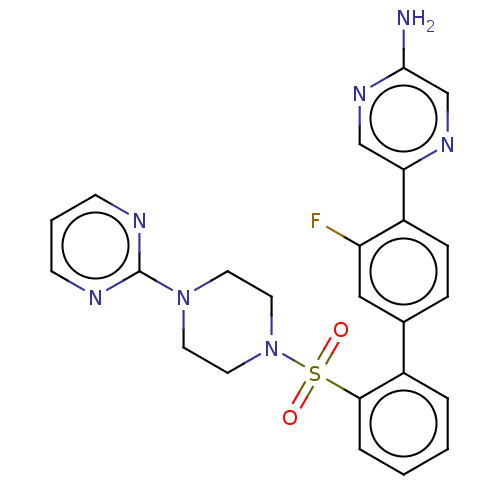 (5-{3-Fluoro-2'-[(4-pyrimidin-2-ylpiperazin-1- yl)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283230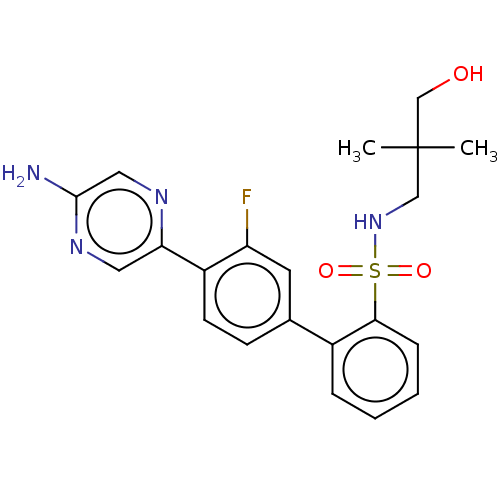 (4'-(5-Aminopyrazin-2-yl)-3'-fluoro-N-(3- hydroxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283306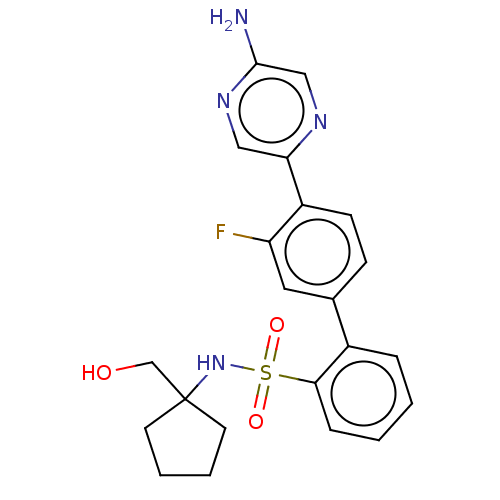 (4'-(5-Aminopyrazin-2-yl)-3'-fluoro-N-[1- (hydroxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302273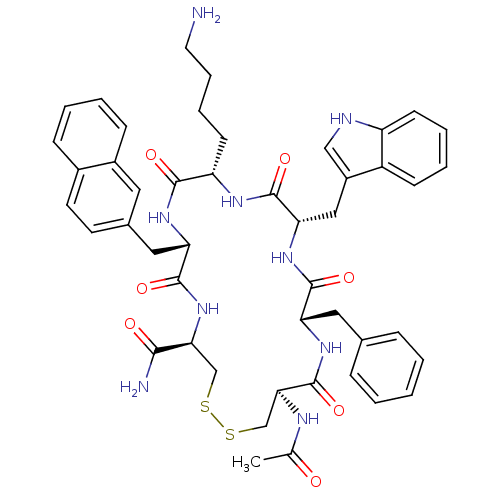 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252993 (CHEMBL4073678) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Non-competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Linew... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252992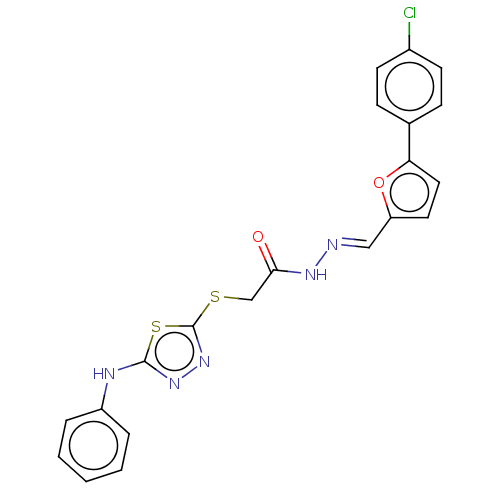 (CHEMBL4100496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007509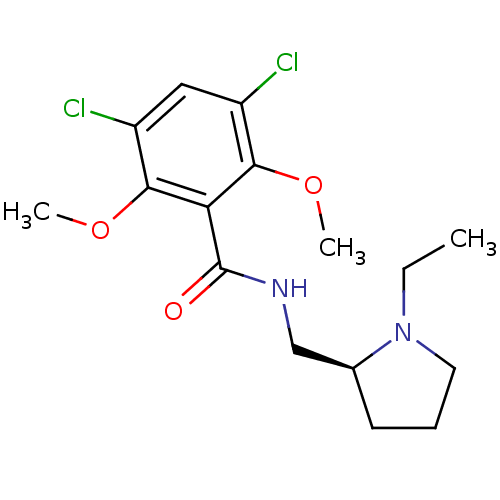 (3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125686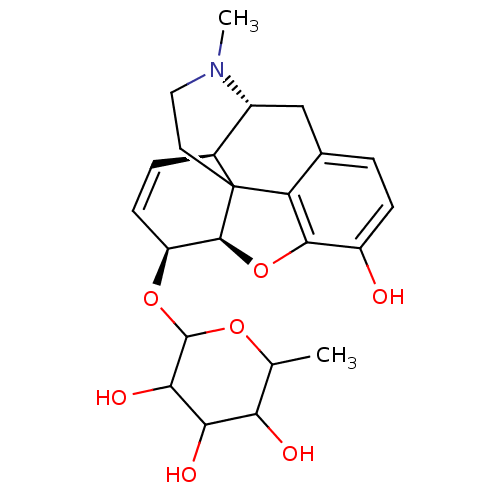 (2-[10-hydroxy-4-methyl-(5R,13R,14S,17R)-12-oxa-4-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222915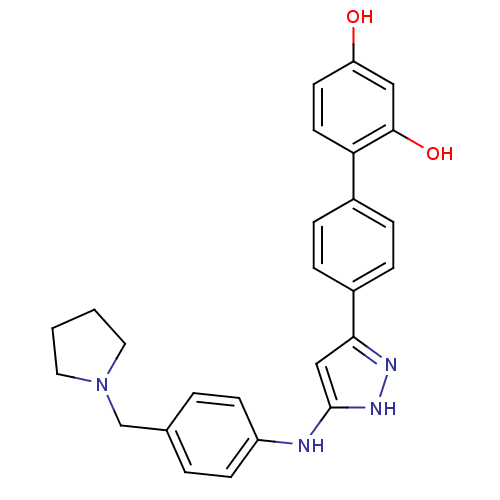 (4'-[5-(4-pyrrolidin-1-ylmethyl-phenylamino)-1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50491017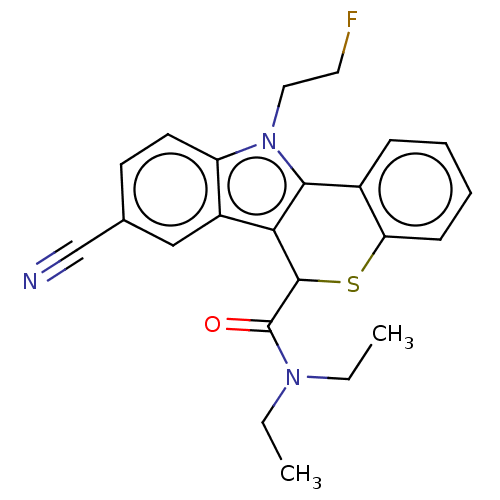 (CHEMBL2377343) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from PBR receptor in Wistar rat heart homogenates | Bioorg Med Chem Lett 23: 2368-72 (2013) Article DOI: 10.1016/j.bmcl.2013.02.057 BindingDB Entry DOI: 10.7270/Q2PR7ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007510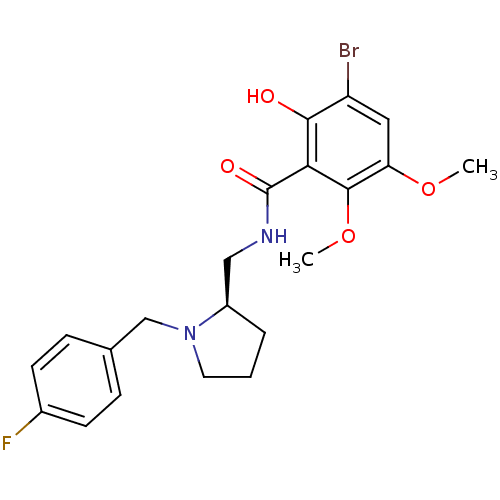 ((R) 3-Bromo-N-[1-(4-fluoro-benzyl)-pyrrolidin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal dopamine receptor D2 was determined in vitro | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222916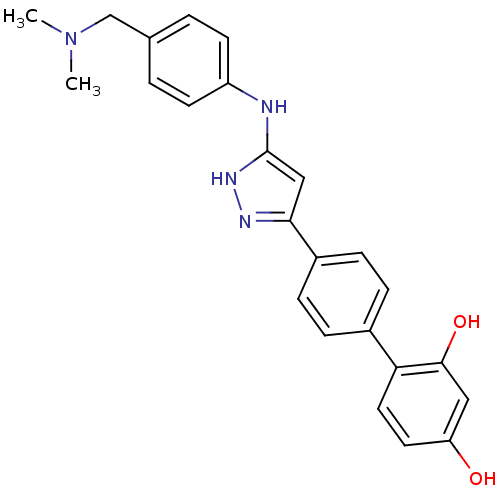 (4'-{5-[4-(dimethylamino-methyl)-phenylamino]-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222920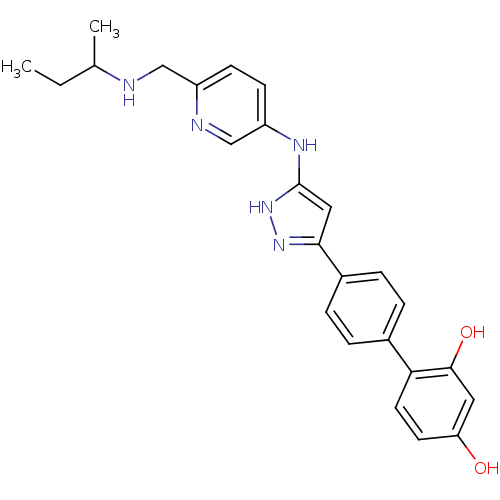 (4'-{5-[6-(sec-butylamino-methyl)-pyridin-3-ylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451820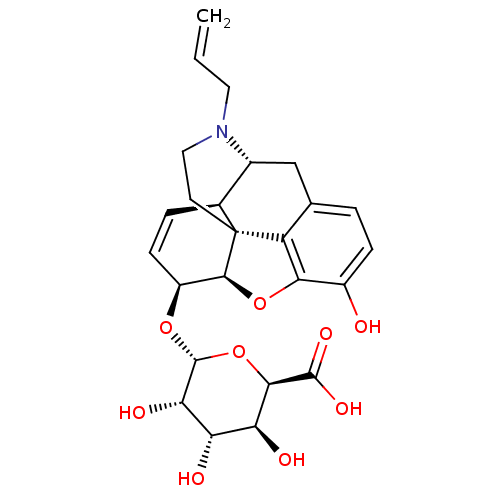 (CHEMBL3085267) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50090529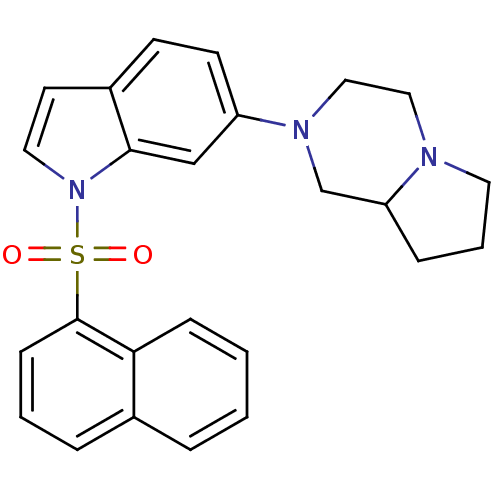 (2-[1-(Naphthalene-1-sulfonyl)-1H-indol-6-yl]-octah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp. Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-hydroxytryptamine 6 receptor expressed in HEK 293 cells | Bioorg Med Chem Lett 10: 1719-21 (2000) BindingDB Entry DOI: 10.7270/Q2BP021W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50401333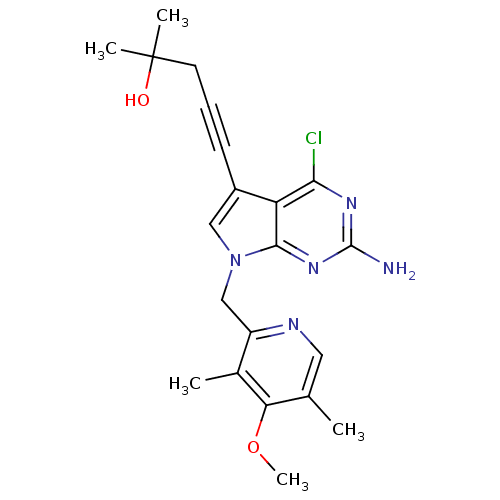 (CHEMBL1230584) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity at recombinant Hsp90alpha incubated for 16 hrs by fluorescence polarization competition assay | J Med Chem 55: 7786-95 (2012) Article DOI: 10.1021/jm300810x BindingDB Entry DOI: 10.7270/Q2V125Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451826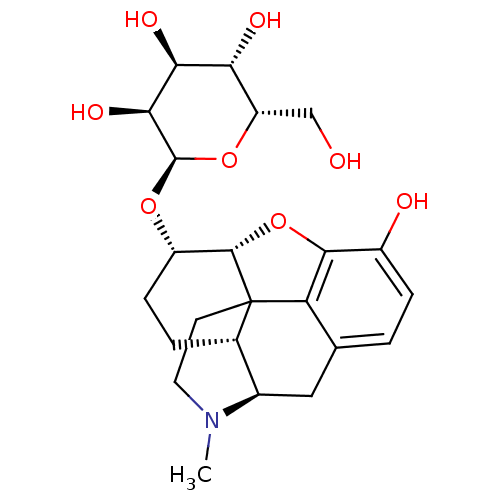 (CHEMBL2112797) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50363527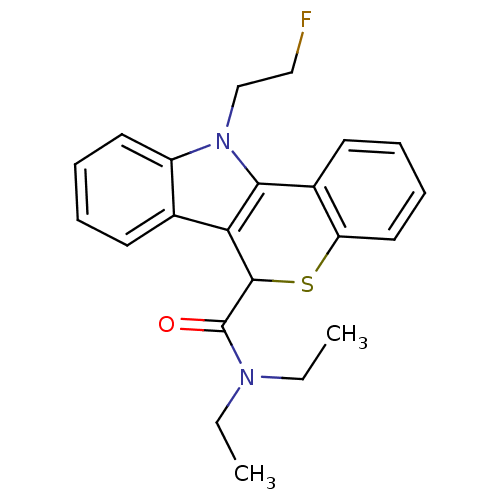 (CHEMBL1945412 | US9481685, [18F]FE-PBR) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from PBR receptor in Wistar rat heart homogenates | Bioorg Med Chem Lett 23: 2368-72 (2013) Article DOI: 10.1016/j.bmcl.2013.02.057 BindingDB Entry DOI: 10.7270/Q2PR7ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50491024 (CHEMBL2377352) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from PBR receptor in Wistar rat heart homogenates | Bioorg Med Chem Lett 23: 2368-72 (2013) Article DOI: 10.1016/j.bmcl.2013.02.057 BindingDB Entry DOI: 10.7270/Q2PR7ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135249 ((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50152456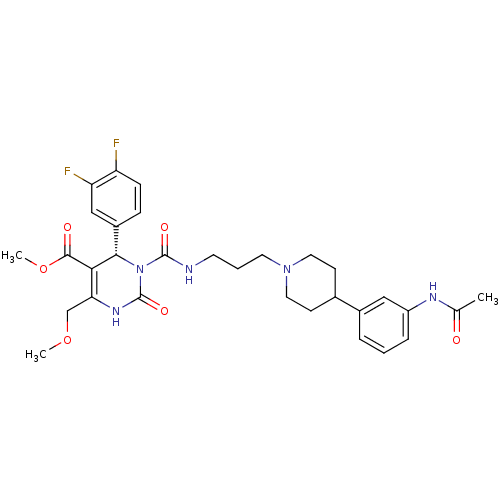 ((S)-3-{3-[4-(3-Acetylamino-phenyl)-piperidin-1-yl]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]T226296 from rat recombinant MCH1 receptor | J Med Chem 50: 3883-90 (2007) Article DOI: 10.1021/jm060383x BindingDB Entry DOI: 10.7270/Q25D8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50318494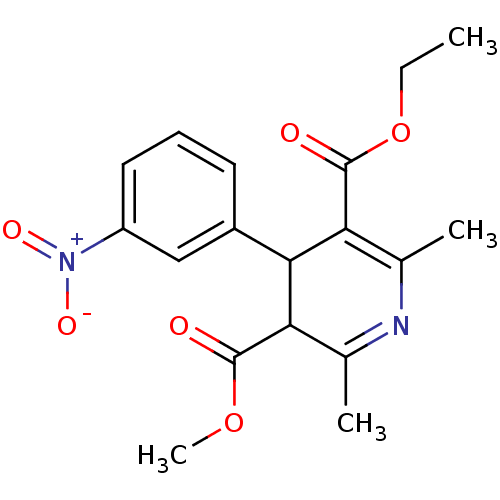 (3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel dihydropyridine site of porcine cardiac sarcolemma membrane vesicles | J Med Chem 30: 690-5 (1987) BindingDB Entry DOI: 10.7270/Q2BC404T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50152456 ((S)-3-{3-[4-(3-Acetylamino-phenyl)-piperidin-1-yl]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells | J Med Chem 50: 3870-82 (2007) Article DOI: 10.1021/jm060381c BindingDB Entry DOI: 10.7270/Q2930SWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451822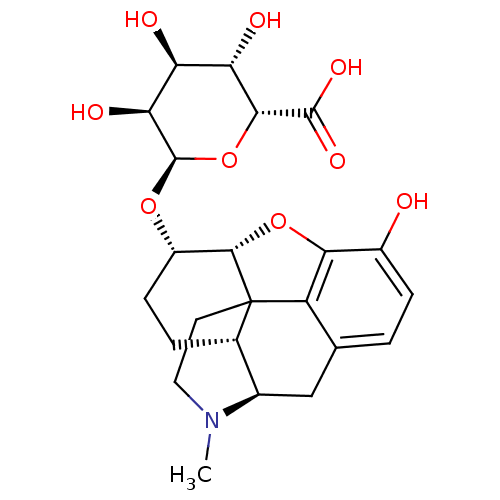 (CHEMBL2112839) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50491021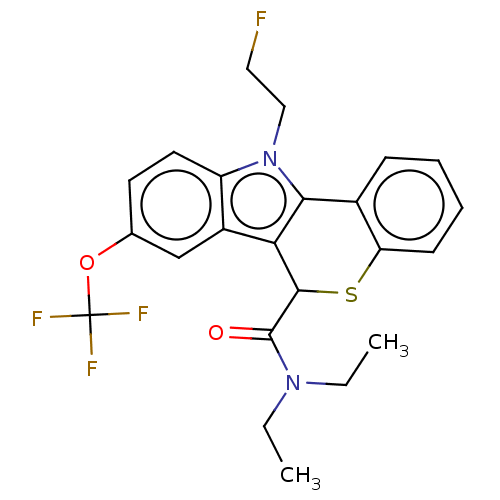 (CHEMBL2377344) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from PBR receptor in Wistar rat heart homogenates | Bioorg Med Chem Lett 23: 2368-72 (2013) Article DOI: 10.1016/j.bmcl.2013.02.057 BindingDB Entry DOI: 10.7270/Q2PR7ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130163 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252992 (CHEMBL4100496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451819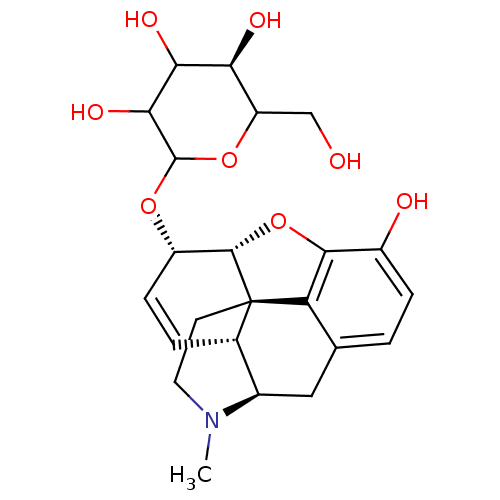 (CHEMBL2079659) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 19457 total ) | Next | Last >> |