

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313984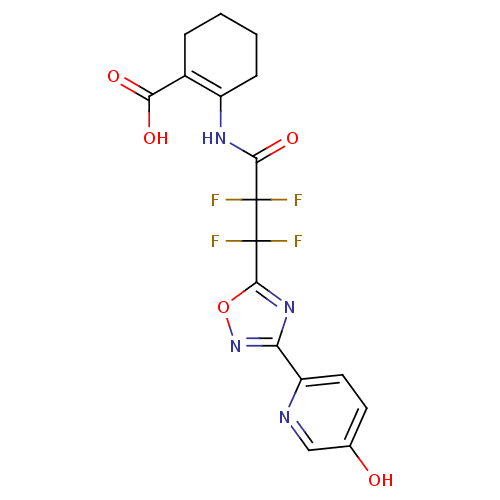 (2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313977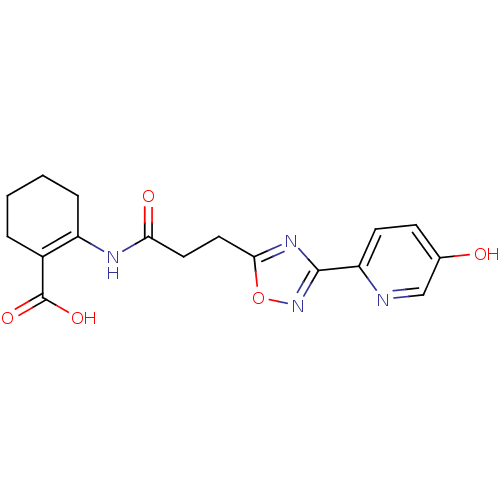 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23533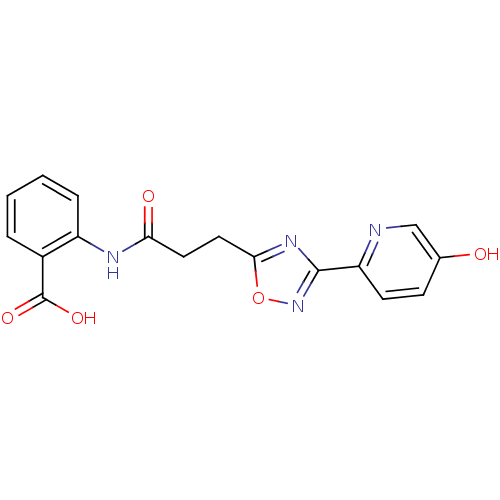 (2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313976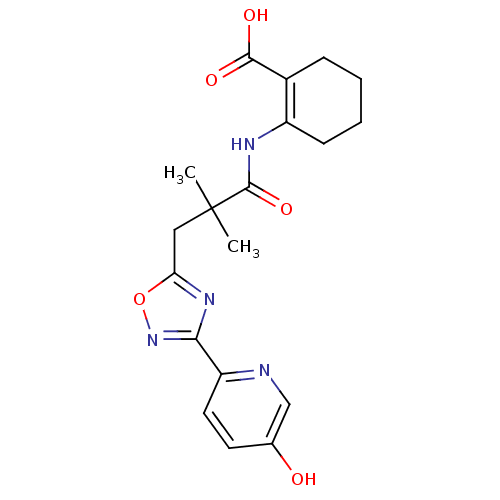 (2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002823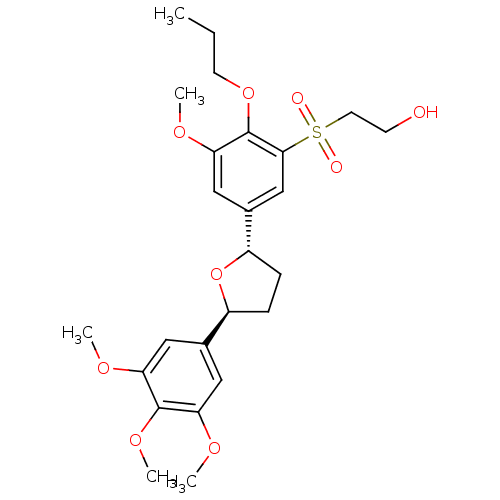 (2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to PMN membrane receptors | Bioorg Med Chem Lett 1: 327-332 (1991) Article DOI: 10.1016/S0960-894X(01)80818-0 BindingDB Entry DOI: 10.7270/Q2TM7BKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313979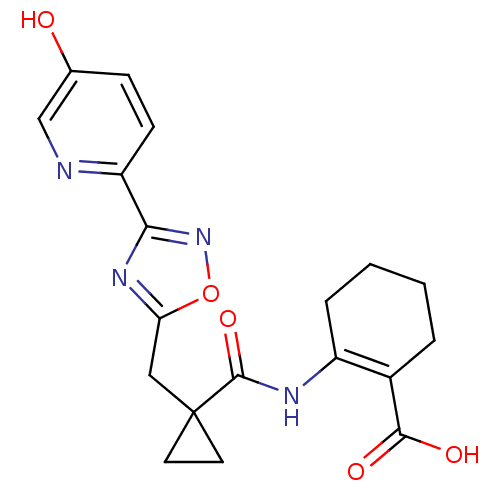 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313981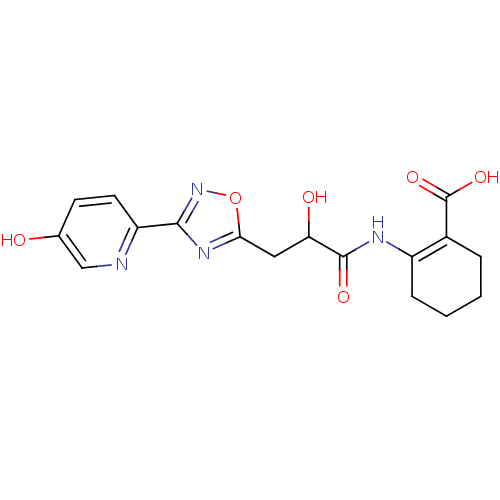 (CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313982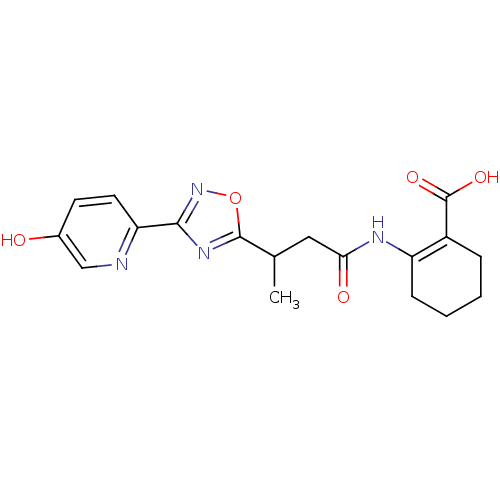 (CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002823 (2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to human platelet | Bioorg Med Chem Lett 1: 327-332 (1991) Article DOI: 10.1016/S0960-894X(01)80818-0 BindingDB Entry DOI: 10.7270/Q2TM7BKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50366241 (CHEMBL297624 | L-652731) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit PAF binding to rabbit platelet membrane | Bioorg Med Chem Lett 1: 327-332 (1991) Article DOI: 10.1016/S0960-894X(01)80818-0 BindingDB Entry DOI: 10.7270/Q2TM7BKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002823 (2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to human platelet | Bioorg Med Chem Lett 1: 327-332 (1991) Article DOI: 10.1016/S0960-894X(01)80818-0 BindingDB Entry DOI: 10.7270/Q2TM7BKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002823 (2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to human platelet | Bioorg Med Chem Lett 1: 327-332 (1991) Article DOI: 10.1016/S0960-894X(01)80818-0 BindingDB Entry DOI: 10.7270/Q2TM7BKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23533 (2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313983 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313977 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313980 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313976 (2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 595 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313981 (CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313984 (2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313980 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313982 (CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313979 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313983 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50175745 (7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type p38alpha (unknown origin) | J Biol Chem 282: 34663-71 (2007) Article DOI: 10.1074/jbc.M704236200 BindingDB Entry DOI: 10.7270/Q2G44Q2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50290861 (5-{3-[1-(3,5-Dichloro-phenyl)-ethoxy]-2-phenyl-pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of NK1 receptor in rat liver microsomes. | Bioorg Med Chem Lett 7: 2959-2962 (1997) Article DOI: 10.1016/S0960-894X(97)10118-4 BindingDB Entry DOI: 10.7270/Q2W66KRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50220136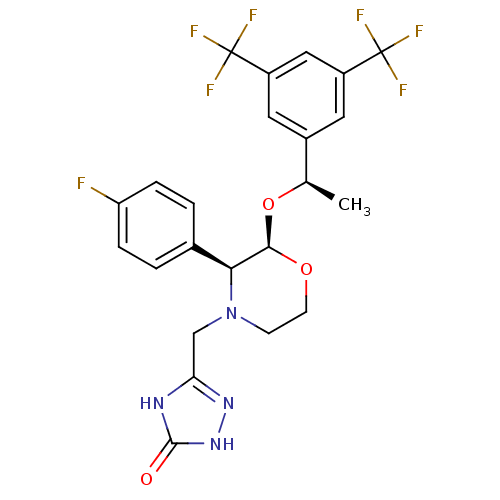 (3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15459 (4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type p38alpha (unknown origin) | J Biol Chem 282: 34663-71 (2007) Article DOI: 10.1074/jbc.M704236200 BindingDB Entry DOI: 10.7270/Q2G44Q2F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM15459 (4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type p38beta (unknown origin) | J Biol Chem 282: 34663-71 (2007) Article DOI: 10.1074/jbc.M704236200 BindingDB Entry DOI: 10.7270/Q2G44Q2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191091 ((S)-5-((((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50290865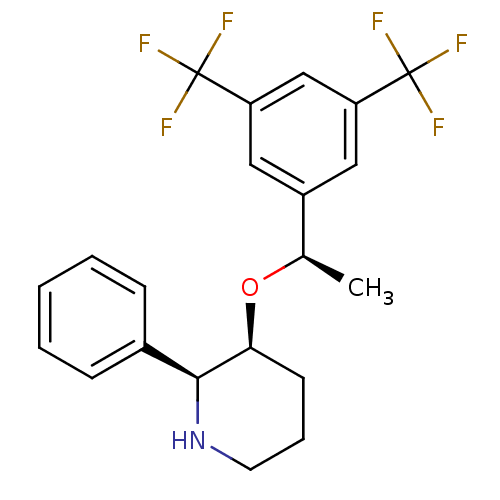 (3-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-2-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of NK1 receptor in rat liver microsomes. | Bioorg Med Chem Lett 7: 2959-2962 (1997) Article DOI: 10.1016/S0960-894X(97)10118-4 BindingDB Entry DOI: 10.7270/Q2W66KRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50290859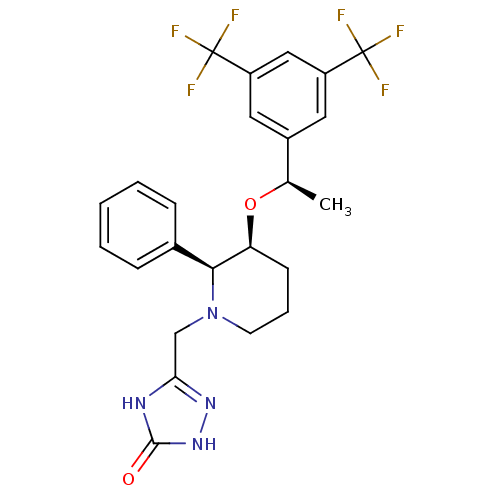 (5-{3-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of NK1 receptor in rat liver microsomes. | Bioorg Med Chem Lett 7: 2959-2962 (1997) Article DOI: 10.1016/S0960-894X(97)10118-4 BindingDB Entry DOI: 10.7270/Q2W66KRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191092 ((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50290856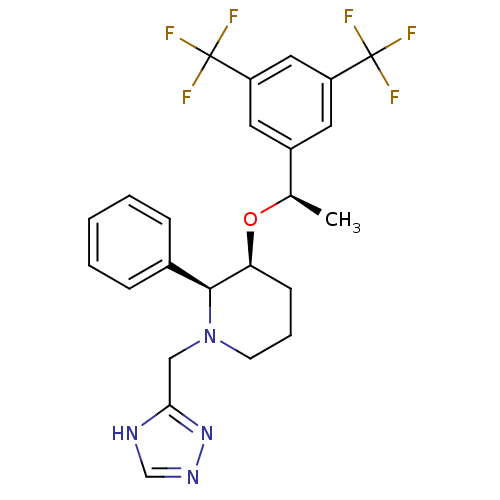 (3-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-2-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of NK1 receptor in rat liver microsomes. | Bioorg Med Chem Lett 7: 2959-2962 (1997) Article DOI: 10.1016/S0960-894X(97)10118-4 BindingDB Entry DOI: 10.7270/Q2W66KRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191087 ((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191079 (2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50052280 ((2S,3S)-3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of NK1 receptor in rat liver microsomes. | Bioorg Med Chem Lett 7: 2959-2962 (1997) Article DOI: 10.1016/S0960-894X(97)10118-4 BindingDB Entry DOI: 10.7270/Q2W66KRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191095 (2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191107 (2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191081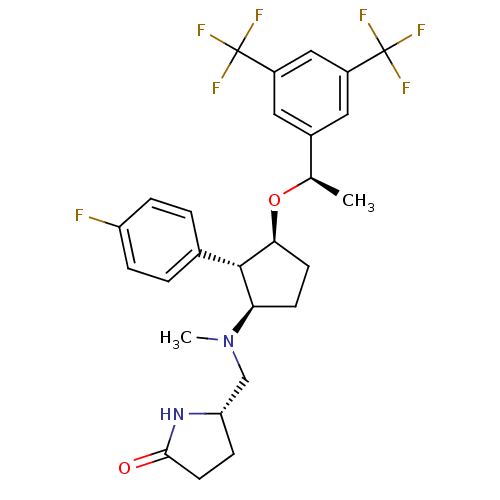 ((S)-5-((((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191082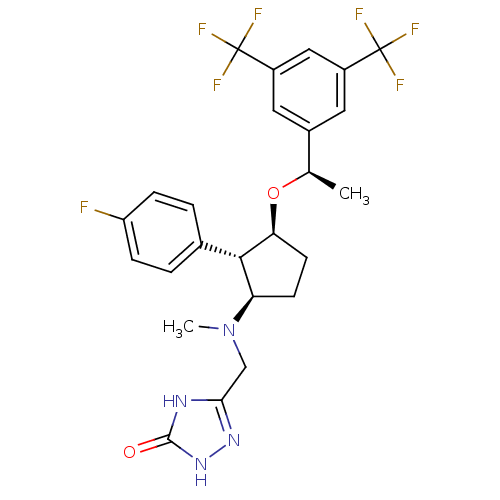 (5-((((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191099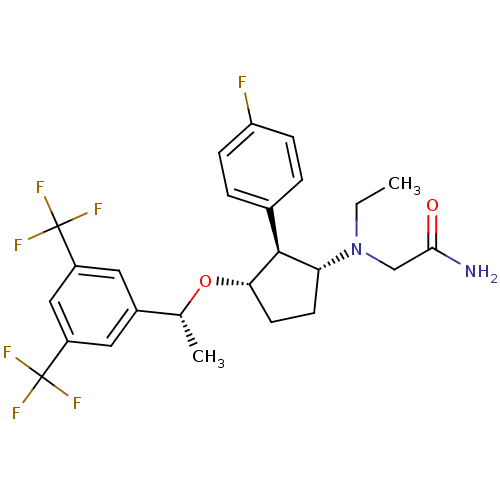 (2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191106 ((R)-5-((((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191096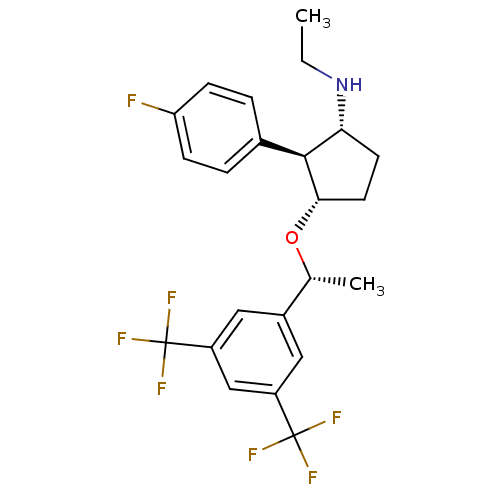 ((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191086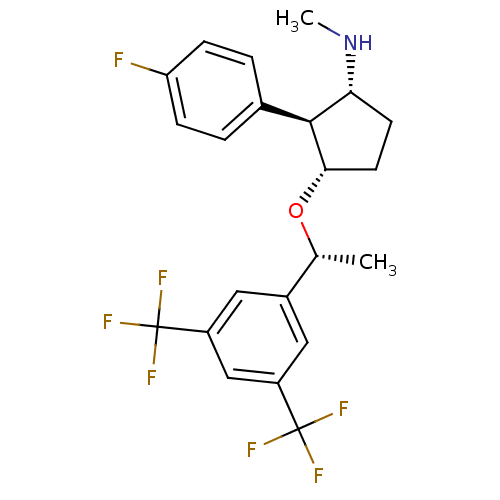 ((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 805 total ) | Next | Last >> |