 Found 188 hits with Last Name = 'matheis' and Initial = 'm'
Found 188 hits with Last Name = 'matheis' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377038
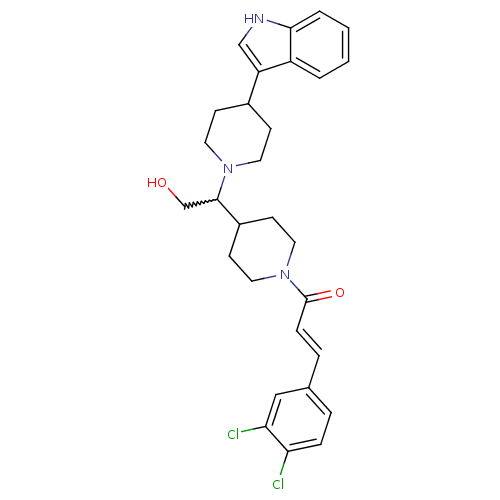
(CHEMBL258205)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C29H33Cl2N3O2/c30-25-7-5-20(17-26(25)31)6-8-29(36)34-15-11-22(12-16-34)28(19-35)33-13-9-21(10-14-33)24-18-32-27-4-2-1-3-23(24)27/h1-8,17-18,21-22,28,32,35H,9-16,19H2/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377039
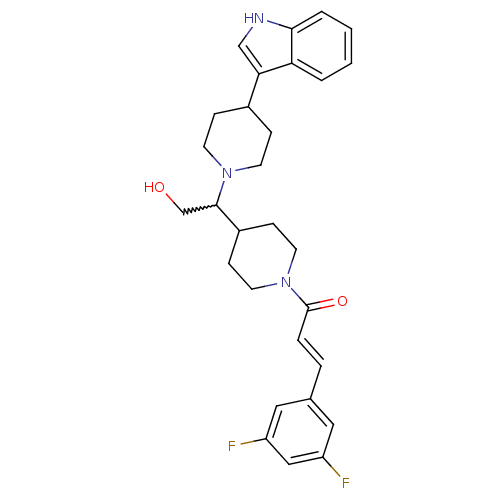
(CHEMBL257191)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1cc(F)cc(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C29H33F2N3O2/c30-23-15-20(16-24(31)17-23)5-6-29(36)34-13-9-22(10-14-34)28(19-35)33-11-7-21(8-12-33)26-18-32-27-4-2-1-3-25(26)27/h1-6,15-18,21-22,28,32,35H,7-14,19H2/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377034
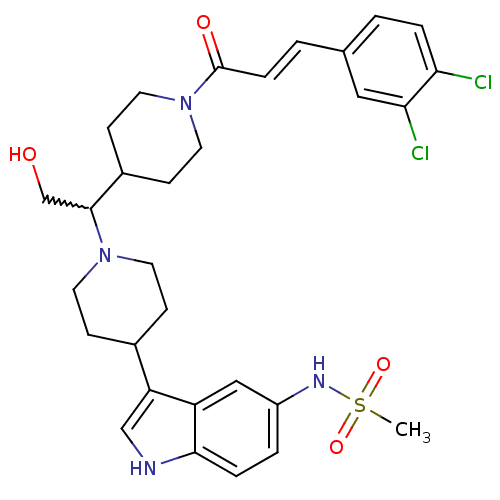
(CHEMBL256301)Show SMILES CS(=O)(=O)Nc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3ccc(Cl)c(Cl)c3)c2c1 |w:18.19| Show InChI InChI=1S/C30H36Cl2N4O4S/c1-41(39,40)34-23-4-6-28-24(17-23)25(18-33-28)21-8-12-35(13-9-21)29(19-37)22-10-14-36(15-11-22)30(38)7-3-20-2-5-26(31)27(32)16-20/h2-7,16-18,21-22,29,33-34,37H,8-15,19H2,1H3/b7-3+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377025

(CHEMBL403889)Show SMILES Nc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)c2c1 |w:14.15| Show InChI InChI=1S/C29H33F3N4O2/c30-24-13-18(14-25(31)29(24)32)1-4-28(38)36-11-7-20(8-12-36)27(17-37)35-9-5-19(6-10-35)23-16-34-26-3-2-21(33)15-22(23)26/h1-4,13-16,19-20,27,34,37H,5-12,17,33H2/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377026
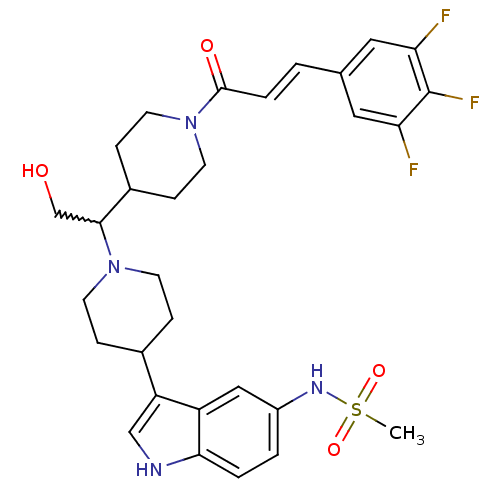
(CHEMBL254772)Show SMILES CS(=O)(=O)Nc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)c2c1 |w:18.19| Show InChI InChI=1S/C30H35F3N4O4S/c1-42(40,41)35-22-3-4-27-23(16-22)24(17-34-27)20-6-10-36(11-7-20)28(18-38)21-8-12-37(13-9-21)29(39)5-2-19-14-25(31)30(33)26(32)15-19/h2-5,14-17,20-21,28,34-35,38H,6-13,18H2,1H3/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377027
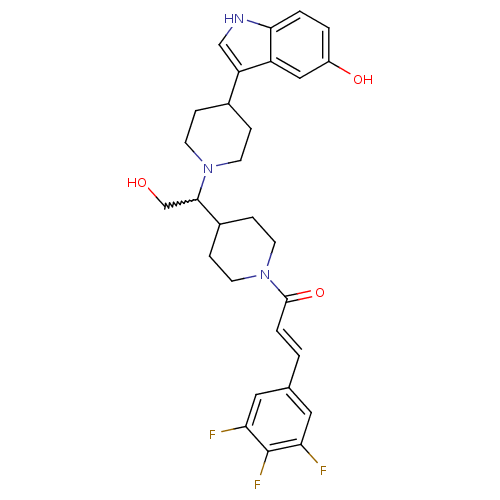
(CHEMBL404904)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccc(O)cc12 |w:2.1| Show InChI InChI=1S/C29H32F3N3O3/c30-24-13-18(14-25(31)29(24)32)1-4-28(38)35-11-7-20(8-12-35)27(17-36)34-9-5-19(6-10-34)23-16-33-26-3-2-21(37)15-22(23)26/h1-4,13-16,19-20,27,33,36-37H,5-12,17H2/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224496
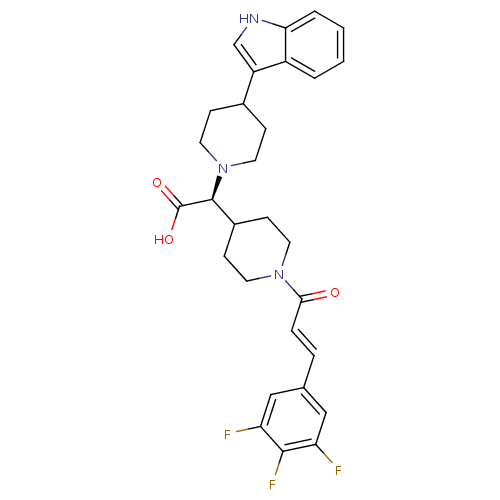
((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 expressed in THP1 cells assessed as MCP1-induced calcium flux |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377028

(CHEMBL255302)Show SMILES CC(=O)Nc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)c2c1 |w:17.18| Show InChI InChI=1S/C31H35F3N4O3/c1-19(40)36-23-3-4-28-24(16-23)25(17-35-28)21-6-10-37(11-7-21)29(18-39)22-8-12-38(13-9-22)30(41)5-2-20-14-26(32)31(34)27(33)15-20/h2-5,14-17,21-22,29,35,39H,6-13,18H2,1H3,(H,36,40)/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377040
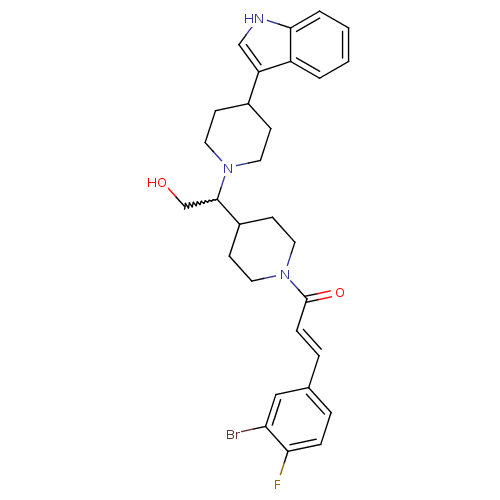
(CHEMBL402442)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1ccc(F)c(Br)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C29H33BrFN3O2/c30-25-17-20(5-7-26(25)31)6-8-29(36)34-15-11-22(12-16-34)28(19-35)33-13-9-21(10-14-33)24-18-32-27-4-2-1-3-23(24)27/h1-8,17-18,21-22,28,32,35H,9-16,19H2/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50239941

((S)-1-(4-(1-(4-(1H-indol-3-yl)piperidin-1-yl)-2-hy...)Show SMILES OC[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H32F3N3O2/c30-24-15-19(16-25(31)29(24)32)5-6-28(37)35-13-9-21(10-14-35)27(18-36)34-11-7-20(8-12-34)23-17-33-26-4-2-1-3-22(23)26/h1-6,15-17,20-21,27,33,36H,7-14,18H2/b6-5+/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224501
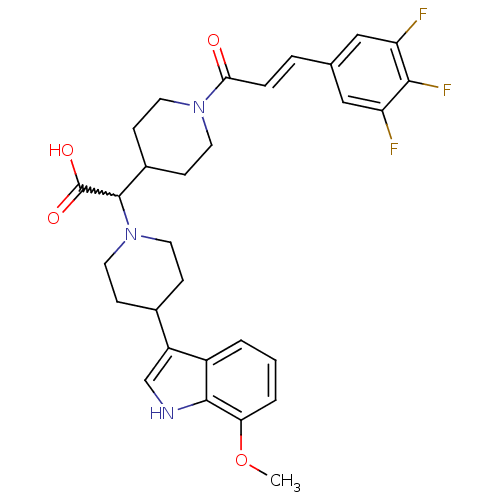
((E)-2-(4-(7-methoxy-1H-indol-3-yl)piperidin-1-yl)-...)Show SMILES COc1cccc2c(c[nH]c12)C1CCN(CC1)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)C(O)=O |w:17.41| Show InChI InChI=1S/C30H32F3N3O4/c1-40-25-4-2-3-21-22(17-34-28(21)25)19-7-13-36(14-8-19)29(30(38)39)20-9-11-35(12-10-20)26(37)6-5-18-15-23(31)27(33)24(32)16-18/h2-6,15-17,19-20,29,34H,7-14H2,1H3,(H,38,39)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50239941

((S)-1-(4-(1-(4-(1H-indol-3-yl)piperidin-1-yl)-2-hy...)Show SMILES OC[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H32F3N3O2/c30-24-15-19(16-25(31)29(24)32)5-6-28(37)35-13-9-21(10-14-35)27(18-36)34-11-7-20(8-12-34)23-17-33-26-4-2-1-3-22(23)26/h1-6,15-17,20-21,27,33,36H,7-14,18H2/b6-5+/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor expressed in THP1 cells assessed as MCP-1-induced calcium flux by chemotoxis assay |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224496

((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224523
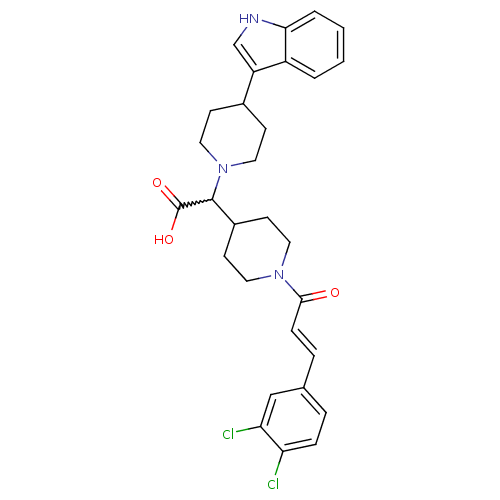
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C29H31Cl2N3O3/c30-24-7-5-19(17-25(24)31)6-8-27(35)33-13-11-21(12-14-33)28(29(36)37)34-15-9-20(10-16-34)23-18-32-26-4-2-1-3-22(23)26/h1-8,17-18,20-21,28,32H,9-16H2,(H,36,37)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224500

((E)-2-(4-(5-amino-1H-indol-3-yl)piperidin-1-yl)-2-...)Show SMILES Nc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:14.36| Show InChI InChI=1S/C29H31F3N4O3/c30-23-13-17(14-24(31)27(23)32)1-4-26(37)35-9-7-19(8-10-35)28(29(38)39)36-11-5-18(6-12-36)22-16-34-25-3-2-20(33)15-21(22)25/h1-4,13-16,18-19,28,34H,5-12,33H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50239941

((S)-1-(4-(1-(4-(1H-indol-3-yl)piperidin-1-yl)-2-hy...)Show SMILES OC[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H32F3N3O2/c30-24-15-19(16-25(31)29(24)32)5-6-28(37)35-13-9-21(10-14-35)27(18-36)34-11-7-20(8-12-34)23-17-33-26-4-2-1-3-22(23)26/h1-6,15-17,20-21,27,33,36H,7-14,18H2/b6-5+/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50246354
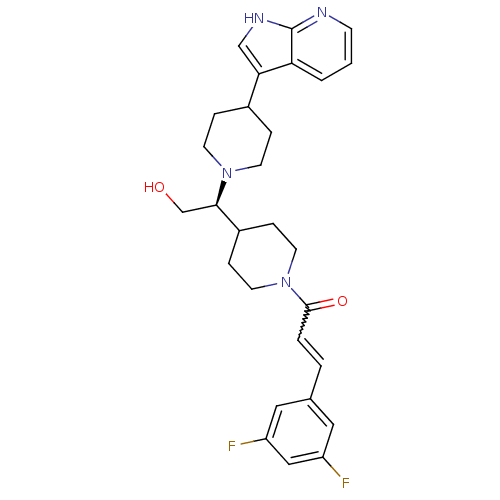
((S)-1-(4-(1-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)pipe...)Show SMILES OC[C@H](C1CCN(CC1)C(=O)C=Cc1cc(F)cc(F)c1)N1CCC(CC1)c1c[nH]c2ncccc12 |r,w:11.11| Show InChI InChI=1S/C28H32F2N4O2/c29-22-14-19(15-23(30)16-22)3-4-27(36)34-12-7-21(8-13-34)26(18-35)33-10-5-20(6-11-33)25-17-32-28-24(25)2-1-9-31-28/h1-4,9,14-17,20-21,26,35H,5-8,10-13,18H2,(H,31,32)/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 |
Bioorg Med Chem Lett 18: 6468-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.061
BindingDB Entry DOI: 10.7270/Q2V40V1J |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224502
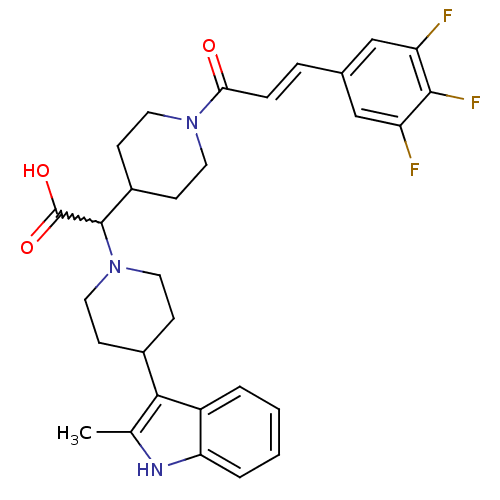
((E)-2-(4-(2-methyl-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES Cc1[nH]c2ccccc2c1C1CCN(CC1)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)C(O)=O |w:16.40| Show InChI InChI=1S/C30H32F3N3O3/c1-18-27(22-4-2-3-5-25(22)34-18)20-8-14-36(15-9-20)29(30(38)39)21-10-12-35(13-11-21)26(37)7-6-19-16-23(31)28(33)24(32)17-19/h2-7,16-17,20-21,29,34H,8-15H2,1H3,(H,38,39)/b7-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224511
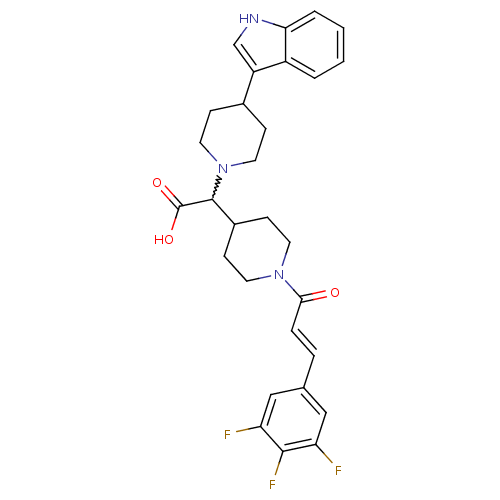
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.24| Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224511

((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.24| Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50246352
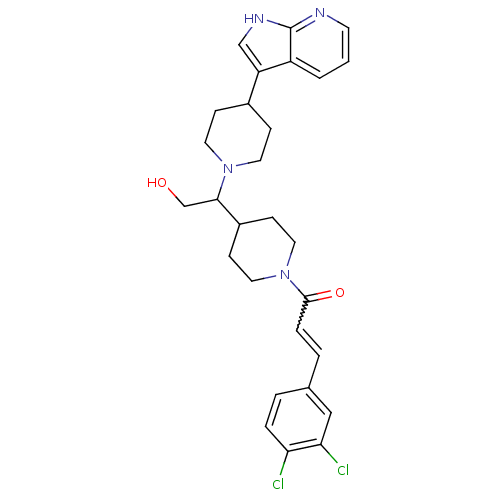
((+/-)-1-(4-(1-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)pi...)Show SMILES OCC(C1CCN(CC1)C(=O)C=Cc1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ncccc12 |w:11.11| Show InChI InChI=1S/C28H32Cl2N4O2/c29-24-5-3-19(16-25(24)30)4-6-27(36)34-14-9-21(10-15-34)26(18-35)33-12-7-20(8-13-33)23-17-32-28-22(23)2-1-11-31-28/h1-6,11,16-17,20-21,26,35H,7-10,12-15,18H2,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 |
Bioorg Med Chem Lett 18: 6468-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.061
BindingDB Entry DOI: 10.7270/Q2V40V1J |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50246351
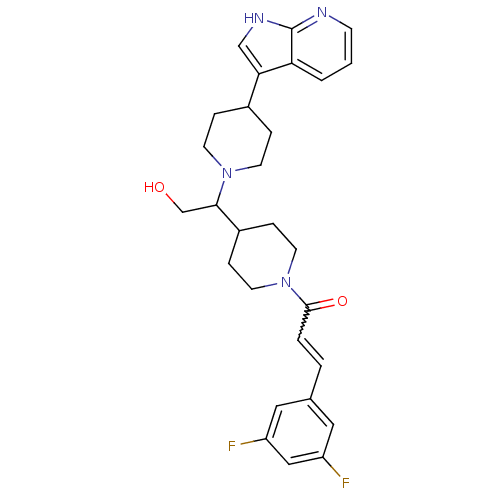
((+/-)-1-(4-(1-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)pi...)Show SMILES OCC(C1CCN(CC1)C(=O)C=Cc1cc(F)cc(F)c1)N1CCC(CC1)c1c[nH]c2ncccc12 |w:11.11| Show InChI InChI=1S/C28H32F2N4O2/c29-22-14-19(15-23(30)16-22)3-4-27(36)34-12-7-21(8-13-34)26(18-35)33-10-5-20(6-11-33)25-17-32-28-24(25)2-1-9-31-28/h1-4,9,14-17,20-21,26,35H,5-8,10-13,18H2,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 |
Bioorg Med Chem Lett 18: 6468-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.061
BindingDB Entry DOI: 10.7270/Q2V40V1J |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377041

(CHEMBL257173)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1ccc(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C29H33F2N3O2/c30-25-7-5-20(17-26(25)31)6-8-29(36)34-15-11-22(12-16-34)28(19-35)33-13-9-21(10-14-33)24-18-32-27-4-2-1-3-23(24)27/h1-8,17-18,21-22,28,32,35H,9-16,19H2/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377029

(CHEMBL255499)Show SMILES COc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)c2c1 |w:15.16| Show InChI InChI=1S/C30H34F3N3O3/c1-39-22-3-4-27-23(16-22)24(17-34-27)20-6-10-35(11-7-20)28(18-37)21-8-12-36(13-9-21)29(38)5-2-19-14-25(31)30(33)26(32)15-19/h2-5,14-17,20-21,28,34,37H,6-13,18H2,1H3/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224524
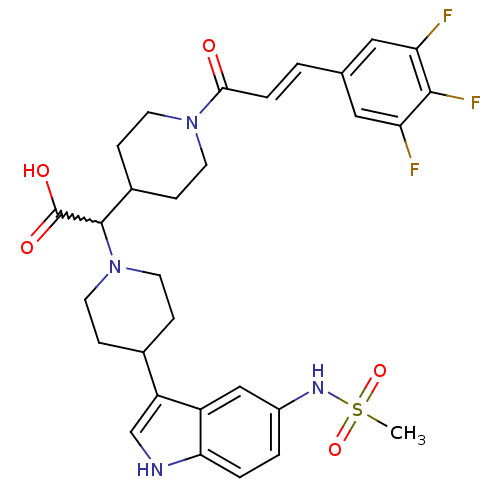
((E)-2-(4-(5-(methylsulfonamido)-1H-indol-3-yl)pipe...)Show SMILES CS(=O)(=O)Nc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:18.40| Show InChI InChI=1S/C30H33F3N4O5S/c1-43(41,42)35-21-3-4-26-22(16-21)23(17-34-26)19-6-12-37(13-7-19)29(30(39)40)20-8-10-36(11-9-20)27(38)5-2-18-14-24(31)28(33)25(32)15-18/h2-5,14-17,19-20,29,34-35H,6-13H2,1H3,(H,39,40)/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50246304
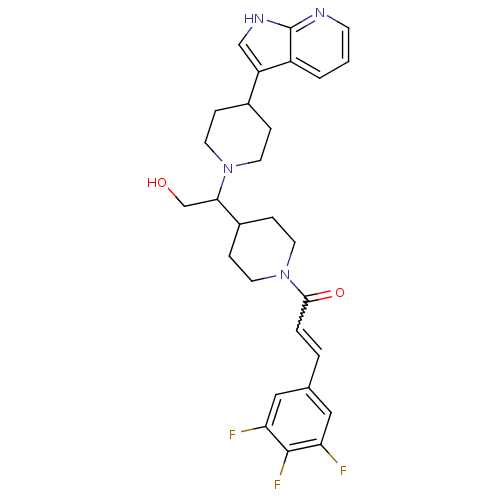
((+/-)-1-(4-(1-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)pi...)Show SMILES OCC(C1CCN(CC1)C(=O)C=Cc1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ncccc12 |w:11.11| Show InChI InChI=1S/C28H31F3N4O2/c29-23-14-18(15-24(30)27(23)31)3-4-26(37)35-12-7-20(8-13-35)25(17-36)34-10-5-19(6-11-34)22-16-33-28-21(22)2-1-9-32-28/h1-4,9,14-16,19-20,25,36H,5-8,10-13,17H2,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 |
Bioorg Med Chem Lett 18: 6468-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.061
BindingDB Entry DOI: 10.7270/Q2V40V1J |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377035
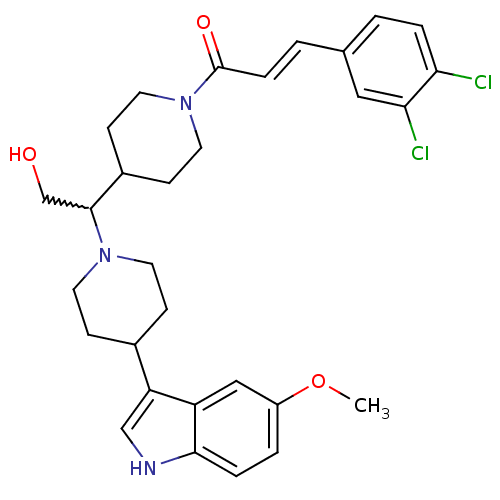
(CHEMBL257628)Show SMILES COc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3ccc(Cl)c(Cl)c3)c2c1 |w:15.16| Show InChI InChI=1S/C30H35Cl2N3O3/c1-38-23-4-6-28-24(17-23)25(18-33-28)21-8-12-34(13-9-21)29(19-36)22-10-14-35(15-11-22)30(37)7-3-20-2-5-26(31)27(32)16-20/h2-7,16-18,21-22,29,33,36H,8-15,19H2,1H3/b7-3+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224519
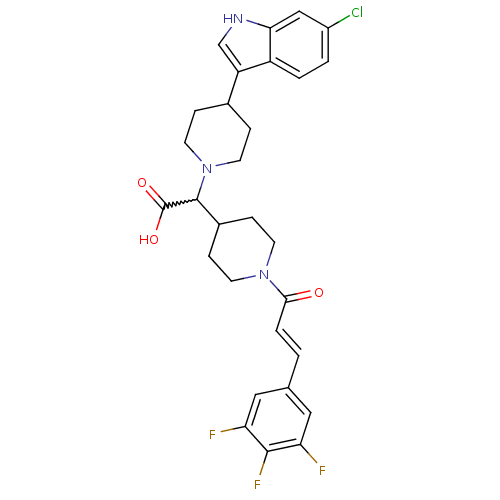
((E)-2-(4-(6-chloro-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2cc(Cl)ccc12 |w:3.2| Show InChI InChI=1S/C29H29ClF3N3O3/c30-20-2-3-21-22(16-34-25(21)15-20)18-5-11-36(12-6-18)28(29(38)39)19-7-9-35(10-8-19)26(37)4-1-17-13-23(31)27(33)24(32)14-17/h1-4,13-16,18-19,28,34H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377023
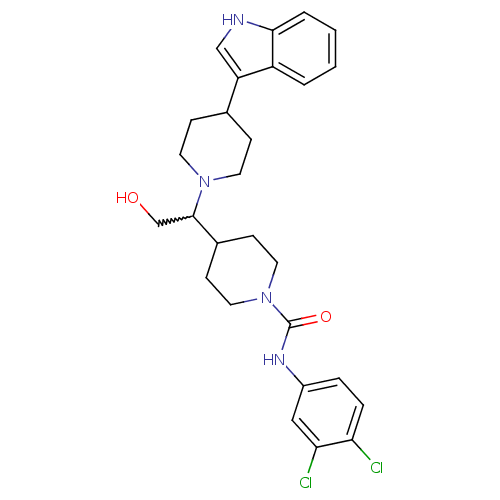
(CHEMBL438353)Show SMILES OCC(C1CCN(CC1)C(=O)Nc1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C27H32Cl2N4O2/c28-23-6-5-20(15-24(23)29)31-27(35)33-13-9-19(10-14-33)26(17-34)32-11-7-18(8-12-32)22-16-30-25-4-2-1-3-21(22)25/h1-6,15-16,18-19,26,30,34H,7-14,17H2,(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377042

(CHEMBL257385)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1cccc(Br)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C29H34BrN3O2/c30-24-5-3-4-21(18-24)8-9-29(35)33-16-12-23(13-17-33)28(20-34)32-14-10-22(11-15-32)26-19-31-27-7-2-1-6-25(26)27/h1-9,18-19,22-23,28,31,34H,10-17,20H2/b9-8+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377020
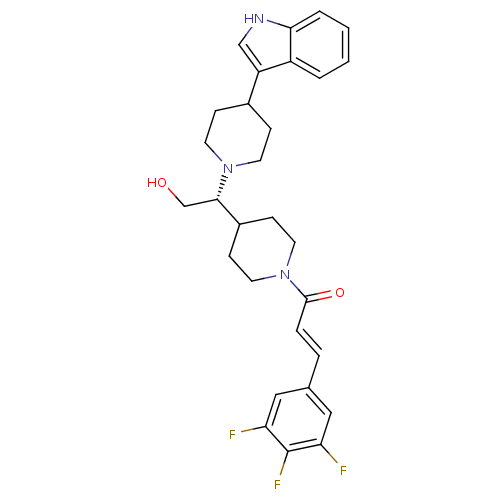
(CHEMBL257768)Show SMILES OC[C@@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H32F3N3O2/c30-24-15-19(16-25(31)29(24)32)5-6-28(37)35-13-9-21(10-14-35)27(18-36)34-11-7-20(8-12-34)23-17-33-26-4-2-1-3-22(23)26/h1-6,15-17,20-21,27,33,36H,7-14,18H2/b6-5+/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224512
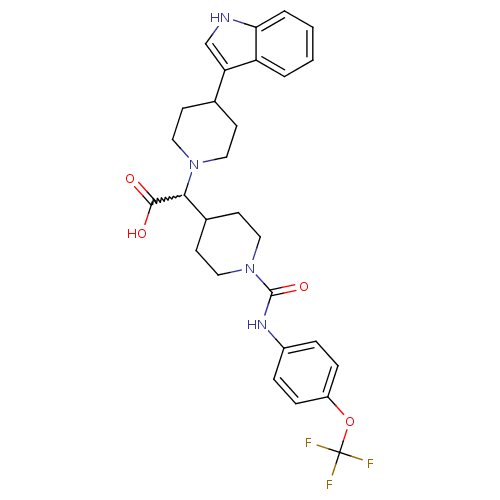
(2-(1-((4-(trifluoromethoxy)phenyl)carbamoyl)piperi...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1ccc(OC(F)(F)F)cc1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C28H31F3N4O4/c29-28(30,31)39-21-7-5-20(6-8-21)33-27(38)35-15-11-19(12-16-35)25(26(36)37)34-13-9-18(10-14-34)23-17-32-24-4-2-1-3-22(23)24/h1-8,17-19,25,32H,9-16H2,(H,33,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50216453

(CHEMBL247537 | dimethyl-{4-[(E)-3-(4'-methyl-biphe...)Show SMILES Cc1ccc(cc1)-c1ccc(\C=C\C(=O)Nc2ccc(C[N+](C)(C)C3CCOCC3)cc2)cc1 Show InChI InChI=1S/C30H34N2O2/c1-23-4-11-26(12-5-23)27-13-6-24(7-14-27)10-17-30(33)31-28-15-8-25(9-16-28)22-32(2,3)29-18-20-34-21-19-29/h4-17,29H,18-22H2,1-3H3/p+1/b17-10+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from CCR2 receptor expressed in THP1 cells |
Bioorg Med Chem Lett 17: 4382-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.115
BindingDB Entry DOI: 10.7270/Q2F47NVR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50216434

(CHEMBL245686 | dimethyl-(tetrahydro-pyran-4-yl)-{4...)Show SMILES C[N+](C)(Cc1ccc(NC(=O)\C=C\c2ccc(cc2)-c2ccc(cc2)C(F)(F)F)cc1)C1CCOCC1 Show InChI InChI=1S/C30H31F3N2O2/c1-35(2,28-17-19-37-20-18-28)21-23-5-14-27(15-6-23)34-29(36)16-7-22-3-8-24(9-4-22)25-10-12-26(13-11-25)30(31,32)33/h3-16,28H,17-21H2,1-2H3/p+1/b16-7+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from CCR2 receptor expressed in THP1 cells |
Bioorg Med Chem Lett 17: 4382-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.115
BindingDB Entry DOI: 10.7270/Q2F47NVR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377043

(CHEMBL403250)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1cccc(c1)C(F)(F)F)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C30H34F3N3O2/c31-30(32,33)24-5-3-4-21(18-24)8-9-29(38)36-16-12-23(13-17-36)28(20-37)35-14-10-22(11-15-35)26-19-34-27-7-2-1-6-25(26)27/h1-9,18-19,22-23,28,34,37H,10-17,20H2/b9-8+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377030

(CHEMBL255500)Show SMILES COc1cccc2c(c[nH]c12)C1CCN(CC1)C(CO)C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1 |w:17.20| Show InChI InChI=1S/C30H34F3N3O3/c1-39-27-4-2-3-22-23(17-34-30(22)27)20-7-11-35(12-8-20)26(18-37)21-9-13-36(14-10-21)28(38)6-5-19-15-24(31)29(33)25(32)16-19/h2-6,15-17,20-21,26,34,37H,7-14,18H2,1H3/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377022
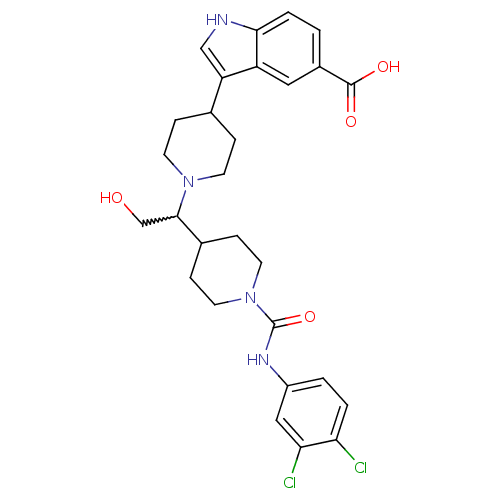
(CHEMBL403581)Show SMILES OCC(C1CCN(CC1)C(=O)Nc1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccc(cc12)C(O)=O |w:2.1| Show InChI InChI=1S/C28H32Cl2N4O4/c29-23-3-2-20(14-24(23)30)32-28(38)34-11-7-18(8-12-34)26(16-35)33-9-5-17(6-10-33)22-15-31-25-4-1-19(27(36)37)13-21(22)25/h1-4,13-15,17-18,26,31,35H,5-12,16H2,(H,32,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50246353
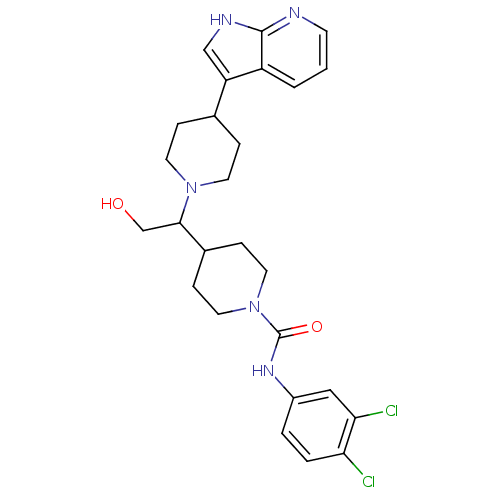
((+/-)-4-(1-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)piper...)Show SMILES OCC(C1CCN(CC1)C(=O)Nc1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ncccc12 Show InChI InChI=1S/C26H31Cl2N5O2/c27-22-4-3-19(14-23(22)28)31-26(35)33-12-7-18(8-13-33)24(16-34)32-10-5-17(6-11-32)21-15-30-25-20(21)2-1-9-29-25/h1-4,9,14-15,17-18,24,34H,5-8,10-13,16H2,(H,29,30)(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 |
Bioorg Med Chem Lett 18: 6468-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.061
BindingDB Entry DOI: 10.7270/Q2V40V1J |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224510
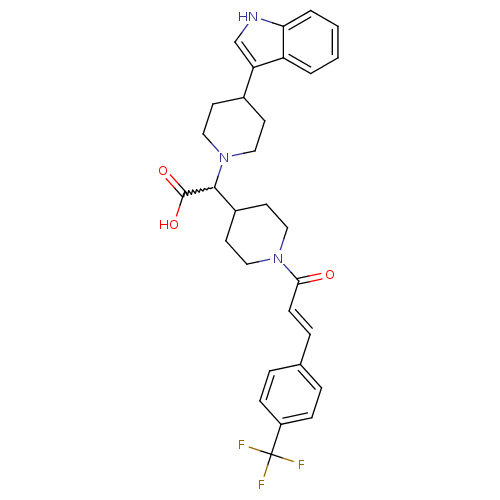
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(4...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1ccc(cc1)C(F)(F)F)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C30H32F3N3O3/c31-30(32,33)23-8-5-20(6-9-23)7-10-27(37)35-15-13-22(14-16-35)28(29(38)39)36-17-11-21(12-18-36)25-19-34-26-4-2-1-3-24(25)26/h1-10,19,21-22,28,34H,11-18H2,(H,38,39)/b10-7+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224508
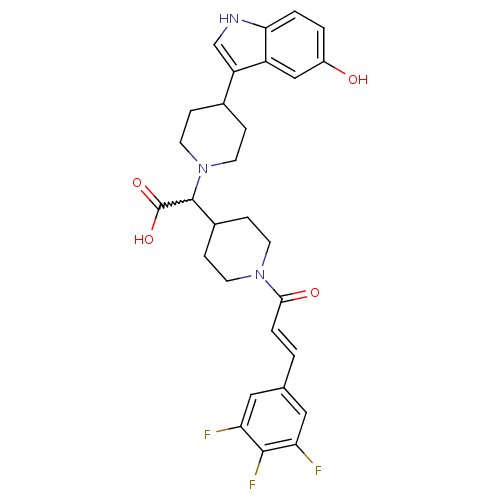
((E)-2-(4-(5-hydroxy-1H-indol-3-yl)piperidin-1-yl)-...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccc(O)cc12 |w:3.2| Show InChI InChI=1S/C29H30F3N3O4/c30-23-13-17(14-24(31)27(23)32)1-4-26(37)34-9-7-19(8-10-34)28(29(38)39)35-11-5-18(6-12-35)22-16-33-25-3-2-20(36)15-21(22)25/h1-4,13-16,18-19,28,33,36H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224521

((E)-2-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)-...)Show SMILES COc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:15.37| Show InChI InChI=1S/C30H32F3N3O4/c1-40-21-3-4-26-22(16-21)23(17-34-26)19-6-12-36(13-7-19)29(30(38)39)20-8-10-35(11-9-20)27(37)5-2-18-14-24(31)28(33)25(32)15-18/h2-5,14-17,19-20,29,34H,6-13H2,1H3,(H,38,39)/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50246303
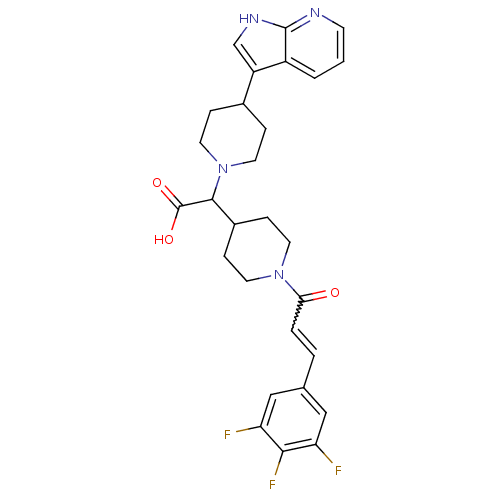
(2-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)piperidin-1-yl...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)C=Cc1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ncccc12 |w:12.12| Show InChI InChI=1S/C28H29F3N4O3/c29-22-14-17(15-23(30)25(22)31)3-4-24(36)34-10-7-19(8-11-34)26(28(37)38)35-12-5-18(6-13-35)21-16-33-27-20(21)2-1-9-32-27/h1-4,9,14-16,18-19,26H,5-8,10-13H2,(H,32,33)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 |
Bioorg Med Chem Lett 18: 6468-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.061
BindingDB Entry DOI: 10.7270/Q2V40V1J |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377036

(CHEMBL255680)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccc(F)cc12 |w:2.1| Show InChI InChI=1S/C29H32Cl2FN3O2/c30-25-4-1-19(15-26(25)31)2-6-29(37)35-13-9-21(10-14-35)28(18-36)34-11-7-20(8-12-34)24-17-33-27-5-3-22(32)16-23(24)27/h1-6,15-17,20-21,28,33,36H,7-14,18H2/b6-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377044

(CHEMBL257143)Show SMILES CC(=O)OCC(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:5.4| Show InChI InChI=1S/C31H34F3N3O3/c1-20(38)40-19-29(36-12-8-22(9-13-36)25-18-35-28-5-3-2-4-24(25)28)23-10-14-37(15-11-23)30(39)7-6-21-16-26(32)31(34)27(33)17-21/h2-7,16-18,22-23,29,35H,8-15,19H2,1H3/b7-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50216435

(CHEMBL397647 | N-(4-(3,4-dichlorobenzamido)benzyl)...)Show SMILES C[N+](C)(Cc1ccc(NC(=O)c2ccc(Cl)c(Cl)c2)cc1)C1CCOCC1 Show InChI InChI=1S/C21H24Cl2N2O2/c1-25(2,18-9-11-27-12-10-18)14-15-3-6-17(7-4-15)24-21(26)16-5-8-19(22)20(23)13-16/h3-8,13,18H,9-12,14H2,1-2H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from CCR2 receptor expressed in THP1 cells |
Bioorg Med Chem Lett 17: 4382-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.115
BindingDB Entry DOI: 10.7270/Q2F47NVR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224499
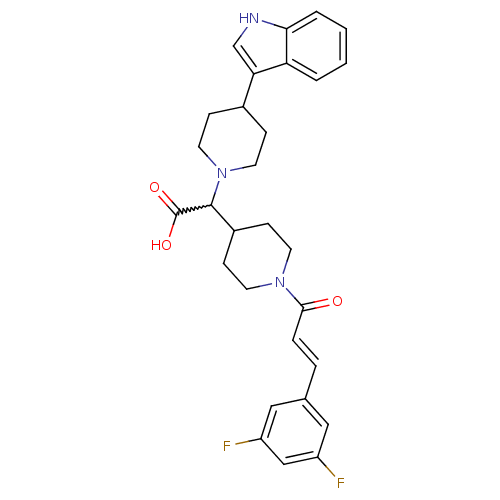
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)cc(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C29H31F2N3O3/c30-22-15-19(16-23(31)17-22)5-6-27(35)33-11-9-21(10-12-33)28(29(36)37)34-13-7-20(8-14-34)25-18-32-26-4-2-1-3-24(25)26/h1-6,15-18,20-21,28,32H,7-14H2,(H,36,37)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224506
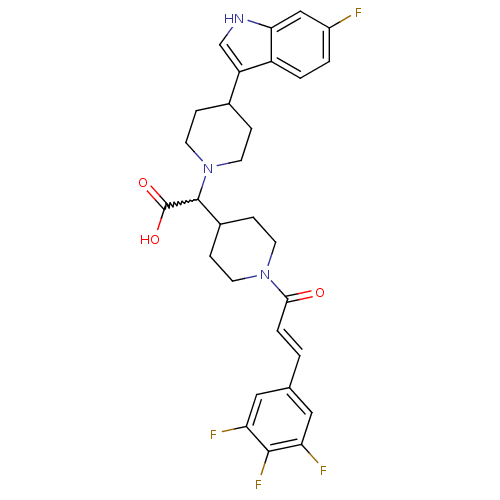
((E)-2-(4-(6-fluoro-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2cc(F)ccc12 |w:3.2| Show InChI InChI=1S/C29H29F4N3O3/c30-20-2-3-21-22(16-34-25(21)15-20)18-5-11-36(12-6-18)28(29(38)39)19-7-9-35(10-8-19)26(37)4-1-17-13-23(31)27(33)24(32)14-17/h1-4,13-16,18-19,28,34H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377031

(CHEMBL402749)Show SMILES COC(=O)c1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)c2c1 |w:17.18| Show InChI InChI=1S/C31H34F3N3O4/c1-41-31(40)22-3-4-27-23(16-22)24(17-35-27)20-6-10-36(11-7-20)28(18-38)21-8-12-37(13-9-21)29(39)5-2-19-14-25(32)30(34)26(33)15-19/h2-5,14-17,20-21,28,35,38H,6-13,18H2,1H3/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50150572

(1-(2-Bromo-phenyl)-3-(2,4-dihydroxy-phenyl)-urea |...)Show InChI InChI=1S/C13H11BrN2O3/c14-9-3-1-2-4-10(9)15-13(19)16-11-6-5-8(17)7-12(11)18/h1-7,17-18H,(H2,15,16,19) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand |
Bioorg Med Chem Lett 14: 4307-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.080
BindingDB Entry DOI: 10.7270/Q23F4P4M |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224516

(2-(1-((3,4-dichlorophenyl)carbamoyl)piperidin-4-yl...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.3| Show InChI InChI=1S/C27H30Cl2N4O3/c28-22-6-5-19(15-23(22)29)31-27(36)33-13-9-18(10-14-33)25(26(34)35)32-11-7-17(8-12-32)21-16-30-24-4-2-1-3-20(21)24/h1-6,15-18,25,30H,7-14H2,(H,31,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data