 Found 2672 hits with Last Name = 'mei' and Initial = 'd'
Found 2672 hits with Last Name = 'mei' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable cofactor NADPH concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50086170
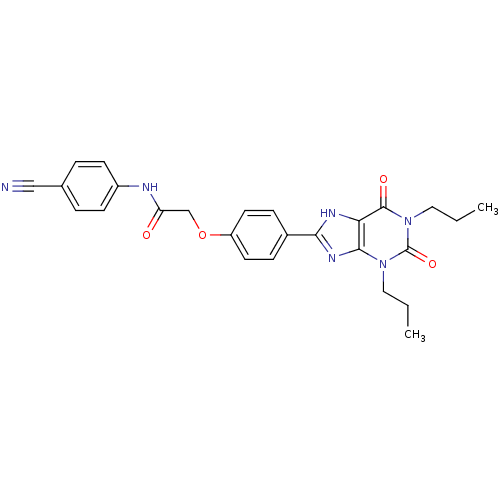
((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)Nc2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C26H26N6O4/c1-3-13-31-24-22(25(34)32(14-4-2)26(31)35)29-23(30-24)18-7-11-20(12-8-18)36-16-21(33)28-19-9-5-17(15-27)6-10-19/h5-12H,3-4,13-14,16H2,1-2H3,(H,28,33)(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human A2B adenosine receptor |
Eur J Med Chem 163: 763-778 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.045
BindingDB Entry DOI: 10.7270/Q24J0JKM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable substrate cortisol concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227864
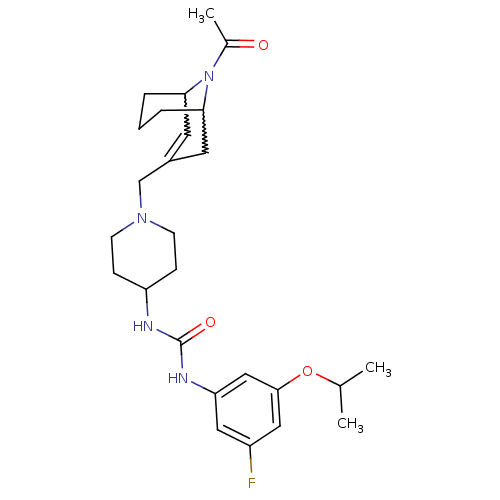
(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(C)Oc1cc(F)cc(NC(=O)NC2CCN(CC3=CC4CCCC(C3)N4C(C)=O)CC2)c1 |w:25.25,21.20,t:19,TLB:28:27:19.26.20:22.24.23| Show InChI InChI=1S/C26H37FN4O3/c1-17(2)34-25-14-20(27)13-22(15-25)29-26(33)28-21-7-9-30(10-8-21)16-19-11-23-5-4-6-24(12-19)31(23)18(3)32/h11,13-15,17,21,23-24H,4-10,12,16H2,1-3H3,(H2,28,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227866
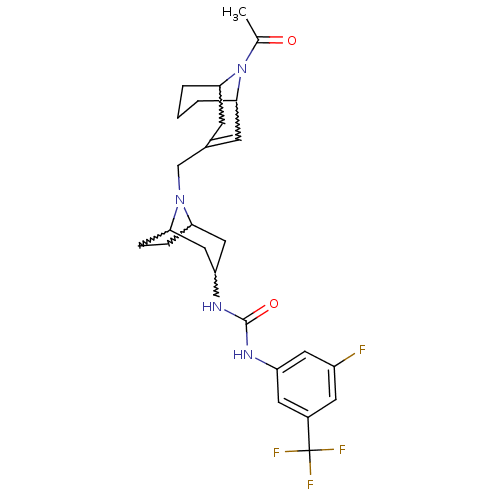
(1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1C3CCC1CC(C3)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.39,8.9,18.22,13.14,16.16,t:10,TLB:1:3:10.35.9:7.5.6,TEB:20:18:12:14.15| Show InChI InChI=1S/C26H32F4N4O2/c1-15(35)34-23-3-2-4-24(34)8-16(7-23)14-33-21-5-6-22(33)13-20(12-21)32-25(36)31-19-10-17(26(28,29)30)9-18(27)11-19/h7,9-11,20-24H,2-6,8,12-14H2,1H3,(H2,31,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227864

(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(C)Oc1cc(F)cc(NC(=O)NC2CCN(CC3=CC4CCCC(C3)N4C(C)=O)CC2)c1 |w:25.25,21.20,t:19,TLB:28:27:19.26.20:22.24.23| Show InChI InChI=1S/C26H37FN4O3/c1-17(2)34-25-14-20(27)13-22(15-25)29-26(33)28-21-7-9-30(10-8-21)16-19-11-23-5-4-6-24(12-19)31(23)18(3)32/h11,13-15,17,21,23-24H,4-10,12,16H2,1-3H3,(H2,28,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227866

(1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1C3CCC1CC(C3)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.39,8.9,18.22,13.14,16.16,t:10,TLB:1:3:10.35.9:7.5.6,TEB:20:18:12:14.15| Show InChI InChI=1S/C26H32F4N4O2/c1-15(35)34-23-3-2-4-24(34)8-16(7-23)14-33-21-5-6-22(33)13-20(12-21)32-25(36)31-19-10-17(26(28,29)30)9-18(27)11-19/h7,9-11,20-24H,2-6,8,12-14H2,1H3,(H2,31,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227863
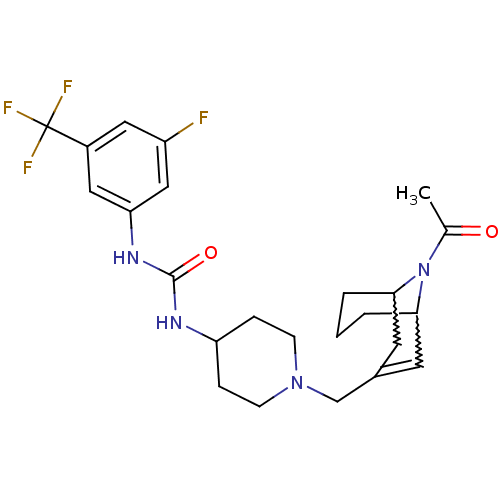
(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.36,8.9,t:10,TLB:1:3:10.33.9:7.5.6| Show InChI InChI=1S/C24H30F4N4O2/c1-15(33)32-21-3-2-4-22(32)10-16(9-21)14-31-7-5-19(6-8-31)29-23(34)30-20-12-17(24(26,27)28)11-18(25)13-20/h9,11-13,19,21-22H,2-8,10,14H2,1H3,(H2,29,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227861

(1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((9-pi...)Show SMILES CC(C)(C)C(=O)N1C2CCCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:7.39,11.12,t:13,TLB:4:6:13.36.12:10.8.9| Show InChI InChI=1S/C27H36F4N4O2/c1-26(2,3)24(36)35-22-5-4-6-23(35)12-17(11-22)16-34-9-7-20(8-10-34)32-25(37)33-21-14-18(27(29,30)31)13-19(28)15-21/h11,13-15,20,22-23H,4-10,12,16H2,1-3H3,(H2,32,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227867
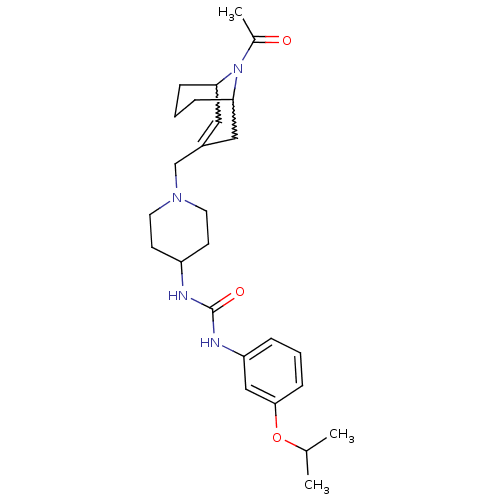
(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(C)Oc1cccc(NC(=O)NC2CCN(CC3=CC4CCCC(C3)N4C(C)=O)CC2)c1 |w:24.24,20.19,t:18,TLB:27:26:18.25.19:21.23.22| Show InChI InChI=1S/C26H38N4O3/c1-18(2)33-25-9-4-6-22(16-25)28-26(32)27-21-10-12-29(13-11-21)17-20-14-23-7-5-8-24(15-20)30(23)19(3)31/h4,6,9,14,16,18,21,23-24H,5,7-8,10-13,15,17H2,1-3H3,(H2,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM520995
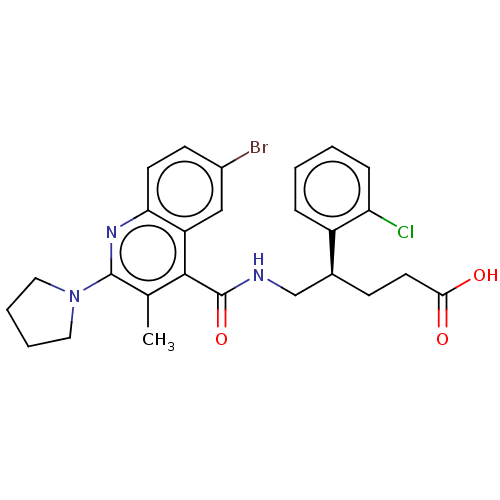
((+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC[C@H](CCC(O)=O)c1ccccc1Cl)N1CCCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGF2alpha from full-length recombinant human FP receptor expressed in HEK293 cell membranes measured after 60 mins by scintillati... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227865
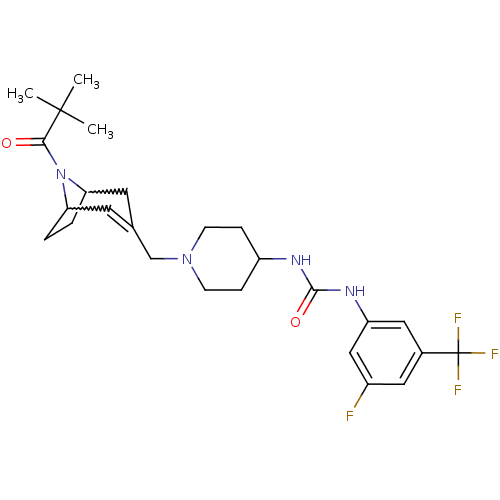
(1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((8-pi...)Show SMILES CC(C)(C)C(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:7.38,10.11,t:12| Show InChI InChI=1S/C26H34F4N4O2/c1-25(2,3)23(35)34-21-4-5-22(34)11-16(10-21)15-33-8-6-19(7-9-33)31-24(36)32-20-13-17(26(28,29)30)12-18(27)14-20/h10,12-14,19,21-22H,4-9,11,15H2,1-3H3,(H2,31,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227862
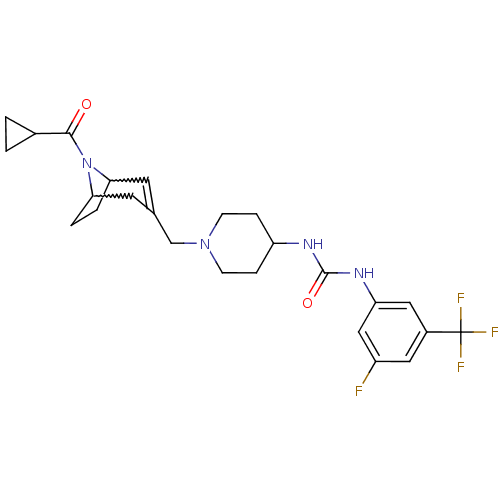
(1-(1-((8-(cyclopropanecarbonyl)-8-aza-bicyclo[3.2....)Show SMILES Fc1cc(NC(=O)NC2CCN(CC3=CC4CCC(C3)N4C(=O)C3CC3)CC2)cc(c1)C(F)(F)F |w:18.18,15.14,t:13| Show InChI InChI=1S/C25H30F4N4O2/c26-18-11-17(25(27,28)29)12-20(13-18)31-24(35)30-19-5-7-32(8-6-19)14-15-9-21-3-4-22(10-15)33(21)23(34)16-1-2-16/h9,11-13,16,19,21-22H,1-8,10,14H2,(H2,30,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50336977
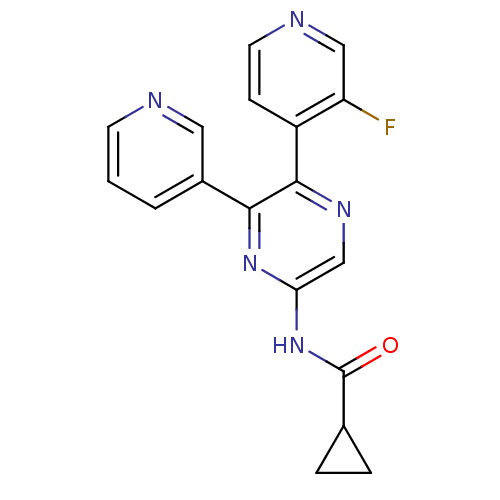
(CHEMBL1672627 | N-[5-(3-Fluoropyridin-4-yl)-6-pyri...)Show InChI InChI=1S/C18H14FN5O/c19-14-9-21-7-5-13(14)17-16(12-2-1-6-20-8-12)23-15(10-22-17)24-18(25)11-3-4-11/h1-2,5-11H,3-4H2,(H,23,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human A2B adenosine receptor |
Eur J Med Chem 163: 763-778 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.045
BindingDB Entry DOI: 10.7270/Q24J0JKM |
More data for this
Ligand-Target Pair | |
4-hydroxy-tetrahydrodipicolinate reductase
(Escherichia coli) | BDBM59098
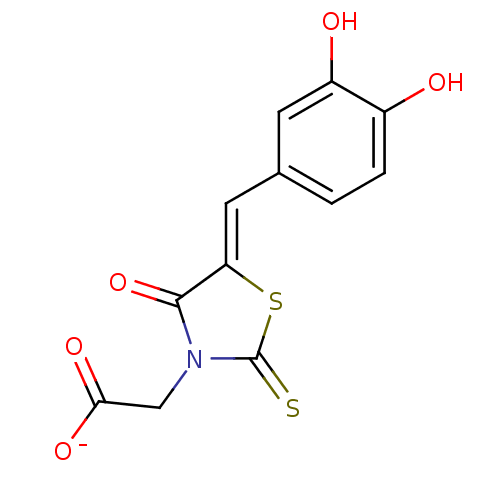
(Bi-ligand, 1)Show InChI InChI=1S/C12H9NO5S2/c14-7-2-1-6(3-8(7)15)4-9-11(18)13(5-10(16)17)12(19)20-9/h1-4,14-15H,5H2,(H,16,17)/p-1/b9-4- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50198392
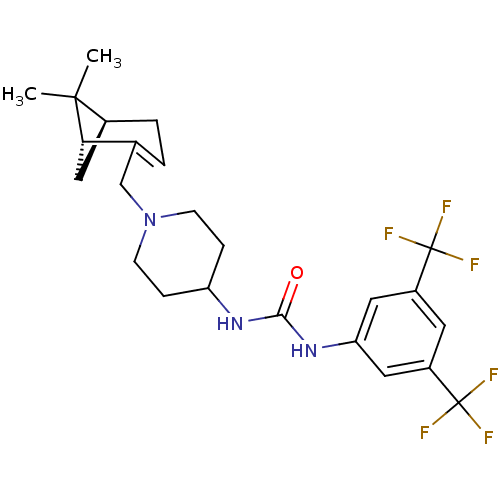
(1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-(((1R,5S)-...)Show SMILES CC1(C)[C@@H]2C[C@H]1C(CN1CCC(CC1)NC(=O)Nc1cc(cc(c1)C(F)(F)F)C(F)(F)F)=CC2 |c:34| Show InChI InChI=1S/C24H29F6N3O/c1-22(2)15-4-3-14(20(22)12-15)13-33-7-5-18(6-8-33)31-21(34)32-19-10-16(23(25,26)27)9-17(11-19)24(28,29)30/h3,9-11,15,18,20H,4-8,12-13H2,1-2H3,(H2,31,32,34)/t15-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227869
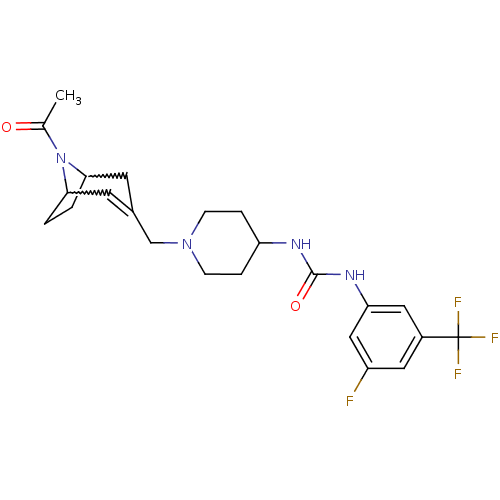
(1-[1-(8-acetyl-8-aza-bicyclo[3.2.1]oct-2-en-3-ylme...)Show SMILES CC(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.35,7.8,t:9| Show InChI InChI=1S/C23H28F4N4O2/c1-14(32)31-20-2-3-21(31)9-15(8-20)13-30-6-4-18(5-7-30)28-22(33)29-19-11-16(23(25,26)27)10-17(24)12-19/h8,10-12,18,20-21H,2-7,9,13H2,1H3,(H2,28,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227873
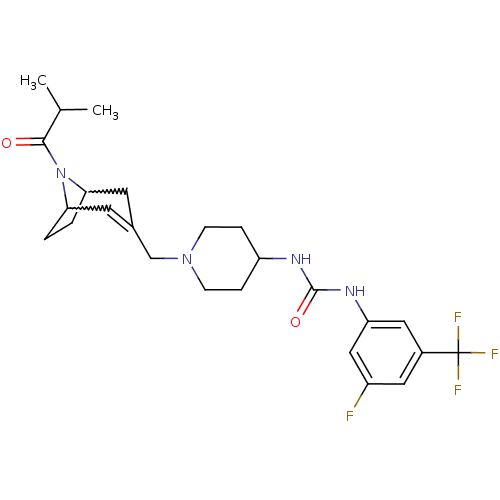
(1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((8-is...)Show SMILES CC(C)C(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:6.37,9.10,t:11| Show InChI InChI=1S/C25H32F4N4O2/c1-15(2)23(34)33-21-3-4-22(33)10-16(9-21)14-32-7-5-19(6-8-32)30-24(35)31-20-12-17(25(27,28)29)11-18(26)13-20/h9,11-13,15,19,21-22H,3-8,10,14H2,1-2H3,(H2,30,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227867

(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(C)Oc1cccc(NC(=O)NC2CCN(CC3=CC4CCCC(C3)N4C(C)=O)CC2)c1 |w:24.24,20.19,t:18,TLB:27:26:18.25.19:21.23.22| Show InChI InChI=1S/C26H38N4O3/c1-18(2)33-25-9-4-6-22(16-25)28-26(32)27-21-10-12-29(13-11-21)17-20-14-23-7-5-8-24(15-20)30(23)19(3)31/h4,6,9,14,16,18,21,23-24H,5,7-8,10-13,15,17H2,1-3H3,(H2,27,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227863

(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.36,8.9,t:10,TLB:1:3:10.33.9:7.5.6| Show InChI InChI=1S/C24H30F4N4O2/c1-15(33)32-21-3-2-4-22(32)10-16(9-21)14-31-7-5-19(6-8-31)29-23(34)30-20-12-17(24(26,27)28)11-18(25)13-20/h9,11-13,19,21-22H,2-8,10,14H2,1H3,(H2,29,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Quinone-dependent D-lactate dehydrogenase
(Escherichia coli) | BDBM59099
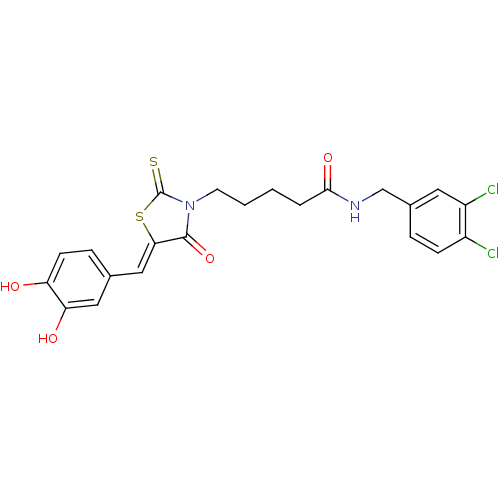
(Bi-ligand, 2)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CCCCC(=O)NCc3ccc(Cl)c(Cl)c3)C2=O)cc1O Show InChI InChI=1S/C22H20Cl2N2O4S2/c23-15-6-4-14(9-16(15)24)12-25-20(29)3-1-2-8-26-21(30)19(32-22(26)31)11-13-5-7-17(27)18(28)10-13/h4-7,9-11,27-28H,1-3,8,12H2,(H,25,29)/b19-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227861

(1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((9-pi...)Show SMILES CC(C)(C)C(=O)N1C2CCCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:7.39,11.12,t:13,TLB:4:6:13.36.12:10.8.9| Show InChI InChI=1S/C27H36F4N4O2/c1-26(2,3)24(36)35-22-5-4-6-23(35)12-17(11-22)16-34-9-7-20(8-10-34)32-25(37)33-21-14-18(27(29,30)31)13-19(28)15-21/h11,13-15,20,22-23H,4-10,12,16H2,1-3H3,(H2,32,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227868
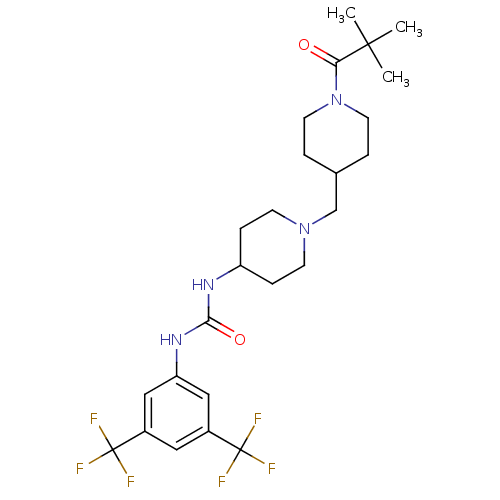
(1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-((1-pivalo...)Show SMILES CC(C)(C)C(=O)N1CCC(CN2CCC(CC2)NC(=O)Nc2cc(cc(c2)C(F)(F)F)C(F)(F)F)CC1 Show InChI InChI=1S/C25H34F6N4O2/c1-23(2,3)21(36)35-10-4-16(5-11-35)15-34-8-6-19(7-9-34)32-22(37)33-20-13-17(24(26,27)28)12-18(14-20)25(29,30)31/h12-14,16,19H,4-11,15H2,1-3H3,(H2,32,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 by steady state kinetic assay |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227862

(1-(1-((8-(cyclopropanecarbonyl)-8-aza-bicyclo[3.2....)Show SMILES Fc1cc(NC(=O)NC2CCN(CC3=CC4CCC(C3)N4C(=O)C3CC3)CC2)cc(c1)C(F)(F)F |w:18.18,15.14,t:13| Show InChI InChI=1S/C25H30F4N4O2/c26-18-11-17(25(27,28)29)12-20(13-18)31-24(35)30-19-5-7-32(8-6-19)14-15-9-21-3-4-22(10-15)33(21)23(34)16-1-2-16/h9,11-13,16,19,21-22H,1-8,10,14H2,(H2,30,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
4-hydroxy-tetrahydrodipicolinate reductase
(Escherichia coli) | BDBM59101
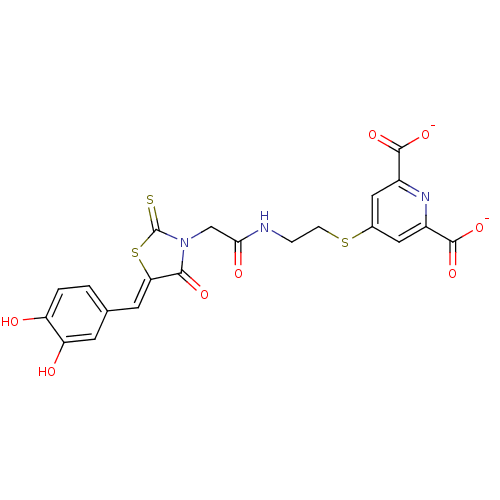
(Bi-ligand, 4)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CC(=O)NCCSc3cc(nc(c3)C([O-])=O)C([O-])=O)C2=O)cc1O Show InChI InChI=1S/C21H17N3O8S3/c25-14-2-1-10(5-15(14)26)6-16-18(28)24(21(33)35-16)9-17(27)22-3-4-34-11-7-12(19(29)30)23-13(8-11)20(31)32/h1-2,5-8,25-26H,3-4,9H2,(H,22,27)(H,29,30)(H,31,32)/p-2/b16-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227865

(1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((8-pi...)Show SMILES CC(C)(C)C(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:7.38,10.11,t:12| Show InChI InChI=1S/C26H34F4N4O2/c1-25(2,3)23(35)34-21-4-5-22(34)11-16(10-21)15-33-8-6-19(7-9-33)31-24(36)32-20-13-17(26(28,29)30)12-18(27)14-20/h10,12-14,19,21-22H,4-9,11,15H2,1-3H3,(H2,31,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50198394
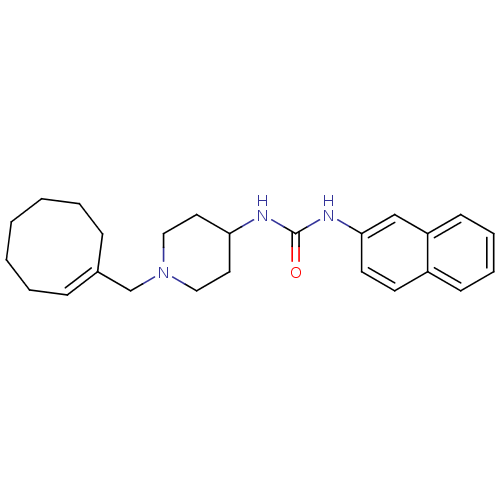
((E)-1-(1-(cyclooctenylmethyl)piperidin-4-yl)-3-(na...)Show SMILES O=C(NC1CCN(C\C2=C\CCCCCC2)CC1)Nc1ccc2ccccc2c1 |t:8| Show InChI InChI=1S/C25H33N3O/c29-25(27-24-13-12-21-10-6-7-11-22(21)18-24)26-23-14-16-28(17-15-23)19-20-8-4-2-1-3-5-9-20/h6-8,10-13,18,23H,1-5,9,14-17,19H2,(H2,26,27,29)/b20-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227871
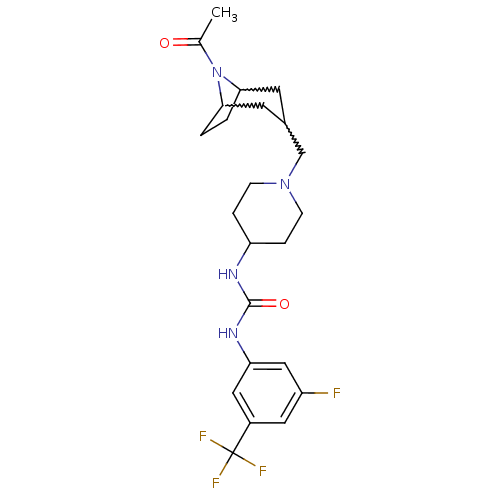
(1-(1-((8-acetyl-8-aza-bicyclo[3.2.1]octan-3-yl)met...)Show SMILES CC(=O)N1C2CCC1CC(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:9.10,7.8,4.35,TLB:1:3:9.8.32:5.6,TEB:10:9:5.6:3| Show InChI InChI=1S/C23H30F4N4O2/c1-14(32)31-20-2-3-21(31)9-15(8-20)13-30-6-4-18(5-7-30)28-22(33)29-19-11-16(23(25,26)27)10-17(24)12-19/h10-12,15,18,20-21H,2-9,13H2,1H3,(H2,28,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227869

(1-[1-(8-acetyl-8-aza-bicyclo[3.2.1]oct-2-en-3-ylme...)Show SMILES CC(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.35,7.8,t:9| Show InChI InChI=1S/C23H28F4N4O2/c1-14(32)31-20-2-3-21(31)9-15(8-20)13-30-6-4-18(5-7-30)28-22(33)29-19-11-16(23(25,26)27)10-17(24)12-19/h8,10-12,18,20-21H,2-7,9,13H2,1H3,(H2,28,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227873

(1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((8-is...)Show SMILES CC(C)C(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:6.37,9.10,t:11| Show InChI InChI=1S/C25H32F4N4O2/c1-15(2)23(34)33-21-3-4-22(33)10-16(9-21)14-32-7-5-19(6-8-32)30-24(35)31-20-12-17(25(27,28)29)11-18(26)13-20/h9,11-13,15,19,21-22H,3-8,10,14H2,1-2H3,(H2,30,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227875
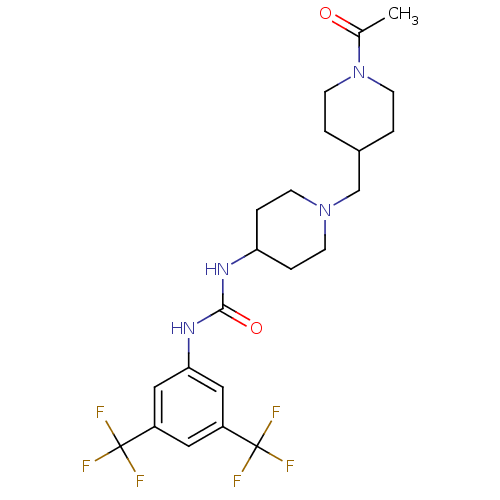
(1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-((1-acetyl...)Show SMILES CC(=O)N1CCC(CN2CCC(CC2)NC(=O)Nc2cc(cc(c2)C(F)(F)F)C(F)(F)F)CC1 Show InChI InChI=1S/C22H28F6N4O2/c1-14(33)32-8-2-15(3-9-32)13-31-6-4-18(5-7-31)29-20(34)30-19-11-16(21(23,24)25)10-17(12-19)22(26,27)28/h10-12,15,18H,2-9,13H2,1H3,(H2,29,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227874
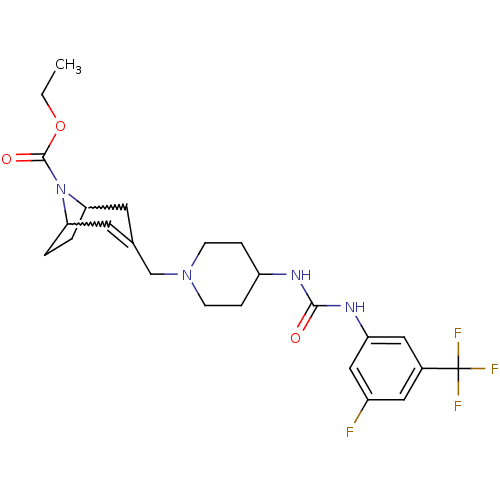
(3-{4-[3-(3-fluoro-5-trifluoromethyl-phenyl)-ureido...)Show SMILES CCOC(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:6.37,9.10,t:11| Show InChI InChI=1S/C24H30F4N4O3/c1-2-35-23(34)32-20-3-4-21(32)10-15(9-20)14-31-7-5-18(6-8-31)29-22(33)30-19-12-16(24(26,27)28)11-17(25)13-19/h9,11-13,18,20-21H,2-8,10,14H2,1H3,(H2,29,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM59100
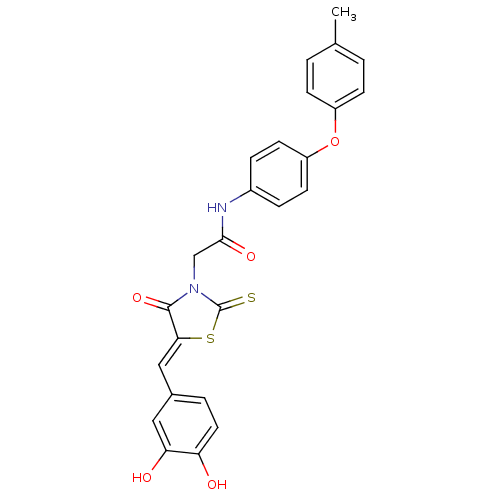
(Bi-ligand, 3)Show SMILES Cc1ccc(Oc2ccc(NC(=O)CN3C(=S)S\C(=C/c4ccc(O)c(O)c4)C3=O)cc2)cc1 Show InChI InChI=1S/C25H20N2O5S2/c1-15-2-7-18(8-3-15)32-19-9-5-17(6-10-19)26-23(30)14-27-24(31)22(34-25(27)33)13-16-4-11-20(28)21(29)12-16/h2-13,28-29H,14H2,1H3,(H,26,30)/b22-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 202 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50227866

(1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1C3CCC1CC(C3)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.39,8.9,18.22,13.14,16.16,t:10,TLB:1:3:10.35.9:7.5.6,TEB:20:18:12:14.15| Show InChI InChI=1S/C26H32F4N4O2/c1-15(35)34-23-3-2-4-24(34)8-16(7-23)14-33-21-5-6-22(33)13-20(12-21)32-25(36)31-19-10-17(26(28,29)30)9-18(27)11-19/h7,9-11,20-24H,2-6,8,12-14H2,1H3,(H2,31,32,36) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Inhibition of muscarinic M1 receptor |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227870
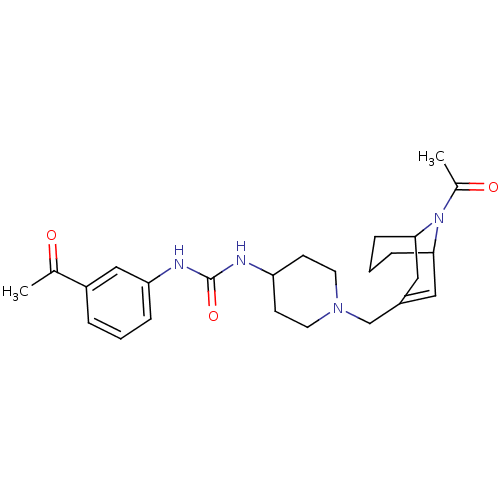
(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1CCC(CC1)NC(=O)Nc1cccc(c1)C(C)=O)C2 |t:10,TLB:1:3:10.31.9:7.5.6| Show InChI InChI=1S/C25H34N4O3/c1-17(30)20-5-3-6-22(15-20)27-25(32)26-21-9-11-28(12-10-21)16-19-13-23-7-4-8-24(14-19)29(23)18(2)31/h3,5-6,13,15,21,23-24H,4,7-12,14,16H2,1-2H3,(H2,26,27,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50227866

(1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1C3CCC1CC(C3)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.39,8.9,18.22,13.14,16.16,t:10,TLB:1:3:10.35.9:7.5.6,TEB:20:18:12:14.15| Show InChI InChI=1S/C26H32F4N4O2/c1-15(35)34-23-3-2-4-24(34)8-16(7-23)14-33-21-5-6-22(33)13-20(12-21)32-25(36)31-19-10-17(26(28,29)30)9-18(27)11-19/h7,9-11,20-24H,2-6,8,12-14H2,1H3,(H2,31,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Inhibition of 5HT1A receptor |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50227866

(1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1C3CCC1CC(C3)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.39,8.9,18.22,13.14,16.16,t:10,TLB:1:3:10.35.9:7.5.6,TEB:20:18:12:14.15| Show InChI InChI=1S/C26H32F4N4O2/c1-15(35)34-23-3-2-4-24(34)8-16(7-23)14-33-21-5-6-22(33)13-20(12-21)32-25(36)31-19-10-17(26(28,29)30)9-18(27)11-19/h7,9-11,20-24H,2-6,8,12-14H2,1H3,(H2,31,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Inhibition of muscarinic M2 receptor |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50198394

((E)-1-(1-(cyclooctenylmethyl)piperidin-4-yl)-3-(na...)Show SMILES O=C(NC1CCN(C\C2=C\CCCCCC2)CC1)Nc1ccc2ccccc2c1 |t:8| Show InChI InChI=1S/C25H33N3O/c29-25(27-24-13-12-21-10-6-7-11-22(21)18-24)26-23-14-16-28(17-15-23)19-20-8-4-2-1-3-5-9-20/h6-8,10-13,18,23H,1-5,9,14-17,19H2,(H2,26,27,29)/b20-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50198392

(1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-(((1R,5S)-...)Show SMILES CC1(C)[C@@H]2C[C@H]1C(CN1CCC(CC1)NC(=O)Nc1cc(cc(c1)C(F)(F)F)C(F)(F)F)=CC2 |c:34| Show InChI InChI=1S/C24H29F6N3O/c1-22(2)15-4-3-14(20(22)12-15)13-33-7-5-18(6-8-33)31-21(34)32-19-10-16(23(25,26)27)9-17(11-19)24(28,29)30/h3,9-11,15,18,20H,4-8,12-13H2,1-2H3,(H2,31,32,34)/t15-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50198394

((E)-1-(1-(cyclooctenylmethyl)piperidin-4-yl)-3-(na...)Show SMILES O=C(NC1CCN(C\C2=C\CCCCCC2)CC1)Nc1ccc2ccccc2c1 |t:8| Show InChI InChI=1S/C25H33N3O/c29-25(27-24-13-12-21-10-6-7-11-22(21)18-24)26-23-14-16-28(17-15-23)19-20-8-4-2-1-3-5-9-20/h6-8,10-13,18,23H,1-5,9,14-17,19H2,(H2,26,27,29)/b20-8+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Quinone-dependent D-lactate dehydrogenase
(Escherichia coli) | BDBM59101

(Bi-ligand, 4)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CC(=O)NCCSc3cc(nc(c3)C([O-])=O)C([O-])=O)C2=O)cc1O Show InChI InChI=1S/C21H17N3O8S3/c25-14-2-1-10(5-15(14)26)6-16-18(28)24(21(33)35-16)9-17(27)22-3-4-34-11-7-12(19(29)30)23-13(8-11)20(31)32/h1-2,5-8,25-26H,3-4,9H2,(H,22,27)(H,29,30)(H,31,32)/p-2/b16-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227871

(1-(1-((8-acetyl-8-aza-bicyclo[3.2.1]octan-3-yl)met...)Show SMILES CC(=O)N1C2CCC1CC(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:9.10,7.8,4.35,TLB:1:3:9.8.32:5.6,TEB:10:9:5.6:3| Show InChI InChI=1S/C23H30F4N4O2/c1-14(32)31-20-2-3-21(31)9-15(8-20)13-30-6-4-18(5-7-30)28-22(33)29-19-11-16(23(25,26)27)10-17(24)12-19/h10-12,15,18,20-21H,2-9,13H2,1H3,(H2,28,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227874

(3-{4-[3-(3-fluoro-5-trifluoromethyl-phenyl)-ureido...)Show SMILES CCOC(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:6.37,9.10,t:11| Show InChI InChI=1S/C24H30F4N4O3/c1-2-35-23(34)32-20-3-4-21(32)10-15(9-20)14-31-7-5-18(6-8-31)29-22(33)30-19-12-16(24(26,27)28)11-17(25)13-19/h9,11-13,18,20-21H,2-8,10,14H2,1H3,(H2,29,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50531422

(CHEMBL4522981)Show SMILES CCn1c(=O)n(CCC(F)(F)F)c2sc(C(=O)N3CCC(O)CC3)c(C)c2c1=O Show InChI InChI=1S/C18H22F3N3O4S/c1-3-23-14(26)12-10(2)13(15(27)22-7-4-11(25)5-8-22)29-16(12)24(17(23)28)9-6-18(19,20)21/h11,25H,3-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human A2A adenosine receptor expressed in HEK293 cell membranes after 120 mins by radioligand displacement assay |
Eur J Med Chem 163: 763-778 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.045
BindingDB Entry DOI: 10.7270/Q24J0JKM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50531422

(CHEMBL4522981)Show SMILES CCn1c(=O)n(CCC(F)(F)F)c2sc(C(=O)N3CCC(O)CC3)c(C)c2c1=O Show InChI InChI=1S/C18H22F3N3O4S/c1-3-23-14(26)12-10(2)13(15(27)22-7-4-11(25)5-8-22)29-16(12)24(17(23)28)9-6-18(19,20)21/h11,25H,3-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins by radioligand displacement assay |
Eur J Med Chem 163: 763-778 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.045
BindingDB Entry DOI: 10.7270/Q24J0JKM |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227872
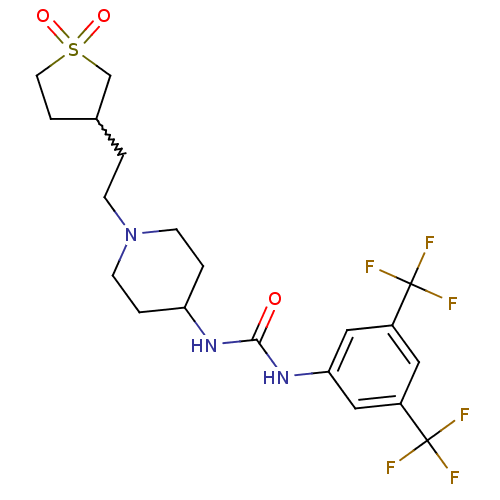
(1-(3,5-bis-trifluoromethyl-phenyl)-3-{1-[2-(1,1-di...)Show SMILES FC(F)(F)c1cc(NC(=O)NC2CCN(CCC3CCS(=O)(=O)C3)CC2)cc(c1)C(F)(F)F |w:17.16| Show InChI InChI=1S/C20H25F6N3O3S/c21-19(22,23)14-9-15(20(24,25)26)11-17(10-14)28-18(30)27-16-2-6-29(7-3-16)5-1-13-4-8-33(31,32)12-13/h9-11,13,16H,1-8,12H2,(H2,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50227866

(1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1C3CCC1CC(C3)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.39,8.9,18.22,13.14,16.16,t:10,TLB:1:3:10.35.9:7.5.6,TEB:20:18:12:14.15| Show InChI InChI=1S/C26H32F4N4O2/c1-15(35)34-23-3-2-4-24(34)8-16(7-23)14-33-21-5-6-22(33)13-20(12-21)32-25(36)31-19-10-17(26(28,29)30)9-18(27)11-19/h7,9-11,20-24H,2-6,8,12-14H2,1H3,(H2,31,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Inhibition of muscarinic M3 receptor |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM50227866

(1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1C3CCC1CC(C3)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.39,8.9,18.22,13.14,16.16,t:10,TLB:1:3:10.35.9:7.5.6,TEB:20:18:12:14.15| Show InChI InChI=1S/C26H32F4N4O2/c1-15(35)34-23-3-2-4-24(34)8-16(7-23)14-33-21-5-6-22(33)13-20(12-21)32-25(36)31-19-10-17(26(28,29)30)9-18(27)11-19/h7,9-11,20-24H,2-6,8,12-14H2,1H3,(H2,31,32,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Inhibition of 5HT5A receptor |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50198392

(1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-(((1R,5S)-...)Show SMILES CC1(C)[C@@H]2C[C@H]1C(CN1CCC(CC1)NC(=O)Nc1cc(cc(c1)C(F)(F)F)C(F)(F)F)=CC2 |c:34| Show InChI InChI=1S/C24H29F6N3O/c1-22(2)15-4-3-14(20(22)12-15)13-33-7-5-18(6-8-33)31-21(34)32-19-10-16(23(25,26)27)9-17(11-19)24(28,29)30/h3,9-11,15,18,20H,4-8,12-13H2,1-2H3,(H2,31,32,34)/t15-,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data