 Found 3419 hits with Last Name = 'mills' and Initial = 'g'
Found 3419 hits with Last Name = 'mills' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50389603

(CHEMBL2069499)Show SMILES CCCCC1(CCCC)N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C28H34N4/c1-3-5-16-28(17-6-4-2)26-22(21-14-10-11-15-23(21)30-26)18-24(32-28)27-29-19-25(31-27)20-12-8-7-9-13-20/h7-15,19,24,30,32H,3-6,16-18H2,1-2H3,(H,29,31)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SST3 receptor expressed in CHO cells after 60 mins by scintillation counting |
ACS Med Chem Lett 3: 289-293 (2012)
Article DOI: 10.1021/ml200272z
BindingDB Entry DOI: 10.7270/Q2K938MH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against specific binding of [125I]-MIP-1 alpha to human CCR5 receptor |
Bioorg Med Chem Lett 11: 265-70 (2001)
BindingDB Entry DOI: 10.7270/Q2668CFZ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288674
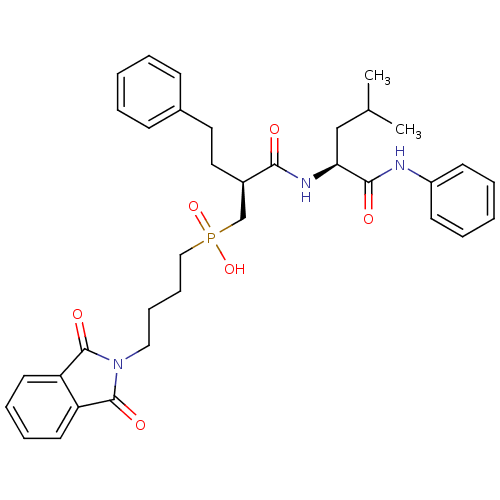
(CHEMBL420674 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50389592
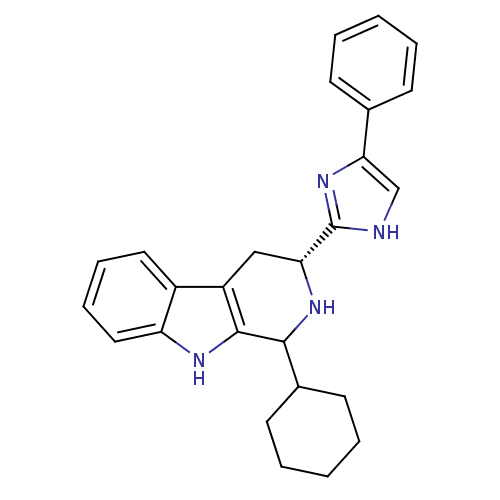
(CHEMBL2069500)Show SMILES C1CCC(CC1)C1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C26H28N4/c1-3-9-17(10-4-1)23-16-27-26(30-23)22-15-20-19-13-7-8-14-21(19)28-25(20)24(29-22)18-11-5-2-6-12-18/h1,3-4,7-10,13-14,16,18,22,24,28-29H,2,5-6,11-12,15H2,(H,27,30)/t22-,24?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human SST3 receptor |
ACS Med Chem Lett 3: 289-293 (2012)
Article DOI: 10.1021/ml200272z
BindingDB Entry DOI: 10.7270/Q2K938MH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288673

(CHEMBL264455 | {4-[((S)-1-Benzoyl-pyrrolidine-2-ca...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C39H51N4O6P/c1-29(2)27-34(37(45)41-33-19-10-5-11-20-33)42-36(44)32(23-22-30-15-6-3-7-16-30)28-50(48,49)26-13-12-24-40-38(46)35-21-14-25-43(35)39(47)31-17-8-4-9-18-31/h3-11,15-20,29,32,34-35H,12-14,21-28H2,1-2H3,(H,40,46)(H,41,45)(H,42,44)(H,48,49)/t32-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288672

(CHEMBL113362 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1cccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)22-32(34(41)37-28-13-5-4-6-14-28)38-33(40)27(19-18-26-12-11-15-29(23-26)46-3)24-47(44,45)21-10-9-20-39-35(42)30-16-7-8-17-31(30)36(39)43/h4-8,11-17,23,25,27,32H,9-10,18-22,24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288683
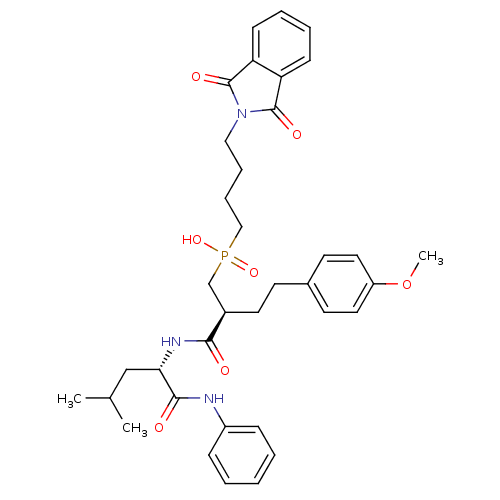
(CHEMBL109438 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1ccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)23-32(34(41)37-28-11-5-4-6-12-28)38-33(40)27(18-15-26-16-19-29(46-3)20-17-26)24-47(44,45)22-10-9-21-39-35(42)30-13-7-8-14-31(30)36(39)43/h4-8,11-14,16-17,19-20,25,27,32H,9-10,15,18,21-24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human NK3 receptor |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human NK2 receptor |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288672

(CHEMBL113362 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1cccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)22-32(34(41)37-28-13-5-4-6-14-28)38-33(40)27(19-18-26-12-11-15-29(23-26)46-3)24-47(44,45)21-10-9-20-39-35(42)30-16-7-8-17-31(30)36(39)43/h4-8,11-17,23,25,27,32H,9-10,18-22,24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288677
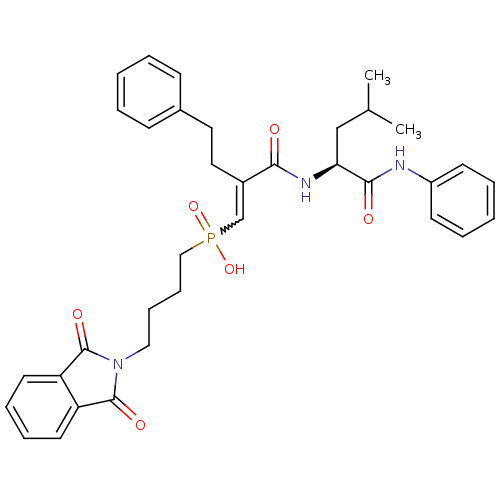
(CHEMBL324691 | [(E)-4-(1,3-Dioxo-1,3-dihydro-isoin...)Show SMILES CC(C)C[C@H](NC(=O)C(CCc1ccccc1)=CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 |w:17.18| Show InChI InChI=1S/C35H40N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,24-25,31H,11-12,19-23H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288677

(CHEMBL324691 | [(E)-4-(1,3-Dioxo-1,3-dihydro-isoin...)Show SMILES CC(C)C[C@H](NC(=O)C(CCc1ccccc1)=CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 |w:17.18| Show InChI InChI=1S/C35H40N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,24-25,31H,11-12,19-23H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288678

(CHEMBL320968 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1Cc2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H44N3O5P/c1-26(2)23-32(34(40)36-30-16-7-4-8-17-30)37-33(39)29(20-19-27-13-5-3-6-14-27)25-44(42,43)22-12-11-21-38-24-28-15-9-10-18-31(28)35(38)41/h3-10,13-18,26,29,32H,11-12,19-25H2,1-2H3,(H,36,40)(H,37,39)(H,42,43)/t29-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288681
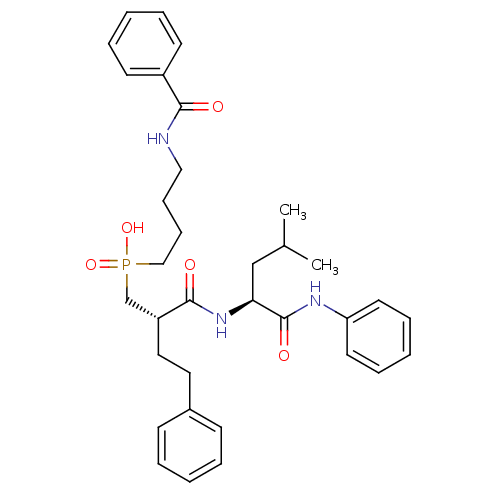
((4-Benzoylamino-butyl)-[(S)-2-((S)-3-methyl-1-phen...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H44N3O5P/c1-26(2)24-31(34(40)36-30-18-10-5-11-19-30)37-33(39)29(21-20-27-14-6-3-7-15-27)25-43(41,42)23-13-12-22-35-32(38)28-16-8-4-9-17-28/h3-11,14-19,26,29,31H,12-13,20-25H2,1-2H3,(H,35,38)(H,36,40)(H,37,39)(H,41,42)/t29-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288687

(CHEMBL111975 | {4-[((S)-1-Acetyl-pyrrolidine-2-car...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288678

(CHEMBL320968 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1Cc2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H44N3O5P/c1-26(2)23-32(34(40)36-30-16-7-4-8-17-30)37-33(39)29(20-19-27-13-5-3-6-14-27)25-44(42,43)22-12-11-21-38-24-28-15-9-10-18-31(28)35(38)41/h3-10,13-18,26,29,32H,11-12,19-25H2,1-2H3,(H,36,40)(H,37,39)(H,42,43)/t29-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288683

(CHEMBL109438 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1ccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)23-32(34(41)37-28-11-5-4-6-12-28)38-33(40)27(18-15-26-16-19-29(46-3)20-17-26)24-47(44,45)22-10-9-21-39-35(42)30-13-7-8-14-31(30)36(39)43/h4-8,11-14,16-17,19-20,25,27,32H,9-10,15,18,21-24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288673

(CHEMBL264455 | {4-[((S)-1-Benzoyl-pyrrolidine-2-ca...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C39H51N4O6P/c1-29(2)27-34(37(45)41-33-19-10-5-11-20-33)42-36(44)32(23-22-30-15-6-3-7-16-30)28-50(48,49)26-13-12-24-40-38(46)35-21-14-25-43(35)39(47)31-17-8-4-9-18-31/h3-11,15-20,29,32,34-35H,12-14,21-28H2,1-2H3,(H,40,46)(H,41,45)(H,42,44)(H,48,49)/t32-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288699
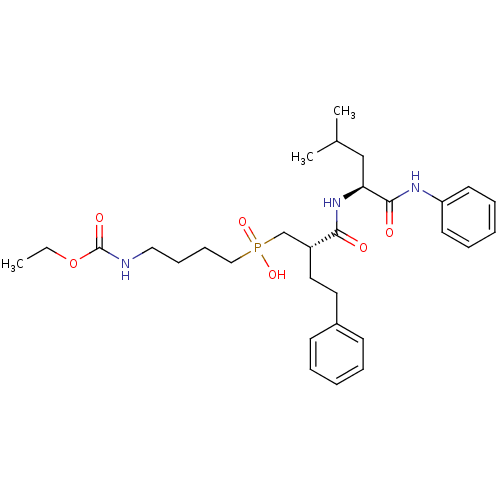
((4-Ethoxycarbonylamino-butyl)-[(S)-2-((S)-3-methyl...)Show SMILES CCOC(=O)NCCCCP(O)(=O)C[C@@H](CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C30H44N3O6P/c1-4-39-30(36)31-19-11-12-20-40(37,38)22-25(18-17-24-13-7-5-8-14-24)28(34)33-27(21-23(2)3)29(35)32-26-15-9-6-10-16-26/h5-10,13-16,23,25,27H,4,11-12,17-22H2,1-3H3,(H,31,36)(H,32,35)(H,33,34)(H,37,38)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288668
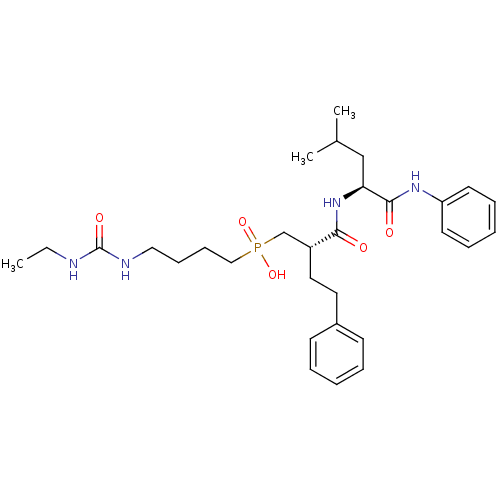
(CHEMBL109829 | [4-(3-Ethyl-ureido)-butyl]-[(S)-2-(...)Show SMILES CCNC(=O)NCCCCP(O)(=O)C[C@@H](CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C30H45N4O5P/c1-4-31-30(37)32-19-11-12-20-40(38,39)22-25(18-17-24-13-7-5-8-14-24)28(35)34-27(21-23(2)3)29(36)33-26-15-9-6-10-16-26/h5-10,13-16,23,25,27H,4,11-12,17-22H2,1-3H3,(H,33,36)(H,34,35)(H,38,39)(H2,31,32,37)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288668

(CHEMBL109829 | [4-(3-Ethyl-ureido)-butyl]-[(S)-2-(...)Show SMILES CCNC(=O)NCCCCP(O)(=O)C[C@@H](CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C30H45N4O5P/c1-4-31-30(37)32-19-11-12-20-40(38,39)22-25(18-17-24-13-7-5-8-14-24)28(35)34-27(21-23(2)3)29(36)33-26-15-9-6-10-16-26/h5-10,13-16,23,25,27H,4,11-12,17-22H2,1-3H3,(H,33,36)(H,34,35)(H,38,39)(H2,31,32,37)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288674

(CHEMBL420674 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288682
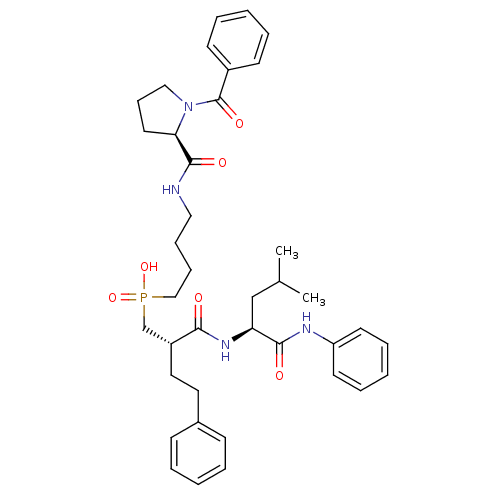
(CHEMBL110991 | {4-[((R)-1-Benzoyl-pyrrolidine-2-ca...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@H]1CCCN1C(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C39H51N4O6P/c1-29(2)27-34(37(45)41-33-19-10-5-11-20-33)42-36(44)32(23-22-30-15-6-3-7-16-30)28-50(48,49)26-13-12-24-40-38(46)35-21-14-25-43(35)39(47)31-17-8-4-9-18-31/h3-11,15-20,29,32,34-35H,12-14,21-28H2,1-2H3,(H,40,46)(H,41,45)(H,42,44)(H,48,49)/t32-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288681

((4-Benzoylamino-butyl)-[(S)-2-((S)-3-methyl-1-phen...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H44N3O5P/c1-26(2)24-31(34(40)36-30-18-10-5-11-19-30)37-33(39)29(21-20-27-14-6-3-7-15-27)25-43(41,42)23-13-12-22-35-32(38)28-16-8-4-9-17-28/h3-11,14-19,26,29,31H,12-13,20-25H2,1-2H3,(H,35,38)(H,36,40)(H,37,39)(H,41,42)/t29-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288675
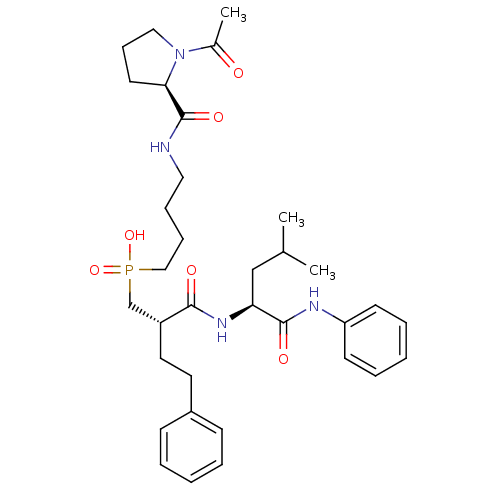
(CHEMBL113234 | {4-[((R)-1-Acetyl-pyrrolidine-2-car...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50187039

(1-(3,4-dichlorobenzyl)-5-hydroxy-1H-indole-2-carbo...)Show InChI InChI=1S/C16H11Cl2NO3/c17-12-3-1-9(5-13(12)18)8-19-14-4-2-11(20)6-10(14)7-15(19)16(21)22/h1-7,20H,8H2,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 3735-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.045
BindingDB Entry DOI: 10.7270/Q2N01645 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288679

(CHEMBL325835 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)Cc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H46N3O5P/c1-27(2)24-32(35(41)37-31-18-10-5-11-19-31)38-34(40)30(21-20-28-14-6-3-7-15-28)26-44(42,43)23-13-12-22-36-33(39)25-29-16-8-4-9-17-29/h3-11,14-19,27,30,32H,12-13,20-26H2,1-2H3,(H,36,39)(H,37,41)(H,38,40)(H,42,43)/t30-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288684
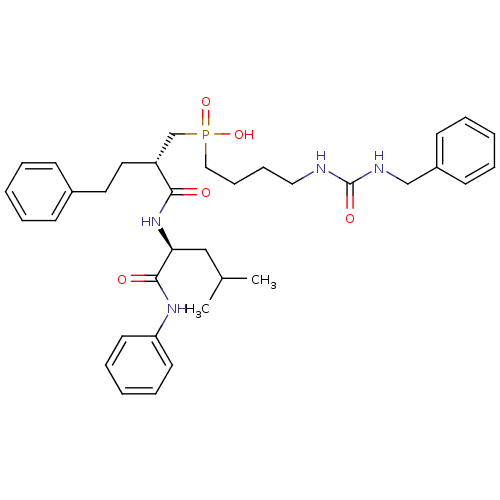
(CHEMBL324004 | [4-(3-Benzyl-ureido)-butyl]-[(S)-2-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)NCc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H47N4O5P/c1-27(2)24-32(34(41)38-31-18-10-5-11-19-31)39-33(40)30(21-20-28-14-6-3-7-15-28)26-45(43,44)23-13-12-22-36-35(42)37-25-29-16-8-4-9-17-29/h3-11,14-19,27,30,32H,12-13,20-26H2,1-2H3,(H,38,41)(H,39,40)(H,43,44)(H2,36,37,42)/t30-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50091438

((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...)Show SMILES Oc1ccc2[nH]cc(C3CCN(CCCCCNC(=O)\C=C\c4ccc(Cl)c(Cl)c4)CC3)c2c1 Show InChI InChI=1S/C27H31Cl2N3O2/c28-24-7-4-19(16-25(24)29)5-9-27(34)30-12-2-1-3-13-32-14-10-20(11-15-32)23-18-31-26-8-6-21(33)17-22(23)26/h4-9,16-18,20,31,33H,1-3,10-15H2,(H,30,34)/b9-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 3735-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.045
BindingDB Entry DOI: 10.7270/Q2N01645 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288693
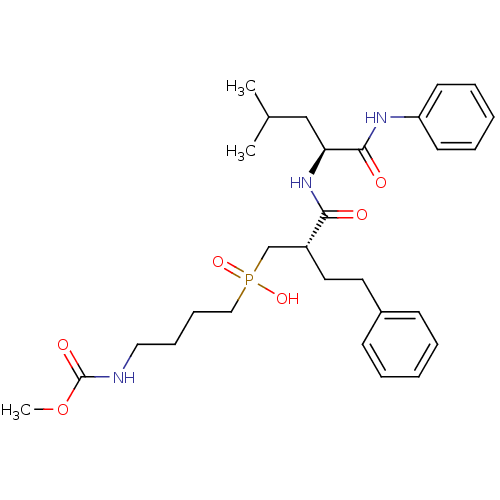
((4-Methoxycarbonylamino-butyl)-[(S)-2-((S)-3-methy...)Show SMILES COC(=O)NCCCCP(O)(=O)C[C@@H](CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C29H42N3O6P/c1-22(2)20-26(28(34)31-25-14-8-5-9-15-25)32-27(33)24(17-16-23-12-6-4-7-13-23)21-39(36,37)19-11-10-18-30-29(35)38-3/h4-9,12-15,22,24,26H,10-11,16-21H2,1-3H3,(H,30,35)(H,31,34)(H,32,33)(H,36,37)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288694
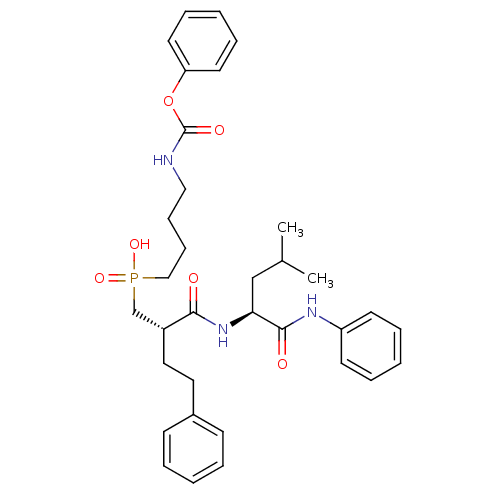
(CHEMBL322142 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)Oc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H44N3O6P/c1-26(2)24-31(33(39)36-29-16-8-4-9-17-29)37-32(38)28(21-20-27-14-6-3-7-15-27)25-44(41,42)23-13-12-22-35-34(40)43-30-18-10-5-11-19-30/h3-11,14-19,26,28,31H,12-13,20-25H2,1-2H3,(H,35,40)(H,36,39)(H,37,38)(H,41,42)/t28-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288687

(CHEMBL111975 | {4-[((S)-1-Acetyl-pyrrolidine-2-car...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288695
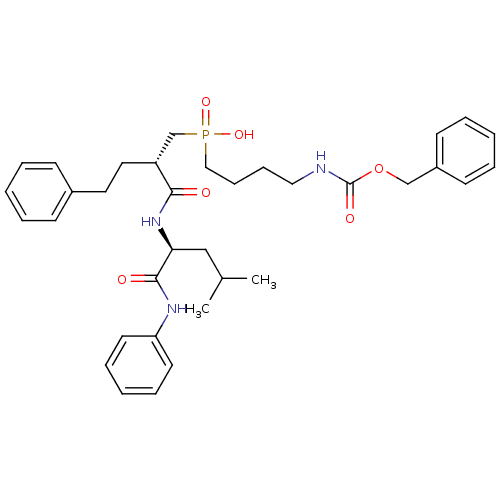
((4-Benzyloxycarbonylamino-butyl)-[(S)-2-((S)-3-met...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)OCc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H46N3O6P/c1-27(2)24-32(34(40)37-31-18-10-5-11-19-31)38-33(39)30(21-20-28-14-6-3-7-15-28)26-45(42,43)23-13-12-22-36-35(41)44-25-29-16-8-4-9-17-29/h3-11,14-19,27,30,32H,12-13,20-26H2,1-2H3,(H,36,41)(H,37,40)(H,38,39)(H,42,43)/t30-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288685
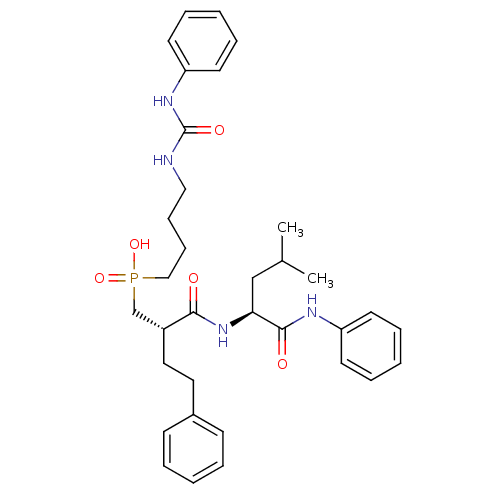
(CHEMBL110253 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H45N4O5P/c1-26(2)24-31(33(40)36-29-16-8-4-9-17-29)38-32(39)28(21-20-27-14-6-3-7-15-27)25-44(42,43)23-13-12-22-35-34(41)37-30-18-10-5-11-19-30/h3-11,14-19,26,28,31H,12-13,20-25H2,1-2H3,(H,36,40)(H,38,39)(H,42,43)(H2,35,37,41)/t28-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288679

(CHEMBL325835 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)Cc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H46N3O5P/c1-27(2)24-32(35(41)37-31-18-10-5-11-19-31)38-34(40)30(21-20-28-14-6-3-7-15-28)26-44(42,43)23-13-12-22-36-33(39)25-29-16-8-4-9-17-29/h3-11,14-19,27,30,32H,12-13,20-26H2,1-2H3,(H,36,39)(H,37,41)(H,38,40)(H,42,43)/t30-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288690

(CHEMBL109670 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CNC(=O)NCCCCP(O)(=O)C[C@@H](CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C29H43N4O5P/c1-22(2)20-26(28(35)32-25-14-8-5-9-15-25)33-27(34)24(17-16-23-12-6-4-7-13-23)21-39(37,38)19-11-10-18-31-29(36)30-3/h4-9,12-15,22,24,26H,10-11,16-21H2,1-3H3,(H,32,35)(H,33,34)(H,37,38)(H2,30,31,36)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288669
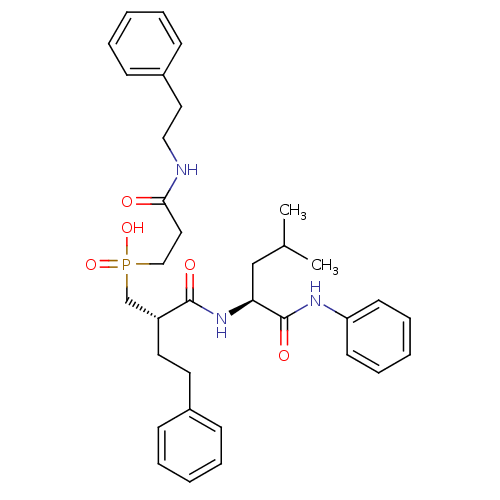
(CHEMBL109671 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCC(=O)NCCc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H44N3O5P/c1-26(2)24-31(34(40)36-30-16-10-5-11-17-30)37-33(39)29(19-18-27-12-6-3-7-13-27)25-43(41,42)23-21-32(38)35-22-20-28-14-8-4-9-15-28/h3-17,26,29,31H,18-25H2,1-2H3,(H,35,38)(H,36,40)(H,37,39)(H,41,42)/t29-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141931

((R)-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl-2...)Show SMILES CCn1nc(Cc2ccc(cc2)-c2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C2CCCCC2)C(O)=O)CC1 Show InChI InChI=1S/C42H51FN4O2/c1-2-47-40(26-38(44-47)24-30-16-18-32(19-17-30)31-10-5-3-6-11-31)33-20-22-45(23-21-33)27-36-28-46(29-39(36)35-14-9-15-37(43)25-35)41(42(48)49)34-12-7-4-8-13-34/h3,5-6,9-11,14-19,25-26,33-34,36,39,41H,2,4,7-8,12-13,20-24,27-29H2,1H3,(H,48,49)/t36-,39+,41+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288698
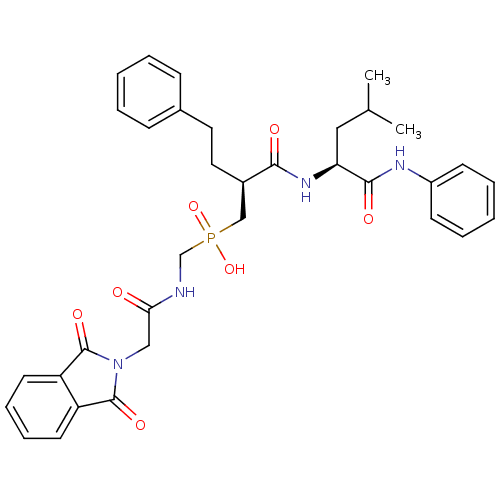
(CHEMBL112724 | {[2-(1,3-Dioxo-1,3-dihydro-isoindol...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CNC(=O)CN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H39N4O7P/c1-23(2)19-29(32(41)36-26-13-7-4-8-14-26)37-31(40)25(18-17-24-11-5-3-6-12-24)21-46(44,45)22-35-30(39)20-38-33(42)27-15-9-10-16-28(27)34(38)43/h3-16,23,25,29H,17-22H2,1-2H3,(H,35,39)(H,36,41)(H,37,40)(H,44,45)/t25-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288691

(CHEMBL111601 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1ccccc1CC[C@H](CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)23-31(34(41)37-28-14-5-4-6-15-28)38-33(40)27(20-19-26-13-7-10-18-32(26)46-3)24-47(44,45)22-12-11-21-39-35(42)29-16-8-9-17-30(29)36(39)43/h4-10,13-18,25,27,31H,11-12,19-24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288696
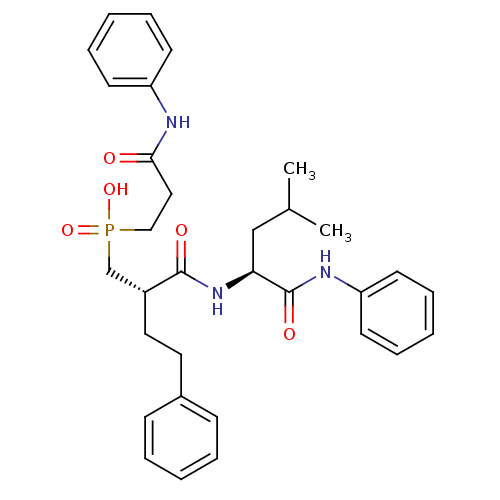
(CHEMBL110075 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCC(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C32H40N3O5P/c1-24(2)22-29(32(38)34-28-16-10-5-11-17-28)35-31(37)26(19-18-25-12-6-3-7-13-25)23-41(39,40)21-20-30(36)33-27-14-8-4-9-15-27/h3-17,24,26,29H,18-23H2,1-2H3,(H,33,36)(H,34,38)(H,35,37)(H,39,40)/t26-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288676

(3-{Hydroxy-[(S)-2-((S)-3-methyl-1-phenylcarbamoyl-...)Show SMILES COC(=O)CCP(O)(=O)C[C@@H](CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C27H37N2O6P/c1-20(2)18-24(27(32)28-23-12-8-5-9-13-23)29-26(31)22(15-14-21-10-6-4-7-11-21)19-36(33,34)17-16-25(30)35-3/h4-13,20,22,24H,14-19H2,1-3H3,(H,28,32)(H,29,31)(H,33,34)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288697
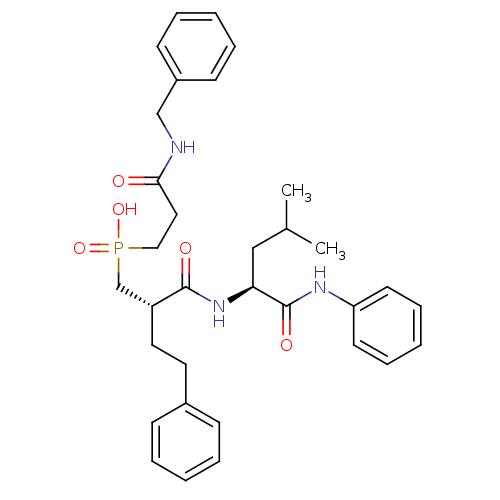
((2-Benzylcarbamoyl-ethyl)-[(S)-2-((S)-3-methyl-1-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCC(=O)NCc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C33H42N3O5P/c1-25(2)22-30(33(39)35-29-16-10-5-11-17-29)36-32(38)28(19-18-26-12-6-3-7-13-26)24-42(40,41)21-20-31(37)34-23-27-14-8-4-9-15-27/h3-17,25,28,30H,18-24H2,1-2H3,(H,34,37)(H,35,39)(H,36,38)(H,40,41)/t28-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3) |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288680
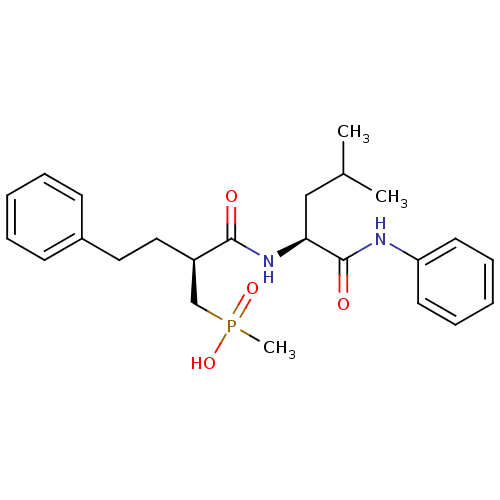
(CHEMBL326018 | Methyl-[(S)-2-((S)-3-methyl-1-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(C)(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C24H33N2O4P/c1-18(2)16-22(24(28)25-21-12-8-5-9-13-21)26-23(27)20(17-31(3,29)30)15-14-19-10-6-4-7-11-19/h4-13,18,20,22H,14-17H2,1-3H3,(H,25,28)(H,26,27)(H,29,30)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288671
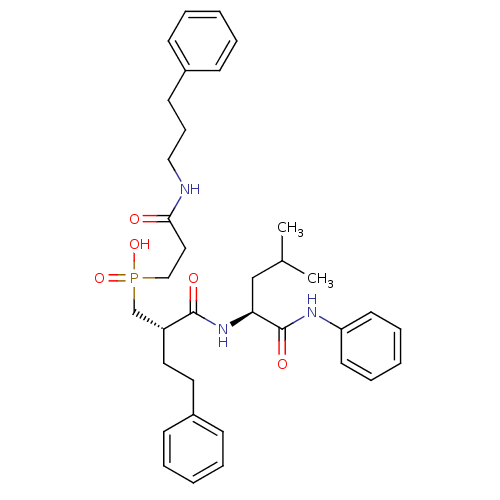
(CHEMBL110997 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCC(=O)NCCCc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H46N3O5P/c1-27(2)25-32(35(41)37-31-18-10-5-11-19-31)38-34(40)30(21-20-29-15-8-4-9-16-29)26-44(42,43)24-22-33(39)36-23-12-17-28-13-6-3-7-14-28/h3-11,13-16,18-19,27,30,32H,12,17,20-26H2,1-2H3,(H,36,39)(H,37,41)(H,38,40)(H,42,43)/t30-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50332461

((R)-3-carba cyclic-phosphatidic acid | CHEMBL16300...)Show SMILES CCCCCCCCCCCCCCCCCC(=O)OC[C@H]1CCP([O-])(=O)O1 |r| Show InChI InChI=1S/C22H43O5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-22(23)26-20-21-18-19-28(24,25)27-21/h21H,2-20H2,1H3,(H,24,25)/p-1/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ATX mediated hydrolysis of FS-3 |
Bioorg Med Chem Lett 20: 7525-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.115
BindingDB Entry DOI: 10.7270/Q2XD11X1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141911

((R)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethyl-2H...)Show SMILES CCn1nc(Cc2ccc(cc2)C#N)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C35H44FN5O2/c1-5-41-32(19-30(38-41)17-24-9-11-25(20-37)12-10-24)26-13-15-39(16-14-26)21-28-22-40(33(34(42)43)35(2,3)4)23-31(28)27-7-6-8-29(36)18-27/h6-12,18-19,26,28,31,33H,5,13-17,21-23H2,1-4H3,(H,42,43)/t28-,31+,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288676

(3-{Hydroxy-[(S)-2-((S)-3-methyl-1-phenylcarbamoyl-...)Show SMILES COC(=O)CCP(O)(=O)C[C@@H](CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C27H37N2O6P/c1-20(2)18-24(27(32)28-23-12-8-5-9-13-23)29-26(31)22(15-14-21-10-6-4-7-11-21)19-36(33,34)17-16-25(30)35-3/h4-13,20,22,24H,14-19H2,1-3H3,(H,28,32)(H,29,31)(H,33,34)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288680

(CHEMBL326018 | Methyl-[(S)-2-((S)-3-methyl-1-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(C)(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C24H33N2O4P/c1-18(2)16-22(24(28)25-21-12-8-5-9-13-21)26-23(27)20(17-31(3,29)30)15-14-19-10-6-4-7-11-19/h4-13,18,20,22H,14-17H2,1-3H3,(H,25,28)(H,26,27)(H,29,30)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50332460
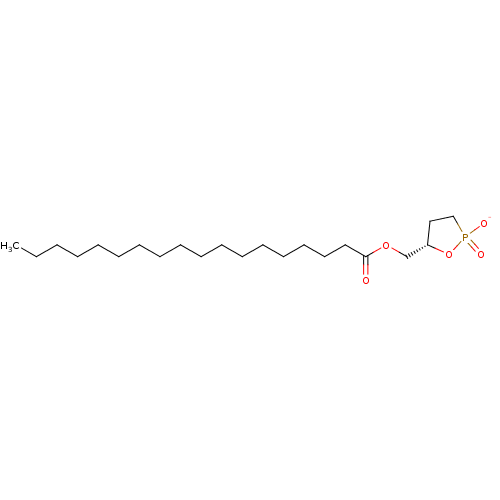
((S)-carba cyclic-phosphatidic acid | CHEMBL1630084)Show SMILES CCCCCCCCCCCCCCCCCC(=O)OC[C@@H]1CCP([O-])(=O)O1 |r| Show InChI InChI=1S/C22H43O5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-22(23)26-20-21-18-19-28(24,25)27-21/h21H,2-20H2,1H3,(H,24,25)/p-1/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ATX mediated hydrolysis of FS-3 |
Bioorg Med Chem Lett 20: 7525-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.115
BindingDB Entry DOI: 10.7270/Q2XD11X1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data