

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27048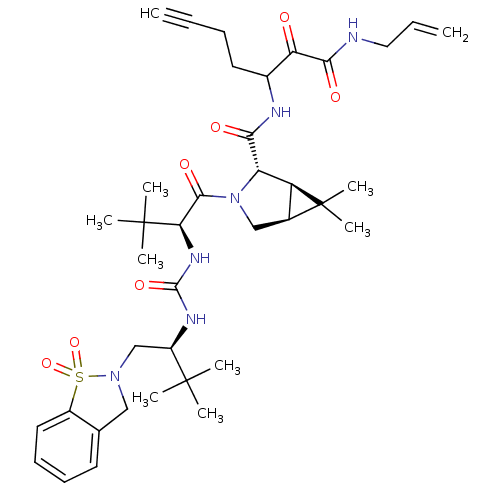 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(1,1-dioxo-2,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | -52.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27043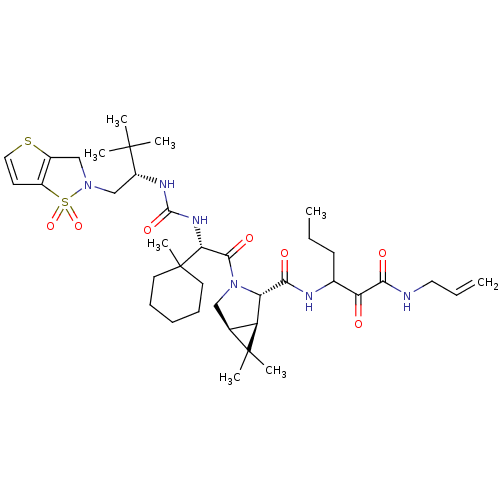 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(1,1-dioxo-2H,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27039 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(1,1-dioxo-2,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27049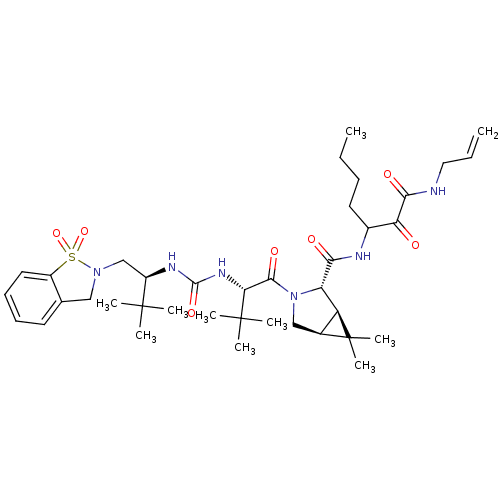 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(1,1-dioxo-2,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27045 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(1,1-dioxo-2,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27033 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(1,1-dioxo-1,2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | -47.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27047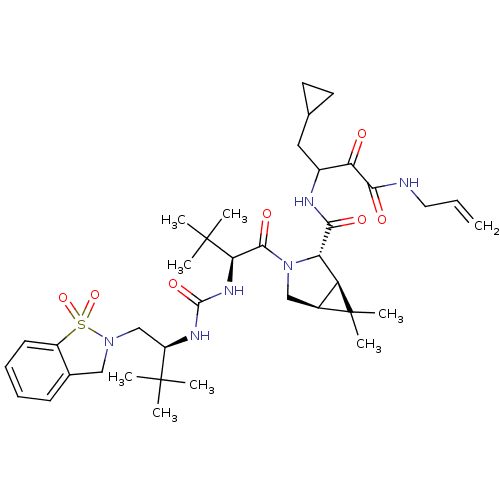 (4-cyclopropyl-3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -47.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27044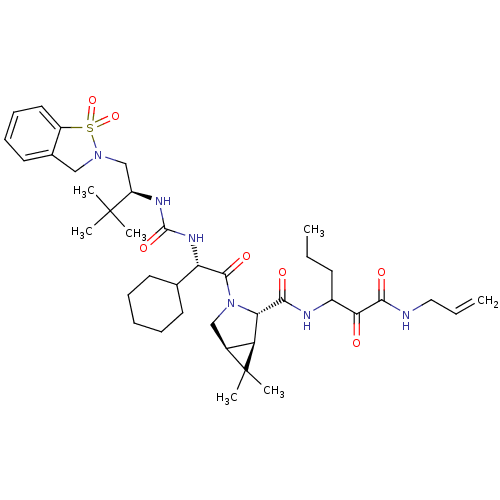 (3-{[(1R,2S,5S)-3-[(2S)-2-cyclohexyl-2-({[(2S)-1-(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -47.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27037 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(1,1-dioxo-2H,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | -46.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27032 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(1,1-dioxo-1,2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | -46.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27042 (3-{[(1R,2S,5S)-3-[(2S)-2-cyclohexyl-2-({[(2S)-1-(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | -45.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27036 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-3,3-dimethyl-1-(6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | -45.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27041 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-3,3-dimethyl-1-(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -45.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27040 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(1S)-1-cyclohexyl-2-(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | -44.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27034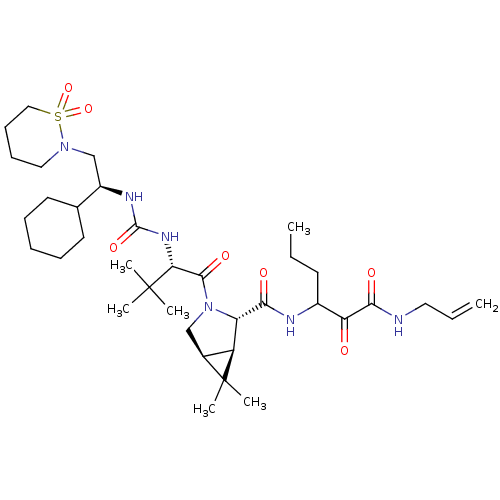 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(1S)-1-cyclohexyl-2-(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | -43.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27046 (4-cyclobutyl-3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | -43.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27038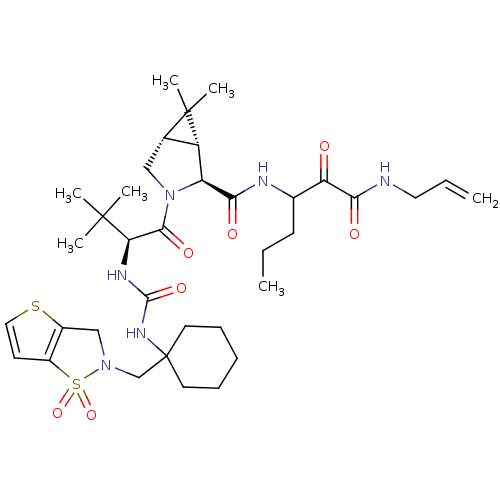 (3-{[(1R,2S,5S)-3-[(2S)-2-[({1-[(1,1-dioxo-2H,3H-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | -42.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27035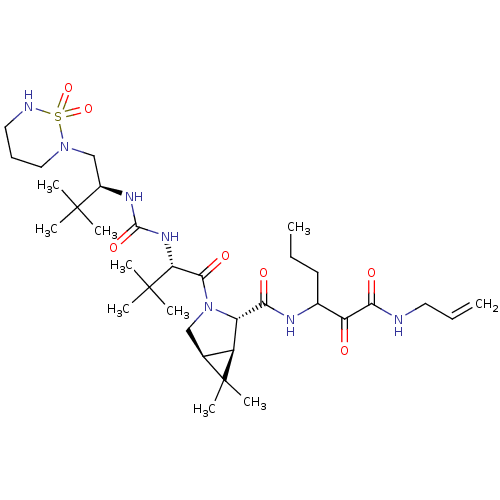 (3-{[(1R,2S,5S)-3-[(2S)-2-({[(2S)-1-(1,1-dioxo-1,2,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 59 | -42.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | Bioorg Med Chem Lett 19: 1105-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.111 BindingDB Entry DOI: 10.7270/Q22B8WBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227653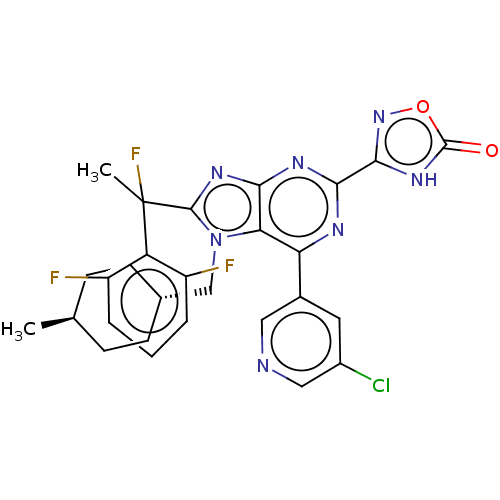 (3-{6-(5-chloropyridin-3- yl)-8-[1-(2,6- difluoroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.156 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227651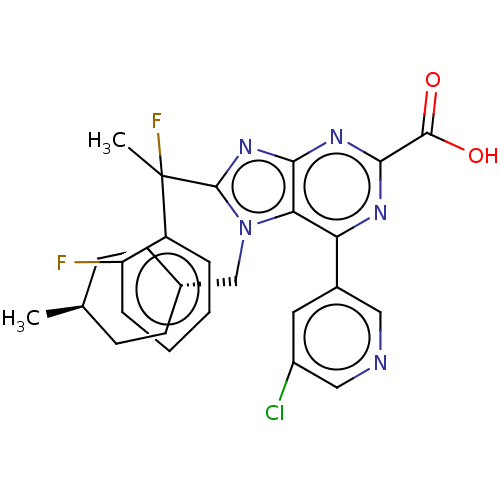 (6-(5-chloropyridin-3-yl)- 8-[1-fluoro-1-(2- fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.176 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM223046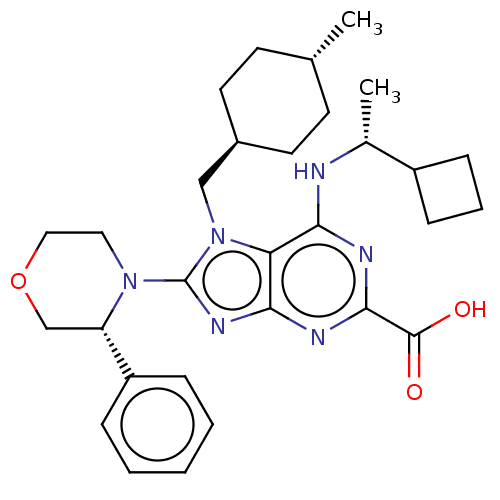 (6-{[(1r)-1- cyclobutylethyl]amino}- 7-[(trans-4- m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.202 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224125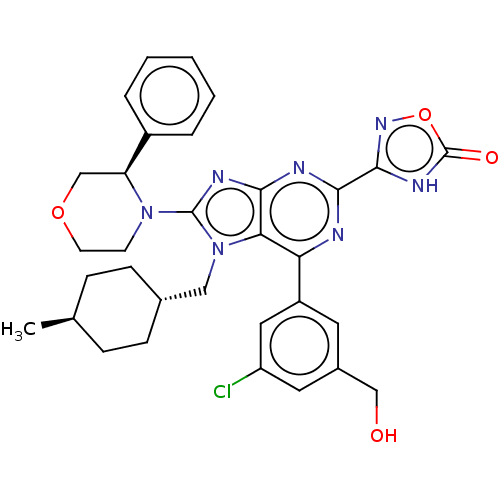 (3-{6-[3-chloro-5- (hydroxymethyl)phen- yl]-7-[(tra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.205 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224261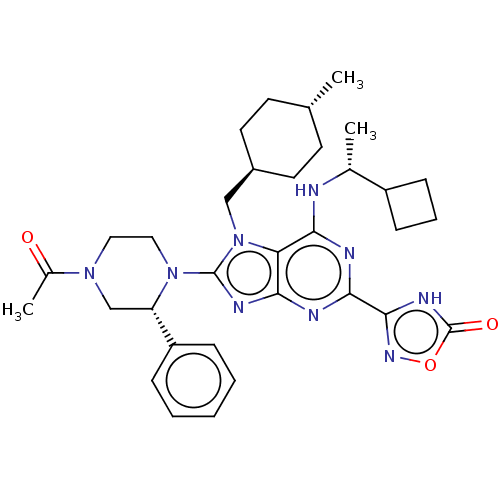 (3-{8-[(2r)-4-acetyl- 2-phenylpiperazin-1- yl]-6-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.215 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227665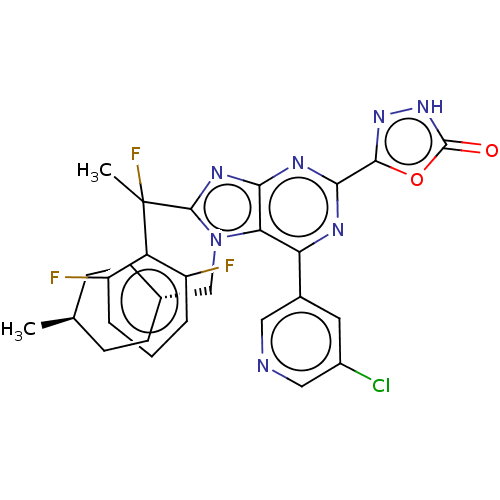 (5-{6-(5-chloropyridin-3- yl)-8-[1-(2,6- difluoroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.289 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227683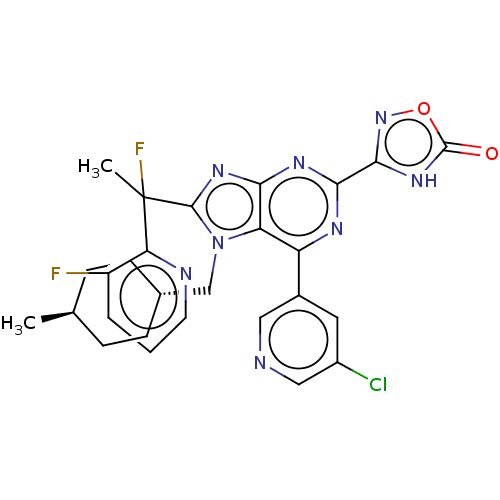 (3-{6-(5-chloropyridin-3- yl)-8-[1-fluoro-1-(3- flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.315 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM223705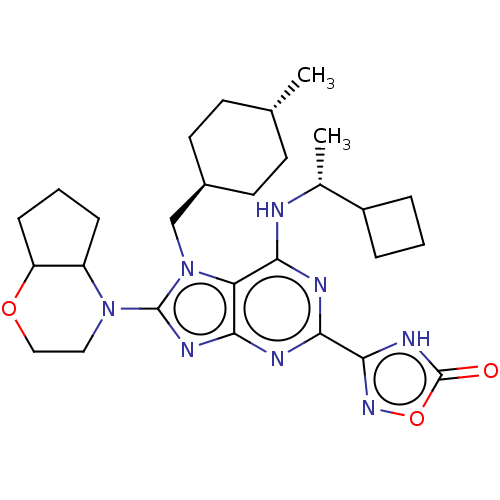 (3-(6-{[(1r)-1- cyclobutylethyl]amino}- 8- (hexahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.344 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227669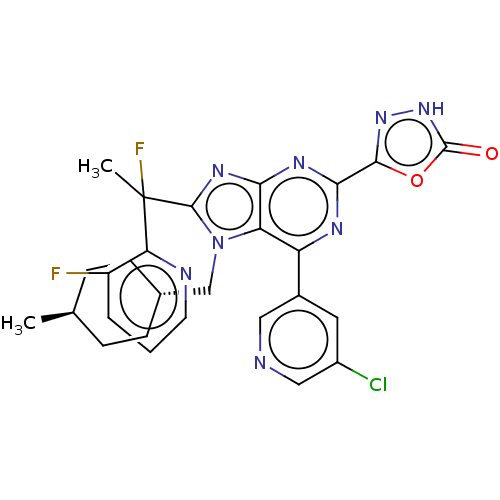 (5-{6-(5-chloropyridin-3- yl)-8-[1-fluoro-1-(3- flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.359 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM223402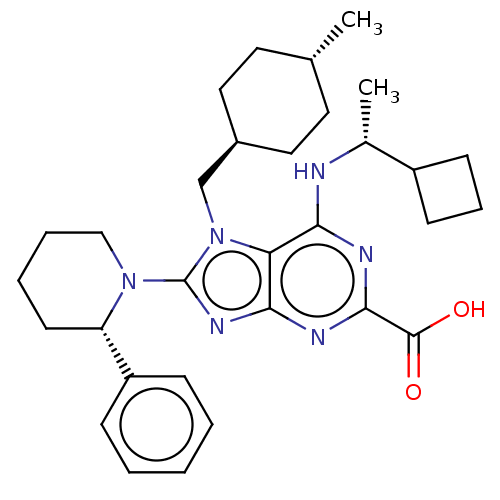 (6-{[(1r)-1- cyclobutylethyl]amino}- 7-[(trans-4- m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.381 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM226428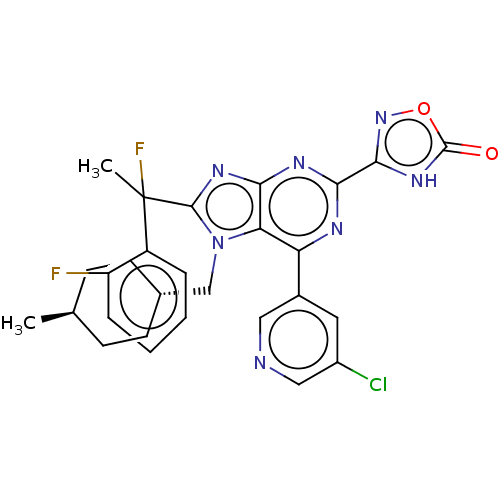 (3-{6-(5-chloropyridin-3- yl)-8-[1-fluoro-1-(2- flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.424 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227696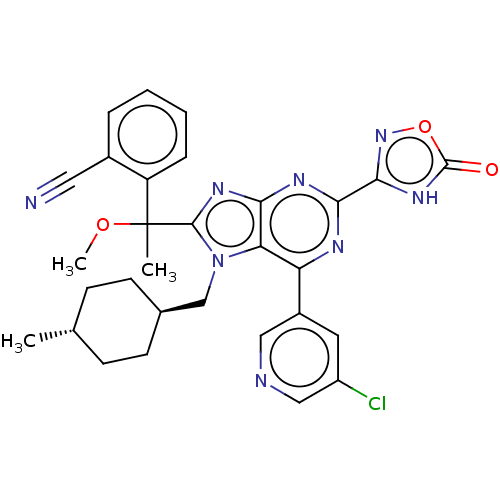 (2-{1-[6-(5-chloropyridin- 3-yl)-7-[(trans-4- methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.435 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227678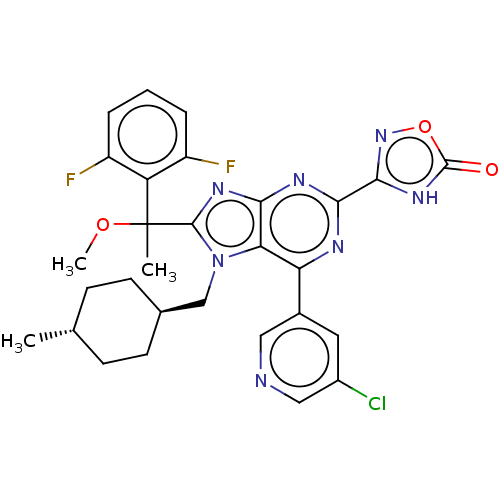 (3-{6-(5-chloropyridin-3- yl)-8-[1-(2,6- difluoroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.452 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM226426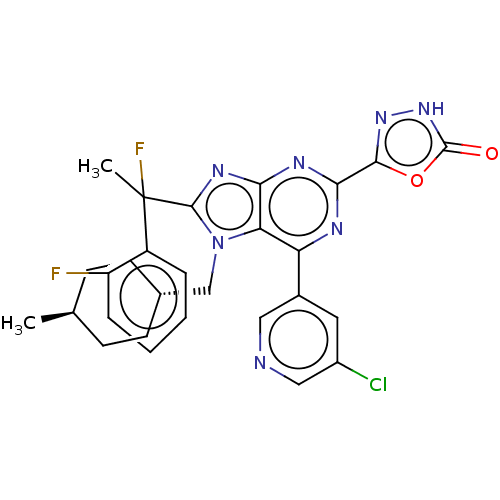 (5-{6-(5-chloropyridin-3- yl)-8-[1-fluoro-1-(2- flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.453 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224176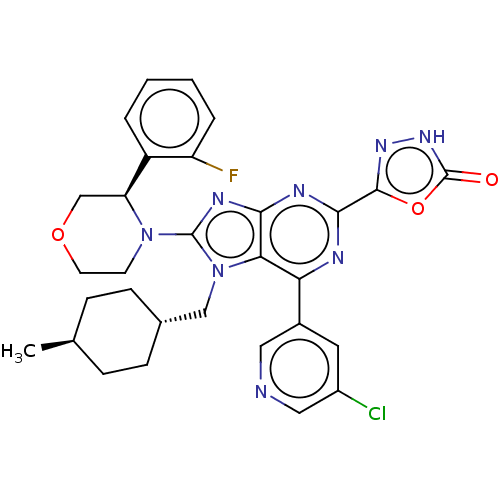 (5-{6-(5- chloropyridin-3-yl)- 8-[(3r)-3-(2- fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.494 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224216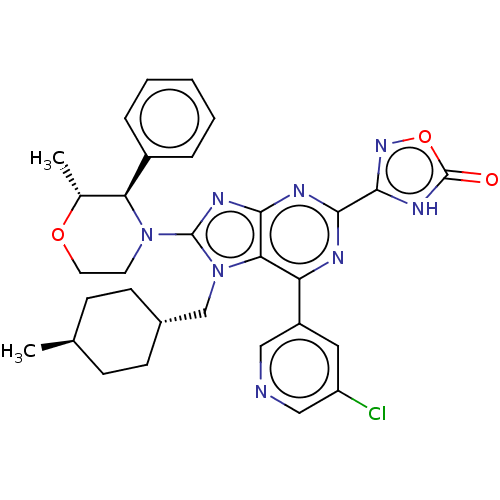 (3-{6-(5- chloropyridin-3-yl)- 7-[(trans-4- methylc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.504 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM223681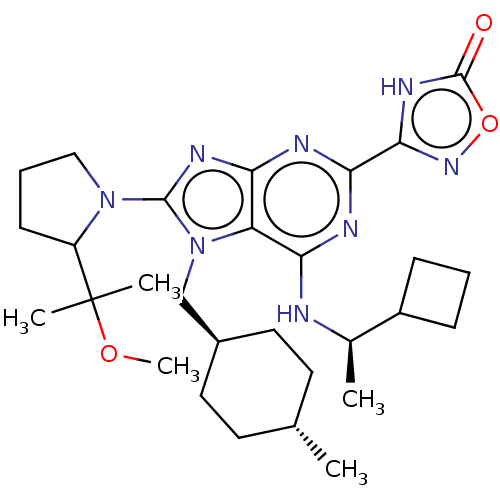 (3-(6-{[(1r)-1- cyclobutylethyl]amino}- 8-[2-(1-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.507 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227695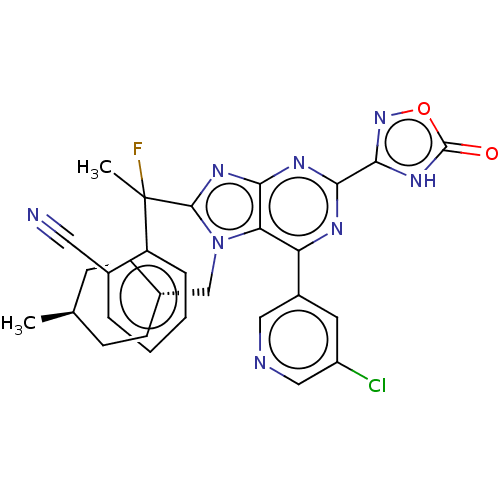 (2-{1-[6-(5-chloropyridin- 3-yl)-7-[(trans-4- methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.515 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224107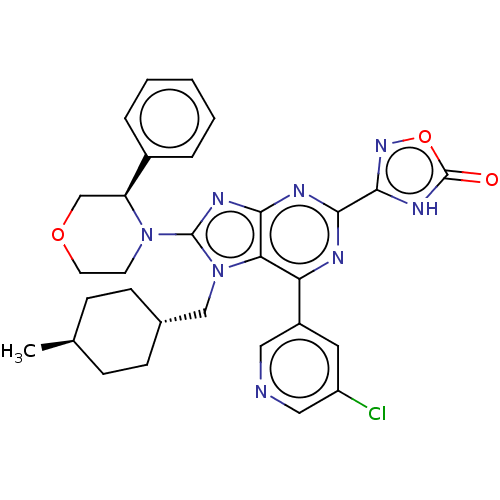 (3-{6-(5- chloropyridin-3-yl)- 7-[(trans-4- methylc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.517 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM221499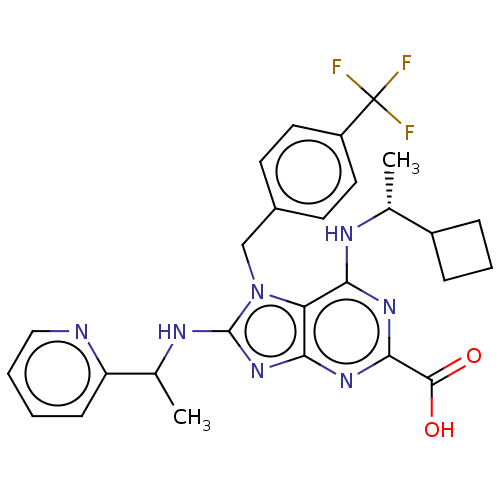 (6-{[(1r)-1- cyclobutylethyl]amino}- 8-[(1-pyridin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.526 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224295 (6-{[(1r)-1-cyclobutylethyl]amino}- 7-[(trans-4-eth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.548 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224851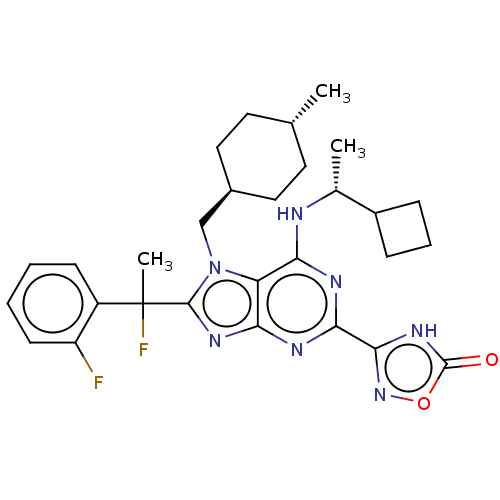 (3-(6-{[(1r)-1- cyclobutylethyl]amino}-8-[1- fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.563 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM223674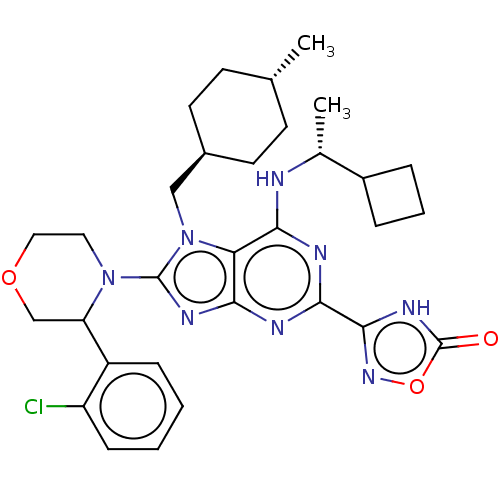 (3-{8-[3-(2- chlorophenyl)morph- olin-4-yl]-6-{[(1r...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.568 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224214 (5-{6-(5- chloropyridin-3-yl)- 7-[(trans-4- methylc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.569 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224173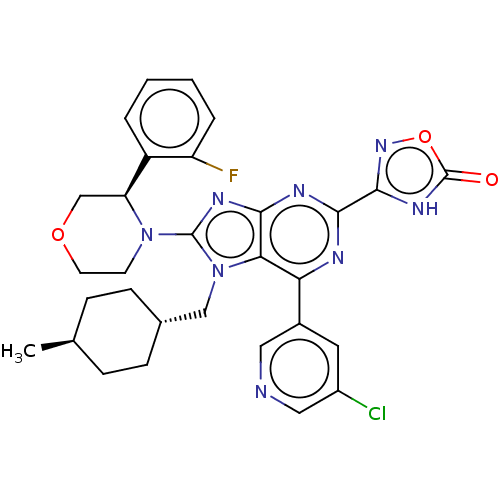 (3-{6-(5- chloropyridin-3-yl)- 8-[(3r)-3-(2- fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.585 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM223756 (6-{[(1r)-1- cyclobutylethyl]amino}- 7-[(trans-4- m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.585 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224155 (3-{6-(5- chloropyridin-3-yl)- 7-[(trans-4- methylc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.585 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227673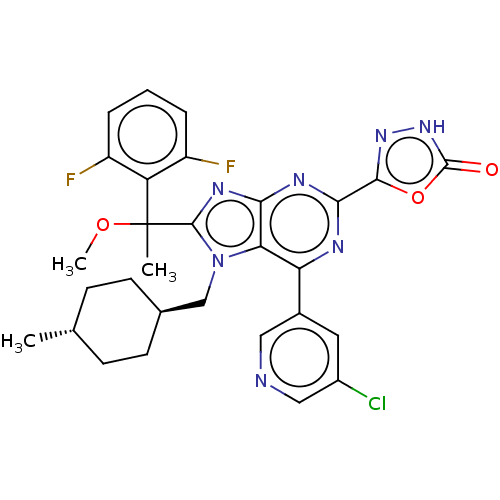 (5-{6-(5-chloropyridin-3- yl)-8-[1-(2,6- difluoroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.598 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224239 (3-{6-(5-chloro-2- methylpyridin-3-yl)- 7-[(trans-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.601 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224288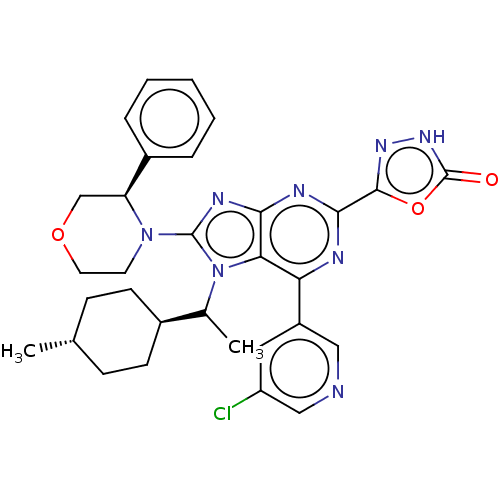 (5-(6-(5-chloropyridin-3-yl)-7-(1- ((trans)-4-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227658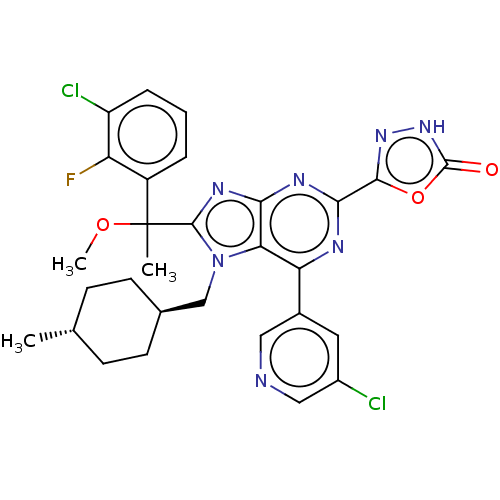 (5-{8-[1-(3-chloro-2- fluorophenyl)-1- methoxyethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.623 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM223200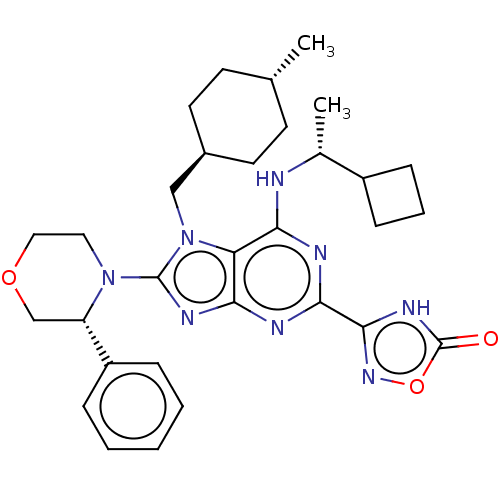 (3-(6-{[(1r)-1- cyclobutylethyl]amino}- 7-[(trans-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.626 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1349 total ) | Next | Last >> |