 Found 5868 hits with Last Name = 'ney' and Initial = 'p'
Found 5868 hits with Last Name = 'ney' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50031895
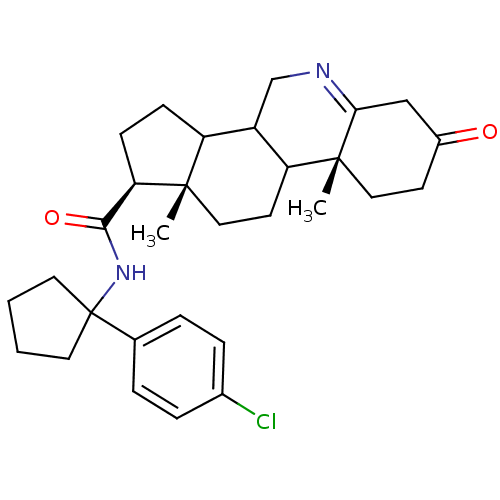
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC1(CCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C30H39ClN2O2/c1-28-16-12-24-22(18-32-26-17-21(34)11-15-29(24,26)2)23(28)9-10-25(28)27(35)33-30(13-3-4-14-30)19-5-7-20(31)8-6-19/h5-8,22-25H,3-4,9-18H2,1-2H3,(H,33,35)/t22?,23?,24?,25-,28+,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity (in vitro) |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318983
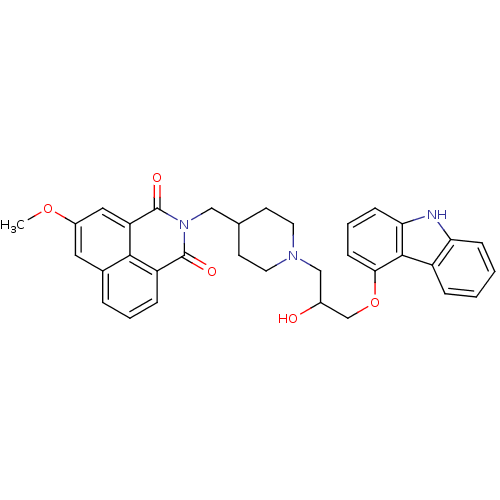
(2-((1-(3-(9H-carbazol-4-yloxy)-2-hydroxypropyl)pip...)Show SMILES COc1cc2C(=O)N(CC3CCN(CC(O)COc4cccc5[nH]c6ccccc6c45)CC3)C(=O)c3cccc(c1)c23 Show InChI InChI=1S/C34H33N3O5/c1-41-24-16-22-6-4-8-26-31(22)27(17-24)34(40)37(33(26)39)18-21-12-14-36(15-13-21)19-23(38)20-42-30-11-5-10-29-32(30)25-7-2-3-9-28(25)35-29/h2-11,16-17,21,23,35,38H,12-15,18-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human adrenergic beta-1 receptor expressed in CHOK1 cells assessed as inhibition of cAMP accumulation by HTRF assay |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50031877
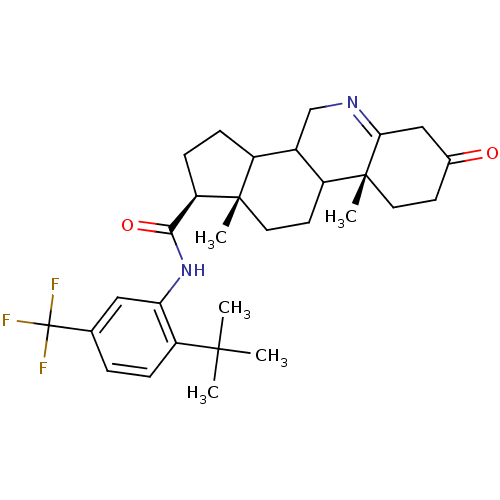
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C)C(F)(F)F |t:20| Show InChI InChI=1S/C30H39F3N2O2/c1-27(2,3)22-7-6-17(30(31,32)33)14-24(22)35-26(37)23-9-8-20-19-16-34-25-15-18(36)10-12-29(25,5)21(19)11-13-28(20,23)4/h6-7,14,19-21,23H,8-13,15-16H2,1-5H3,(H,35,37)/t19?,20?,21?,23-,28+,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318987
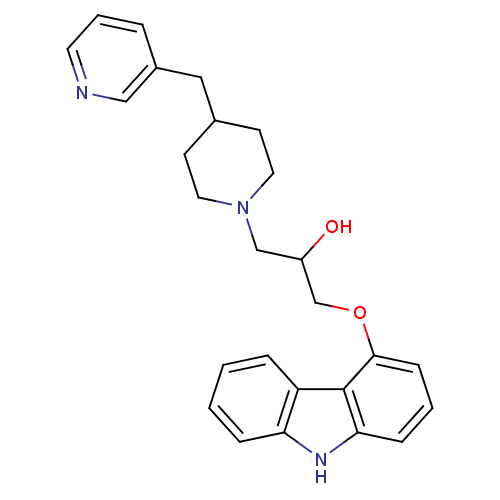
(1-(9H-carbazol-4-yloxy)-3-(4-(pyridin-3-ylmethyl)p...)Show SMILES OC(COc1cccc2[nH]c3ccccc3c12)CN1CCC(Cc2cccnc2)CC1 Show InChI InChI=1S/C26H29N3O2/c30-21(17-29-13-10-19(11-14-29)15-20-5-4-12-27-16-20)18-31-25-9-3-8-24-26(25)22-6-1-2-7-23(22)28-24/h1-9,12,16,19,21,28,30H,10-11,13-15,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Rattus norvegicus) | BDBM50031895

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC1(CCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C30H39ClN2O2/c1-28-16-12-24-22(18-32-26-17-21(34)11-15-29(24,26)2)23(28)9-10-25(28)27(35)33-30(13-3-4-14-30)19-5-7-20(31)8-6-19/h5-8,22-25H,3-4,9-18H2,1-2H3,(H,33,35)/t22?,23?,24?,25-,28+,29-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031874
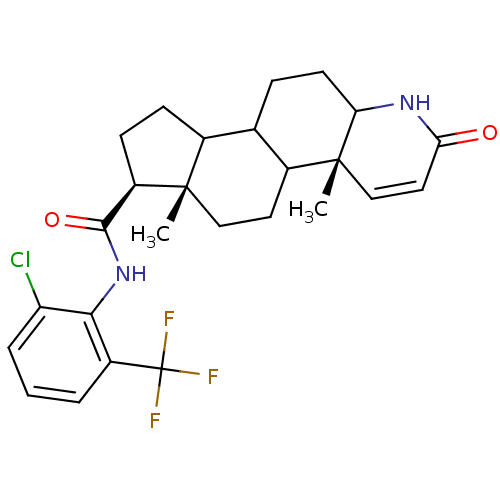
((4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CC[C@@H]2C(=O)Nc1c(Cl)cccc1C(F)(F)F |c:12| Show InChI InChI=1S/C26H30ClF3N2O2/c1-24-12-10-16-14(6-9-20-25(16,2)13-11-21(33)31-20)15(24)7-8-18(24)23(34)32-22-17(26(28,29)30)4-3-5-19(22)27/h3-5,11,13-16,18,20H,6-10,12H2,1-2H3,(H,31,33)(H,32,34)/t14?,15?,16?,18-,20?,24+,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Steroid 5-alpha-reductase type I was evaluated |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Rattus norvegicus) | BDBM50031877

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C)C(F)(F)F |t:20| Show InChI InChI=1S/C30H39F3N2O2/c1-27(2,3)22-7-6-17(30(31,32)33)14-24(22)35-26(37)23-9-8-20-19-16-34-25-15-18(36)10-12-29(25,5)21(19)11-13-28(20,23)4/h6-7,14,19-21,23H,8-13,15-16H2,1-5H3,(H,35,37)/t19?,20?,21?,23-,28+,29-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031883
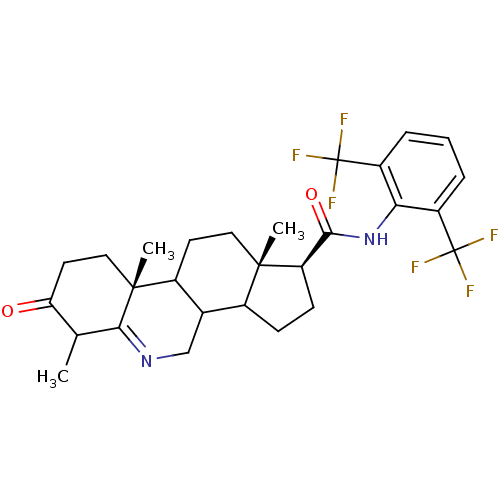
((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CC1C(=O)CC[C@]2(C)C3CC[C@@]4(C)C(CC[C@@H]4C(=O)Nc4c(cccc4C(F)(F)F)C(F)(F)F)C3CN=C12 |t:39| Show InChI InChI=1S/C28H32F6N2O2/c1-14-21(37)10-12-26(3)17-9-11-25(2)16(15(17)13-35-23(14)26)7-8-20(25)24(38)36-22-18(27(29,30)31)5-4-6-19(22)28(32,33)34/h4-6,14-17,20H,7-13H2,1-3H3,(H,36,38)/t14?,15?,16?,17?,20-,25+,26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318988
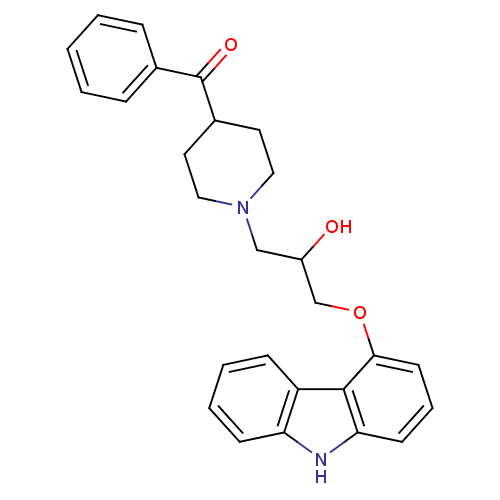
((1-(3-(9H-carbazol-4-yloxy)-2-hydroxypropyl)piperi...)Show SMILES OC(COc1cccc2[nH]c3ccccc3c12)CN1CCC(CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C27H28N2O3/c30-21(17-29-15-13-20(14-16-29)27(31)19-7-2-1-3-8-19)18-32-25-12-6-11-24-26(25)22-9-4-5-10-23(22)28-24/h1-12,20-21,28,30H,13-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50407405
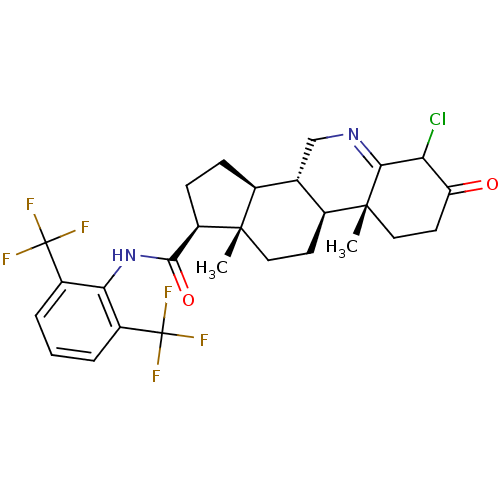
(CHEMBL2115222)Show SMILES C[C@]12CC[C@H]3[C@@H](CN=C4C(Cl)C(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)Nc1c(cccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H29ClF6N2O2/c1-24-10-8-15-13(12-35-22-20(28)19(37)9-11-25(15,22)2)14(24)6-7-18(24)23(38)36-21-16(26(29,30)31)4-3-5-17(21)27(32,33)34/h3-5,13-15,18,20H,6-12H2,1-2H3,(H,36,38)/t13-,14-,15-,18+,20?,24-,25+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318986
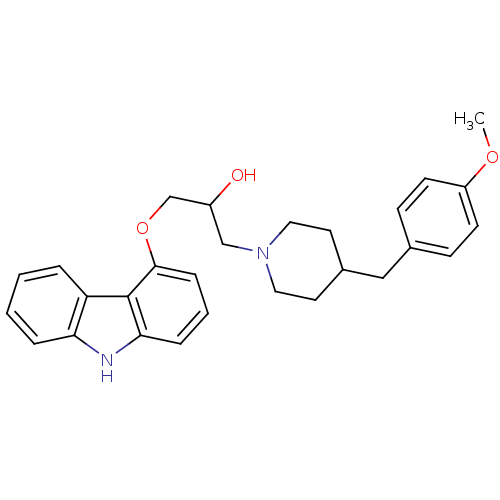
(1-(9H-carbazol-4-yloxy)-3-(4-(4-methoxybenzyl)pipe...)Show SMILES COc1ccc(CC2CCN(CC(O)COc3cccc4[nH]c5ccccc5c34)CC2)cc1 Show InChI InChI=1S/C28H32N2O3/c1-32-23-11-9-20(10-12-23)17-21-13-15-30(16-14-21)18-22(31)19-33-27-8-4-7-26-28(27)24-5-2-3-6-25(24)29-26/h2-12,21-22,29,31H,13-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50407405

(CHEMBL2115222)Show SMILES C[C@]12CC[C@H]3[C@@H](CN=C4C(Cl)C(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)Nc1c(cccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H29ClF6N2O2/c1-24-10-8-15-13(12-35-22-20(28)19(37)9-11-25(15,22)2)14(24)6-7-18(24)23(38)36-21-16(26(29,30)31)4-3-5-17(21)27(32,33)34/h3-5,13-15,18,20H,6-12H2,1-2H3,(H,36,38)/t13-,14-,15-,18+,20?,24-,25+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318989
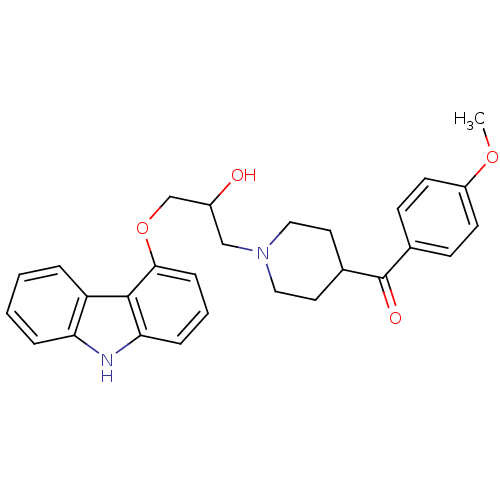
((1-(3-(9H-carbazol-4-yloxy)-2-hydroxypropyl)piperi...)Show SMILES COc1ccc(cc1)C(=O)C1CCN(CC(O)COc2cccc3[nH]c4ccccc4c23)CC1 Show InChI InChI=1S/C28H30N2O4/c1-33-22-11-9-19(10-12-22)28(32)20-13-15-30(16-14-20)17-21(31)18-34-26-8-4-7-25-27(26)23-5-2-3-6-24(23)29-25/h2-12,20-21,29,31H,13-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50031896
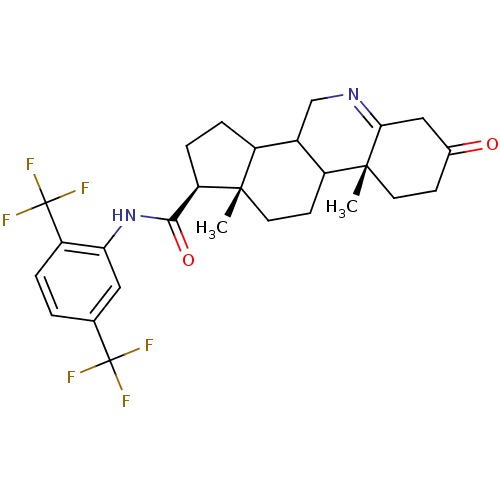
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)Nc1cc(ccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H30F6N2O2/c1-24-10-8-18-16(13-34-22-12-15(36)7-9-25(18,22)2)17(24)5-6-20(24)23(37)35-21-11-14(26(28,29)30)3-4-19(21)27(31,32)33/h3-4,11,16-18,20H,5-10,12-13H2,1-2H3,(H,35,37)/t16?,17?,18?,20-,24+,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35226
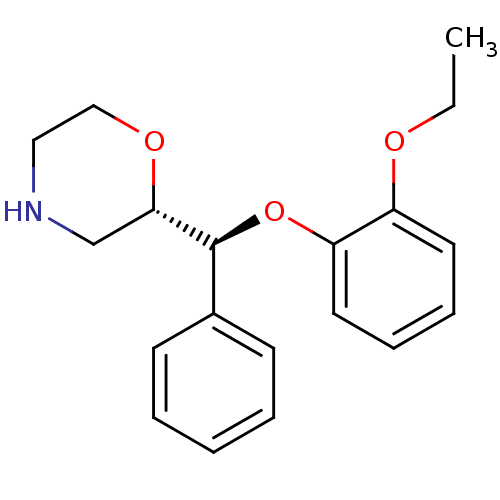
((S,S)-reboxetine | Reboxetine | Vestra)Show SMILES CCOc1ccccc1O[C@H]([C@@H]1CNCCO1)c1ccccc1 |r| Show InChI InChI=1S/C19H23NO3/c1-2-21-16-10-6-7-11-17(16)23-19(15-8-4-3-5-9-15)18-14-20-12-13-22-18/h3-11,18-20H,2,12-14H2,1H3/t18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research
| Assay Description
Compounds were evaluated the inhibition of [3H] nisoxetine binding to MDCK-Net6 cells, stably transfected with the human norepinephrine transporter (... |
Bioorg Med Chem 17: 7802-15 (2009)
Article DOI: 10.1016/j.bmc.2009.09.023
BindingDB Entry DOI: 10.7270/Q26Q1VK2 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318990
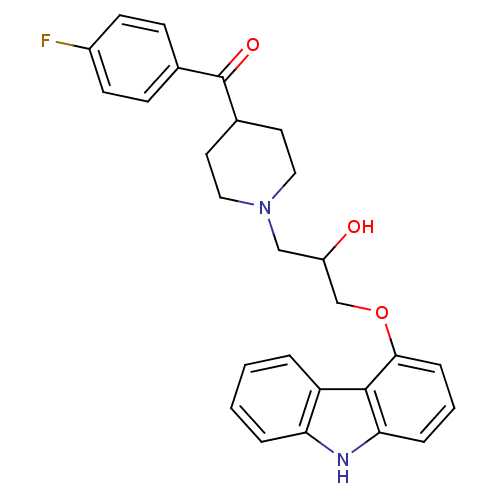
((1-(3-(9H-carbazol-4-yloxy)-2-hydroxypropyl)piperi...)Show SMILES OC(COc1cccc2[nH]c3ccccc3c12)CN1CCC(CC1)C(=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H27FN2O3/c28-20-10-8-18(9-11-20)27(32)19-12-14-30(15-13-19)16-21(31)17-33-25-7-3-6-24-26(25)22-4-1-2-5-23(22)29-24/h1-11,19,21,29,31H,12-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372966
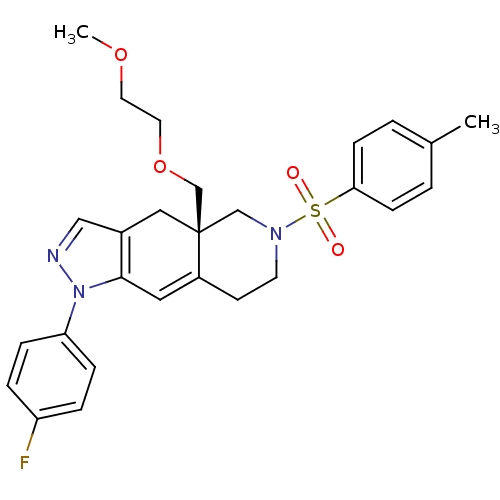
(CHEMBL270668)Show SMILES COCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(C)cc1 |c:12| Show InChI InChI=1S/C27H30FN3O4S/c1-20-3-9-25(10-4-20)36(32,33)30-12-11-22-15-26-21(16-27(22,18-30)19-35-14-13-34-2)17-29-31(26)24-7-5-23(28)6-8-24/h3-10,15,17H,11-14,16,18-19H2,1-2H3/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031889
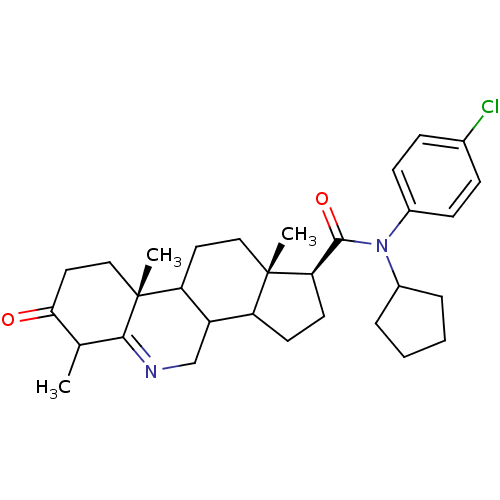
((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CC1C(=O)CC[C@]2(C)C3CC[C@@]4(C)C(CC[C@@H]4C(=O)N(C4CCCC4)c4ccc(Cl)cc4)C3CN=C12 |t:38| Show InChI InChI=1S/C31H41ClN2O2/c1-19-27(35)15-17-31(3)25-14-16-30(2)24(23(25)18-33-28(19)31)12-13-26(30)29(36)34(21-6-4-5-7-21)22-10-8-20(32)9-11-22/h8-11,19,21,23-26H,4-7,12-18H2,1-3H3/t19?,23?,24?,25?,26-,30+,31-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human glucocorticoid receptor expressed in recombinant baculovirus |
Bioorg Med Chem Lett 17: 5704-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.055
BindingDB Entry DOI: 10.7270/Q2FX7B8B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50368883

(CHEMBL1159458)Show SMILES COc1ccc(CCC(=O)c2c(O)cc(O[C@@H]3O[C@](C)(CO)[C@@](C)(O)[C@](C)(O)[C@@]3(C)O[C@]3(C)OC(C)(C)[C@](C)(O)[C@@](C)(O)[C@H]3O)cc2O)cc1O |r| Show InChI InChI=1S/C36H52O15/c1-29(2)34(7,44)31(4,43)27(42)33(6,50-29)51-32(5)28(49-30(3,18-37)35(8,45)36(32,9)46)48-20-16-23(40)26(24(41)17-20)21(38)13-11-19-12-14-25(47-10)22(39)15-19/h12,14-17,27-28,37,39-46H,11,13,18H2,1-10H3/t27-,28-,30-,31+,32+,33+,34+,35-,36-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human type 1 5-alpha reductase |
J Med Chem 37: 2352-60 (1994)
BindingDB Entry DOI: 10.7270/Q228088W |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372977
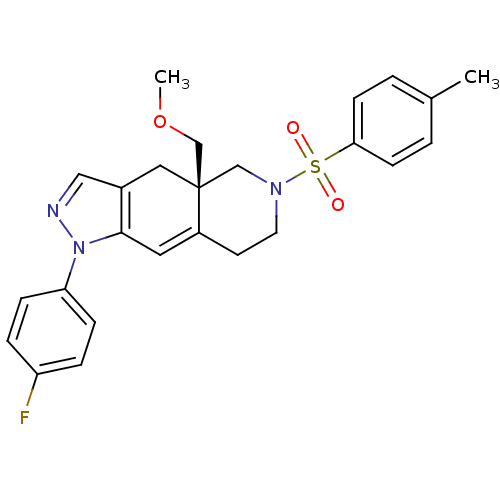
(CHEMBL255369)Show SMILES COC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(C)cc1 |c:9| Show InChI InChI=1S/C25H26FN3O3S/c1-18-3-9-23(10-4-18)33(30,31)28-12-11-20-13-24-19(14-25(20,16-28)17-32-2)15-27-29(24)22-7-5-21(26)6-8-22/h3-10,13,15H,11-12,14,16-17H2,1-2H3/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372975
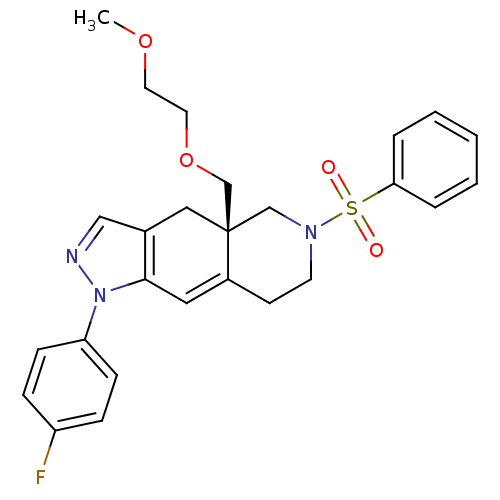
(CHEMBL270667)Show SMILES COCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccccc1 |c:12| Show InChI InChI=1S/C26H28FN3O4S/c1-33-13-14-34-19-26-16-20-17-28-30(23-9-7-22(27)8-10-23)25(20)15-21(26)11-12-29(18-26)35(31,32)24-5-3-2-4-6-24/h2-10,15,17H,11-14,16,18-19H2,1H3/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35255

(2-Chlor-11-(2-dimethylaminoaethoxy)-dibenzo(b,f)-t...)Show InChI InChI=1S/C18H18ClNOS/c1-20(2)9-10-21-16-11-13-5-3-4-6-17(13)22-18-8-7-14(19)12-15(16)18/h3-8,11-12H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Compounds were evaluated the inhibition of [3H] ketanserin binding to membranes from CHO cells, stably transfected with the human 5-HT2A receptor. Da... |
Bioorg Med Chem 17: 7802-15 (2009)
Article DOI: 10.1016/j.bmc.2009.09.023
BindingDB Entry DOI: 10.7270/Q26Q1VK2 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318985

(1-(9H-carbazol-4-yloxy)-3-(4-benzylpiperidin-1-yl)...)Show SMILES OC(COc1cccc2[nH]c3ccccc3c12)CN1CCC(Cc2ccccc2)CC1 Show InChI InChI=1S/C27H30N2O2/c30-22(18-29-15-13-21(14-16-29)17-20-7-2-1-3-8-20)19-31-26-12-6-11-25-27(26)23-9-4-5-10-24(23)28-25/h1-12,21-22,28,30H,13-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031878
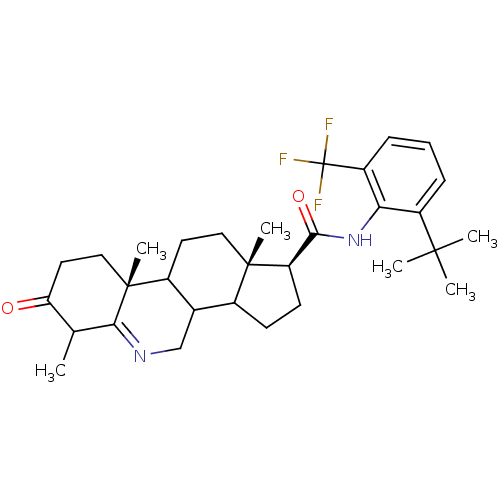
((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CC1C(=O)CC[C@]2(C)C3CC[C@@]4(C)C(CC[C@@H]4C(=O)Nc4c(cccc4C(F)(F)F)C(C)(C)C)C3CN=C12 |t:39| Show InChI InChI=1S/C31H41F3N2O2/c1-17-24(37)13-15-30(6)20-12-14-29(5)19(18(20)16-35-26(17)30)10-11-23(29)27(38)36-25-21(28(2,3)4)8-7-9-22(25)31(32,33)34/h7-9,17-20,23H,10-16H2,1-6H3,(H,36,38)/t17?,18?,19?,20?,23-,29+,30-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031897
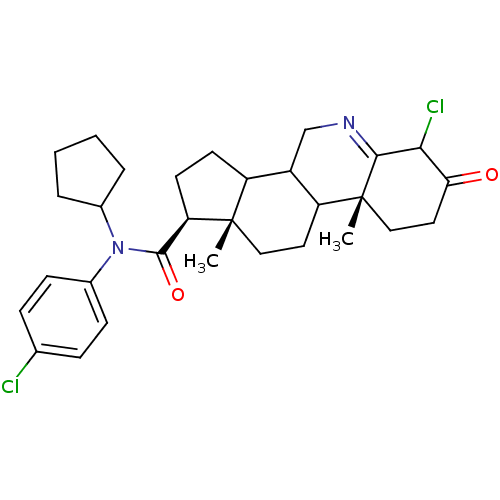
((1S,9aR,11aS)-6-Chloro-9a,11a-dimethyl-7-oxo-2,3,3...)Show SMILES C[C@]12CCC3C(CN=C4C(Cl)C(=O)CC[C@]34C)C1CC[C@@H]2C(=O)N(C1CCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C30H38Cl2N2O2/c1-29-15-13-23-21(17-33-27-26(32)25(35)14-16-30(23,27)2)22(29)11-12-24(29)28(36)34(19-5-3-4-6-19)20-9-7-18(31)8-10-20/h7-10,19,21-24,26H,3-6,11-17H2,1-2H3/t21?,22?,23?,24-,26?,29+,30-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372937
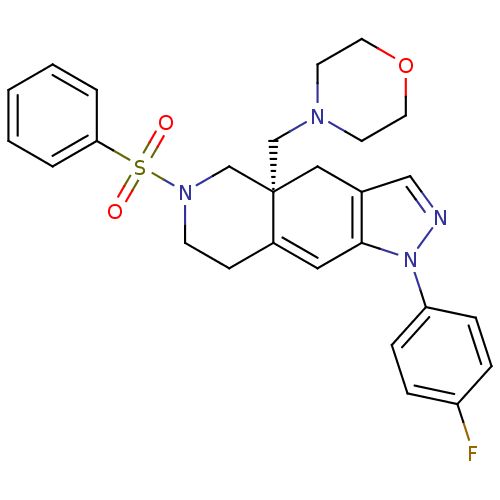
(CHEMBL257461)Show SMILES Fc1ccc(cc1)-n1ncc2C[C@]3(CN4CCOCC4)CN(CCC3=Cc12)S(=O)(=O)c1ccccc1 |c:27| Show InChI InChI=1S/C27H29FN4O3S/c28-23-6-8-24(9-7-23)32-26-16-22-10-11-31(36(33,34)25-4-2-1-3-5-25)20-27(22,17-21(26)18-29-32)19-30-12-14-35-15-13-30/h1-9,16,18H,10-15,17,19-20H2/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372965
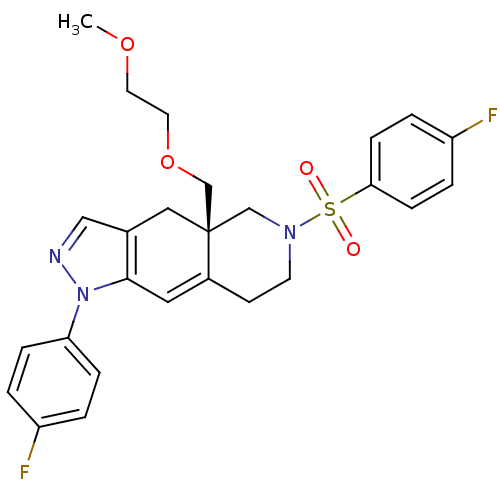
(CHEMBL442803)Show SMILES COCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(F)cc1 |c:12| Show InChI InChI=1S/C26H27F2N3O4S/c1-34-12-13-35-18-26-15-19-16-29-31(23-6-2-21(27)3-7-23)25(19)14-20(26)10-11-30(17-26)36(32,33)24-8-4-22(28)5-9-24/h2-9,14,16H,10-13,15,17-18H2,1H3/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372974
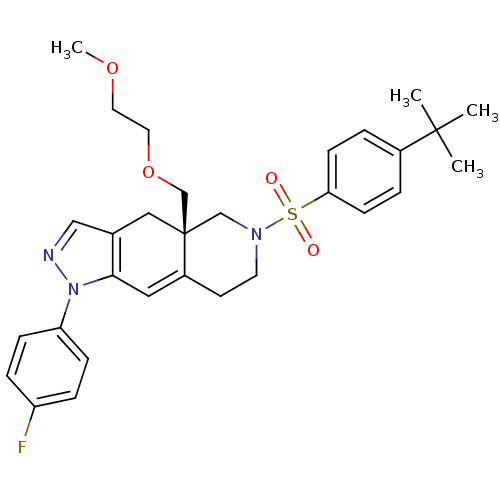
(CHEMBL270575)Show SMILES COCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(C)(C)C |c:12| Show InChI InChI=1S/C30H36FN3O4S/c1-29(2,3)23-5-11-27(12-6-23)39(35,36)33-14-13-24-17-28-22(18-30(24,20-33)21-38-16-15-37-4)19-32-34(28)26-9-7-25(31)8-10-26/h5-12,17,19H,13-16,18,20-21H2,1-4H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at GR in SW1353/MMTV5 cells assessed as inhibition of dexamethasone-induced luciferase expression |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by whole cell binding assay |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research
| Assay Description
Compounds were evaluated the inhibition of [3H] nisoxetine binding to MDCK-Net6 cells, stably transfected with the human norepinephrine transporter (... |
Bioorg Med Chem 17: 7802-15 (2009)
Article DOI: 10.1016/j.bmc.2009.09.023
BindingDB Entry DOI: 10.7270/Q26Q1VK2 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113780
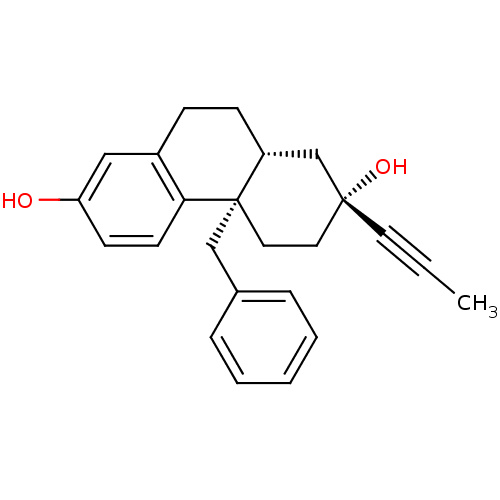
((2R,4aS,10aR)-4a-benzyl-2-(prop-1-ynyl)-1,2,3,4,4a...)Show SMILES CC#C[C@@]1(O)CC[C@]2(Cc3ccccc3)[C@H](CCc3cc(O)ccc23)C1 Show InChI InChI=1S/C24H26O2/c1-2-12-23(26)13-14-24(16-18-6-4-3-5-7-18)20(17-23)9-8-19-15-21(25)10-11-22(19)24/h3-7,10-11,15,20,25-26H,8-9,13-14,16-17H2,1H3/t20-,23-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human glucocorticoid receptor expressed in recombinant baculovirus |
Bioorg Med Chem Lett 17: 5704-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.055
BindingDB Entry DOI: 10.7270/Q2FX7B8B |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372974

(CHEMBL270575)Show SMILES COCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(C)(C)C |c:12| Show InChI InChI=1S/C30H36FN3O4S/c1-29(2,3)23-5-11-27(12-6-23)39(35,36)33-14-13-24-17-28-22(18-30(24,20-33)21-38-16-15-37-4)19-32-34(28)26-9-7-25(31)8-10-26/h5-12,17,19H,13-16,18,20-21H2,1-4H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NPA from rat brain Dopamine receptor D2 |
J Med Chem 39: 143-8 (1996)
Article DOI: 10.1021/jm950625l
BindingDB Entry DOI: 10.7270/Q2ZG6RB0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372970
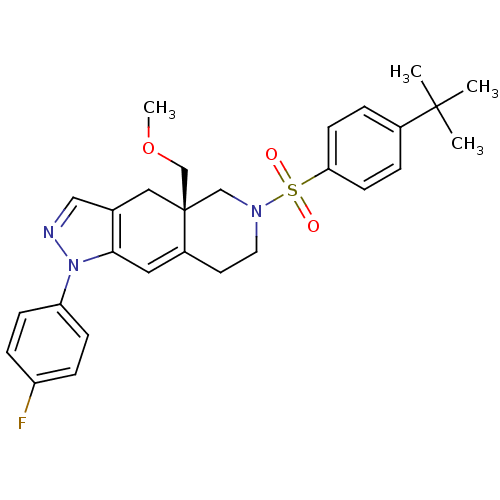
(CHEMBL402893)Show SMILES COC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(C)(C)C |c:9| Show InChI InChI=1S/C28H32FN3O3S/c1-27(2,3)21-5-11-25(12-6-21)36(33,34)31-14-13-22-15-26-20(16-28(22,18-31)19-35-4)17-30-32(26)24-9-7-23(29)8-10-24/h5-12,15,17H,13-14,16,18-19H2,1-4H3/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372976
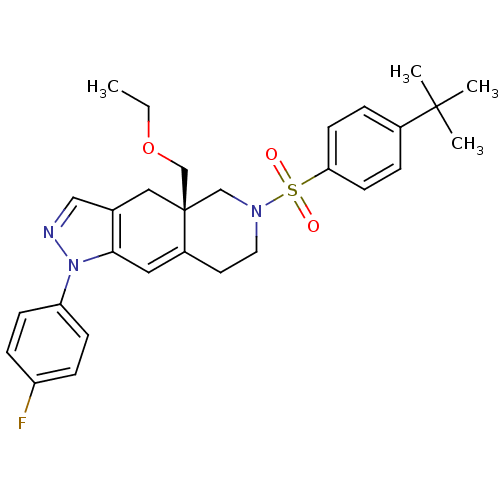
(CHEMBL271221)Show SMILES CCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(C)(C)C |c:10| Show InChI InChI=1S/C29H34FN3O3S/c1-5-36-20-29-17-21-18-31-33(25-10-8-24(30)9-11-25)27(21)16-23(29)14-15-32(19-29)37(34,35)26-12-6-22(7-13-26)28(2,3)4/h6-13,16,18H,5,14-15,17,19-20H2,1-4H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372926
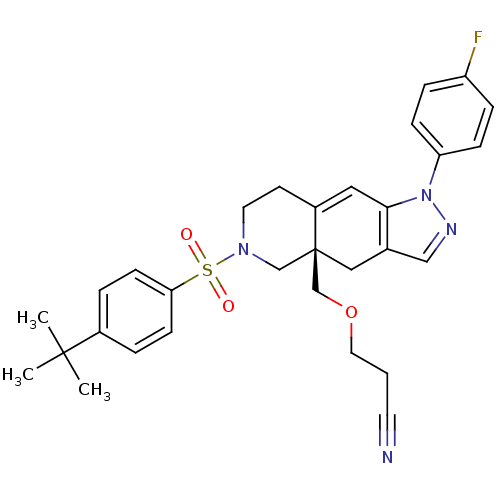
(CHEMBL411852)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1CCC2=Cc3c(C[C@]2(COCCC#N)C1)cnn3-c1ccc(F)cc1 |t:17| Show InChI InChI=1S/C30H33FN4O3S/c1-29(2,3)23-5-11-27(12-6-23)39(36,37)34-15-13-24-17-28-22(18-30(24,20-34)21-38-16-4-14-32)19-33-35(28)26-9-7-25(31)8-10-26/h5-12,17,19H,4,13,15-16,18,20-21H2,1-3H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372964
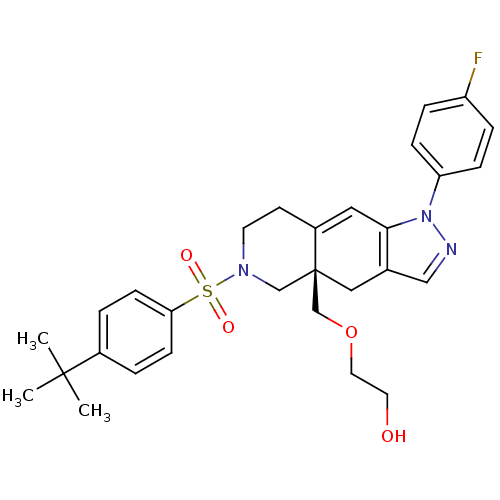
(CHEMBL270568)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1CCC2=Cc3c(C[C@]2(COCCO)C1)cnn3-c1ccc(F)cc1 |t:17| Show InChI InChI=1S/C29H34FN3O4S/c1-28(2,3)22-4-10-26(11-5-22)38(35,36)32-13-12-23-16-27-21(17-29(23,19-32)20-37-15-14-34)18-31-33(27)25-8-6-24(30)7-9-25/h4-11,16,18,34H,12-15,17,19-20H2,1-3H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372969
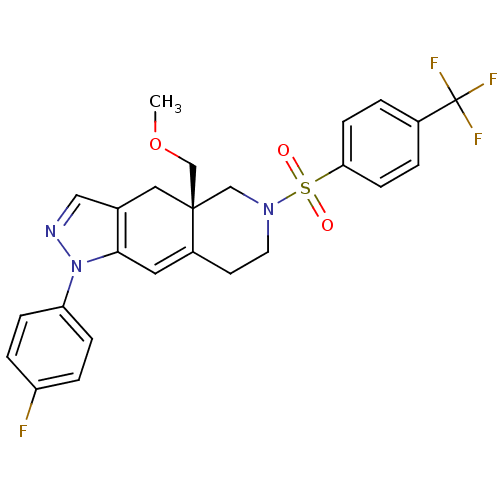
(CHEMBL271428)Show SMILES COC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(F)(F)F |c:9| Show InChI InChI=1S/C25H23F4N3O3S/c1-35-16-24-13-17-14-30-32(21-6-4-20(26)5-7-21)23(17)12-19(24)10-11-31(15-24)36(33,34)22-8-2-18(3-9-22)25(27,28)29/h2-9,12,14H,10-11,13,15-16H2,1H3/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372945
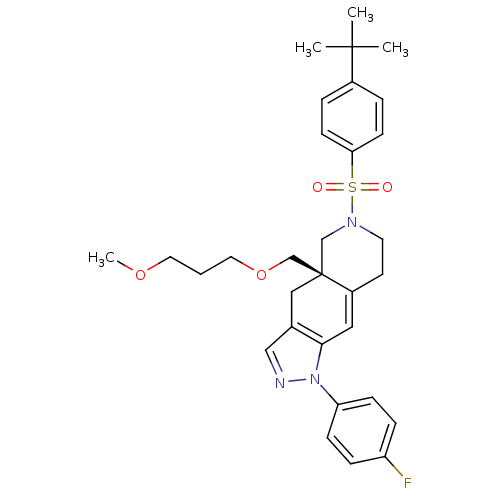
(CHEMBL404183)Show SMILES COCCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(C)(C)C |c:13| Show InChI InChI=1S/C31H38FN3O4S/c1-30(2,3)24-6-12-28(13-7-24)40(36,37)34-15-14-25-18-29-23(19-31(25,21-34)22-39-17-5-16-38-4)20-33-35(29)27-10-8-26(32)9-11-27/h6-13,18,20H,5,14-17,19,21-22H2,1-4H3/t31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372931
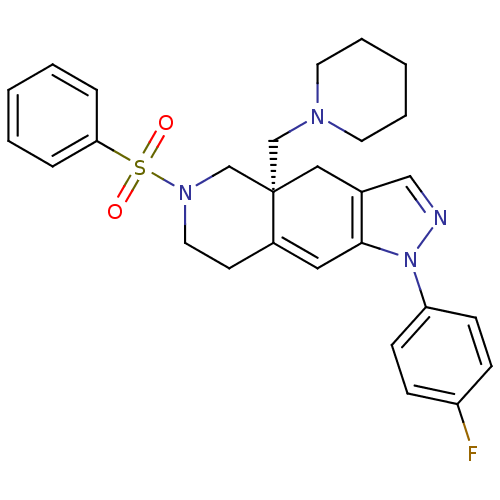
(CHEMBL409835)Show SMILES Fc1ccc(cc1)-n1ncc2C[C@]3(CN4CCCCC4)CN(CCC3=Cc12)S(=O)(=O)c1ccccc1 |c:27| Show InChI InChI=1S/C28H31FN4O2S/c29-24-9-11-25(12-10-24)33-27-17-23-13-16-32(36(34,35)26-7-3-1-4-8-26)21-28(23,18-22(27)19-30-33)20-31-14-5-2-6-15-31/h1,3-4,7-12,17,19H,2,5-6,13-16,18,20-21H2/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor in SW1353 cells by whole-cell binding assay |
Bioorg Med Chem Lett 17: 5704-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.055
BindingDB Entry DOI: 10.7270/Q2FX7B8B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372967
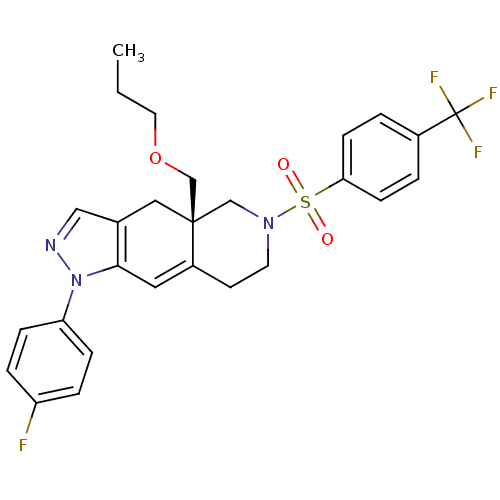
(CHEMBL270879)Show SMILES CCCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(F)(F)F |c:11| Show InChI InChI=1S/C27H27F4N3O3S/c1-2-13-37-18-26-15-19-16-32-34(23-7-5-22(28)6-8-23)25(19)14-21(26)11-12-33(17-26)38(35,36)24-9-3-20(4-10-24)27(29,30)31/h3-10,14,16H,2,11-13,15,17-18H2,1H3/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372918
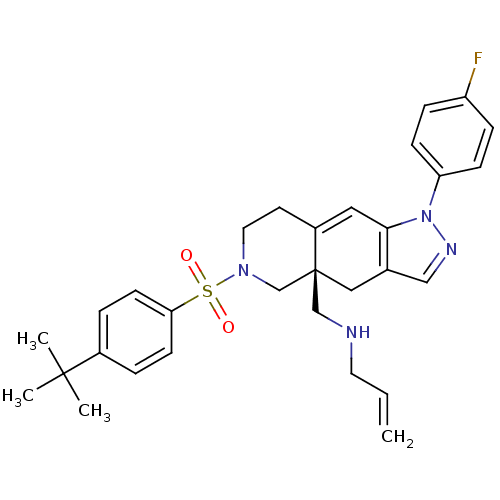
(CHEMBL401864)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1CCC2=Cc3c(C[C@]2(CNCC=C)C1)cnn3-c1ccc(F)cc1 |t:17| Show InChI InChI=1S/C30H35FN4O2S/c1-5-15-32-20-30-18-22-19-33-35(26-10-8-25(31)9-11-26)28(22)17-24(30)14-16-34(21-30)38(36,37)27-12-6-23(7-13-27)29(2,3)4/h5-13,17,19,32H,1,14-16,18,20-21H2,2-4H3/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372929
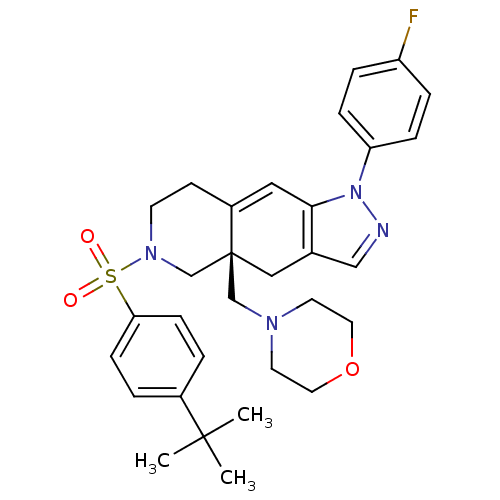
(CHEMBL270838)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1CCC2=Cc3c(C[C@]2(CN2CCOCC2)C1)cnn3-c1ccc(F)cc1 |t:17| Show InChI InChI=1S/C31H37FN4O3S/c1-30(2,3)24-4-10-28(11-5-24)40(37,38)35-13-12-25-18-29-23(20-33-36(29)27-8-6-26(32)7-9-27)19-31(25,22-35)21-34-14-16-39-17-15-34/h4-11,18,20H,12-17,19,21-22H2,1-3H3/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372968
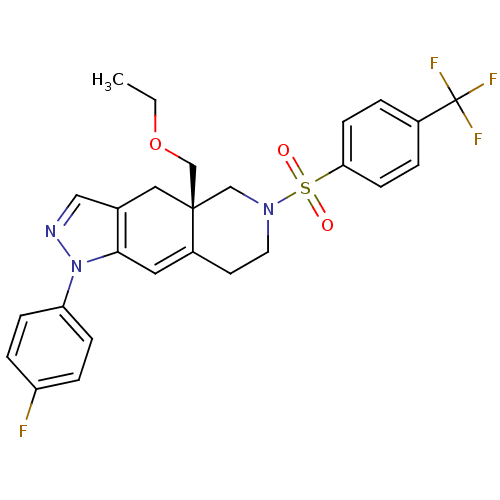
(CHEMBL271220)Show SMILES CCOC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(F)(F)F |c:10| Show InChI InChI=1S/C26H25F4N3O3S/c1-2-36-17-25-14-18-15-31-33(22-7-5-21(27)6-8-22)24(18)13-20(25)11-12-32(16-25)37(34,35)23-9-3-19(4-10-23)26(28,29)30/h3-10,13,15H,2,11-12,14,16-17H2,1H3/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50372921
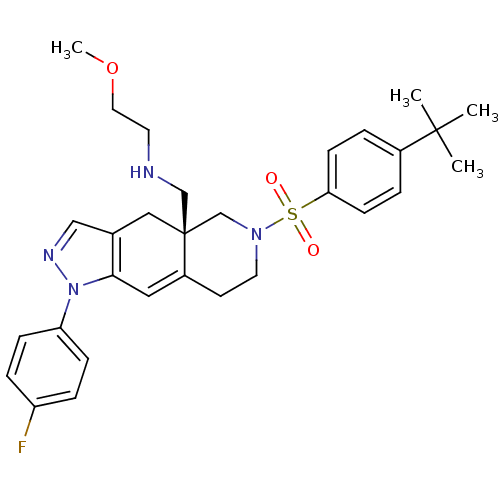
(CHEMBL270143)Show SMILES COCCNC[C@@]12CN(CCC1=Cc1c(C2)cnn1-c1ccc(F)cc1)S(=O)(=O)c1ccc(cc1)C(C)(C)C |c:12| Show InChI InChI=1S/C30H37FN4O3S/c1-29(2,3)23-5-11-27(12-6-23)39(36,37)34-15-13-24-17-28-22(18-30(24,21-34)20-32-14-16-38-4)19-33-35(28)26-9-7-25(31)8-10-26/h5-12,17,19,32H,13-16,18,20-21H2,1-4H3/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [3H]dexamethasone from human recombinant GR |
Bioorg Med Chem Lett 18: 1312-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.027
BindingDB Entry DOI: 10.7270/Q22Z16CP |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318987

(1-(9H-carbazol-4-yloxy)-3-(4-(pyridin-3-ylmethyl)p...)Show SMILES OC(COc1cccc2[nH]c3ccccc3c12)CN1CCC(Cc2cccnc2)CC1 Show InChI InChI=1S/C26H29N3O2/c30-21(17-29-13-10-19(11-14-29)15-20-5-4-12-27-16-20)18-31-25-9-3-8-24-26(25)22-6-1-2-7-23(22)28-24/h1-9,12,16,19,21,28,30H,10-11,13-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human adrenergic beta-1 receptor expressed in CHOK1 cells assessed as inhibition of cAMP accumulation by HTRF assay |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50328300
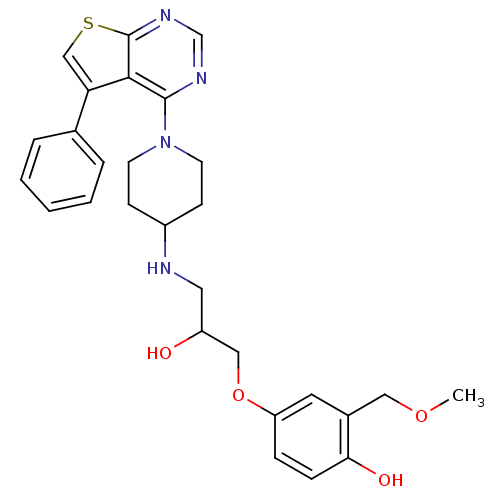
(4-(2-hydroxy-3-(1-(5-phenylthieno[2,3-d]pyrimidin-...)Show SMILES COCc1cc(OCC(O)CNC2CCN(CC2)c2ncnc3scc(-c4ccccc4)c23)ccc1O Show InChI InChI=1S/C28H32N4O4S/c1-35-15-20-13-23(7-8-25(20)34)36-16-22(33)14-29-21-9-11-32(12-10-21)27-26-24(19-5-3-2-4-6-19)17-37-28(26)31-18-30-27/h2-8,13,17-18,21-22,29,33-34H,9-12,14-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]-cyanopindolol from human adrenergic beta3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 6108-15 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.039
BindingDB Entry DOI: 10.7270/Q2WM1DNR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data