 Found 349 hits with Last Name = 'pace' and Initial = 'p'
Found 349 hits with Last Name = 'pace' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089450
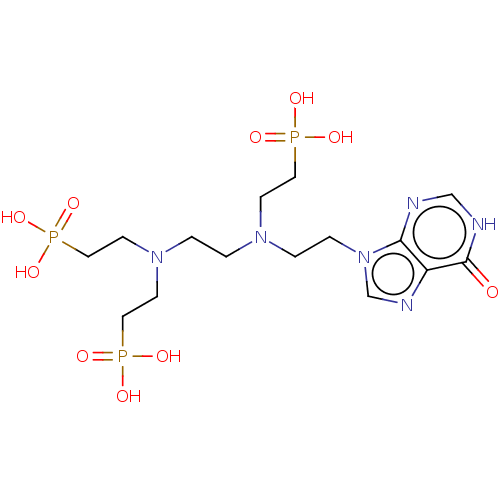
(CHEMBL3578115)Show SMILES OP(O)(=O)CCN(CCN(CCP(O)(O)=O)CCP(O)(O)=O)CCn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C15H29N6O10P3/c22-15-13-14(16-11-17-15)21(12-18-13)4-3-19(5-8-32(23,24)25)1-2-20(6-9-33(26,27)28)7-10-34(29,30)31/h11-12H,1-10H2,(H,16,17,22)(H2,23,24,25)(H2,26,27,28)(H2,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089444
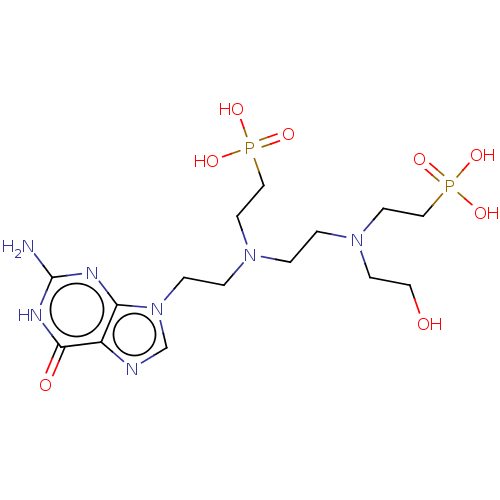
(CHEMBL3578110)Show SMILES Nc1nc2n(CCN(CCN(CCO)CCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C15H29N7O8P2/c16-15-18-13-12(14(24)19-15)17-11-22(13)4-3-20(6-9-31(25,26)27)1-2-21(5-8-23)7-10-32(28,29)30/h11,23H,1-10H2,(H2,25,26,27)(H2,28,29,30)(H3,16,18,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50427810
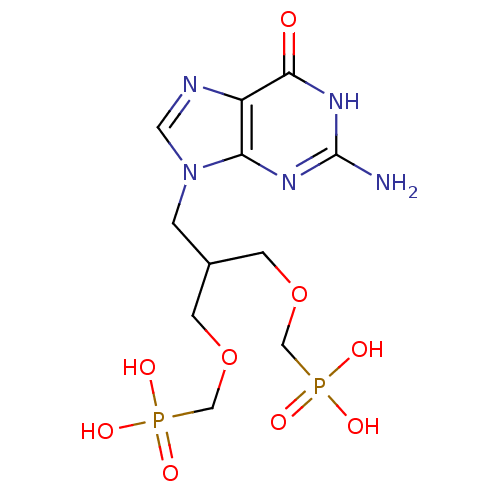
(CHEMBL2325752)Show SMILES Nc1nc2n(CC(COCP(O)(O)=O)COCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C11H19N5O9P2/c12-11-14-9-8(10(17)15-11)13-4-16(9)1-7(2-24-5-26(18,19)20)3-25-6-27(21,22)23/h4,7H,1-3,5-6H2,(H2,18,19,20)(H2,21,22,23)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged HGPRT |
J Med Chem 56: 2513-26 (2013)
Article DOI: 10.1021/jm301893b
BindingDB Entry DOI: 10.7270/Q2MW2JGT |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089449

(CHEMBL3578114)Show SMILES Nc1nc2n(CCN(CCN(CCP(O)(O)=O)CCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C15H30N7O10P3/c16-15-18-13-12(14(23)19-15)17-11-22(13)4-3-20(5-8-33(24,25)26)1-2-21(6-9-34(27,28)29)7-10-35(30,31)32/h11H,1-10H2,(H2,24,25,26)(H2,27,28,29)(H2,30,31,32)(H3,16,18,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089446

(CHEMBL3578112)Show SMILES Nc1nc2n(CCN(CCN(CCCO)CCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C16H31N7O8P2/c17-16-19-14-13(15(25)20-16)18-12-23(14)6-5-22(8-11-33(29,30)31)4-3-21(2-1-9-24)7-10-32(26,27)28/h12,24H,1-11H2,(H2,26,27,28)(H2,29,30,31)(H3,17,19,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181460
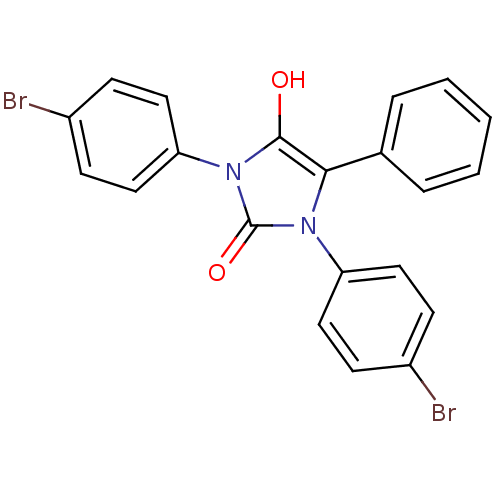
(1,3-bis(4-bromophenyl)-5-phenylimidazolidine-2,4-d...)Show SMILES Oc1c(-c2ccccc2)n(-c2ccc(Br)cc2)c(=O)n1-c1ccc(Br)cc1 Show InChI InChI=1S/C21H14Br2N2O2/c22-15-6-10-17(11-7-15)24-19(14-4-2-1-3-5-14)20(26)25(21(24)27)18-12-8-16(23)9-13-18/h1-13,26H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50181463
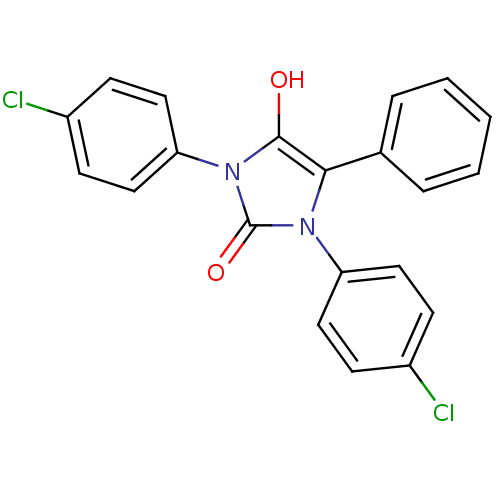
(1,3-bis(4-chlorophenyl)-5-phenylimidazolidine-2,4-...)Show SMILES Oc1c(-c2ccccc2)n(-c2ccc(Cl)cc2)c(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H14Cl2N2O2/c22-15-6-10-17(11-7-15)24-19(14-4-2-1-3-5-14)20(26)25(21(24)27)18-12-8-16(23)9-13-18/h1-13,26H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50181460

(1,3-bis(4-bromophenyl)-5-phenylimidazolidine-2,4-d...)Show SMILES Oc1c(-c2ccccc2)n(-c2ccc(Br)cc2)c(=O)n1-c1ccc(Br)cc1 Show InChI InChI=1S/C21H14Br2N2O2/c22-15-6-10-17(11-7-15)24-19(14-4-2-1-3-5-14)20(26)25(21(24)27)18-12-8-16(23)9-13-18/h1-13,26H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181463

(1,3-bis(4-chlorophenyl)-5-phenylimidazolidine-2,4-...)Show SMILES Oc1c(-c2ccccc2)n(-c2ccc(Cl)cc2)c(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H14Cl2N2O2/c22-15-6-10-17(11-7-15)24-19(14-4-2-1-3-5-14)20(26)25(21(24)27)18-12-8-16(23)9-13-18/h1-13,26H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089445
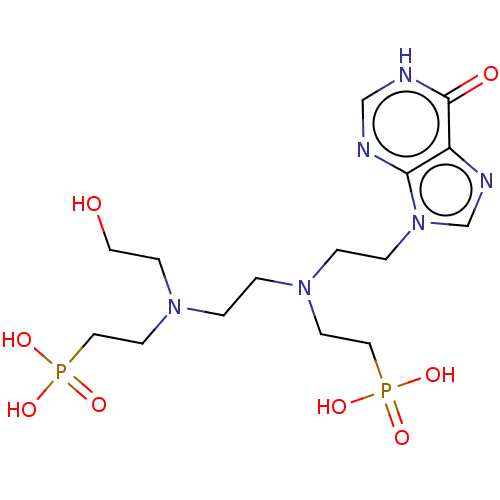
(CHEMBL3578111)Show SMILES OCCN(CCN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O)CCP(O)(O)=O Show InChI InChI=1S/C15H28N6O8P2/c22-8-5-20(7-10-31(27,28)29)2-1-19(6-9-30(24,25)26)3-4-21-12-18-13-14(21)16-11-17-15(13)23/h11-12,22H,1-10H2,(H,16,17,23)(H2,24,25,26)(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50427808
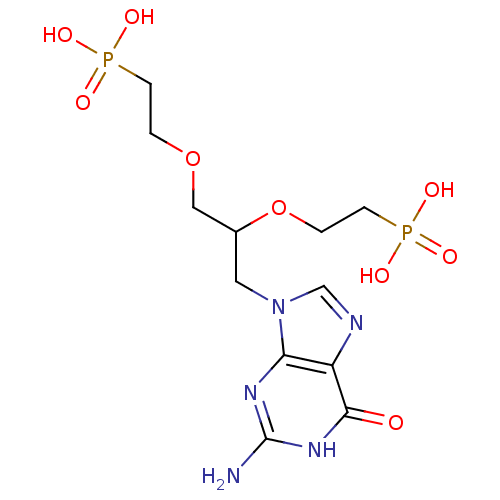
(CHEMBL2325754)Show SMILES Nc1nc2n(CC(COCCP(O)(O)=O)OCCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H21N5O9P2/c13-12-15-10-9(11(18)16-12)14-7-17(10)5-8(26-2-4-28(22,23)24)6-25-1-3-27(19,20)21/h7-8H,1-6H2,(H2,19,20,21)(H2,22,23,24)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged HGPRT |
J Med Chem 56: 2513-26 (2013)
Article DOI: 10.1021/jm301893b
BindingDB Entry DOI: 10.7270/Q2MW2JGT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181462
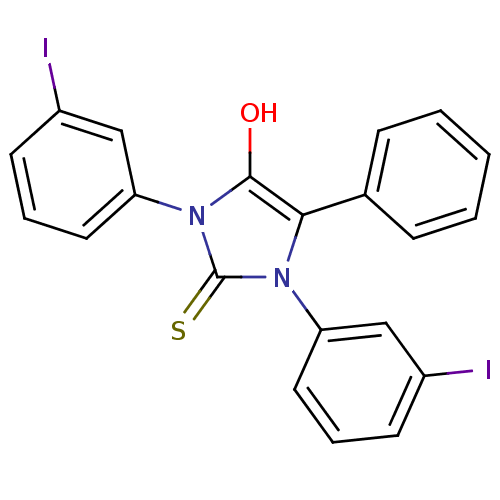
(1,3-bis(3-iodophenyl)-5-phenyl-2-thioxoimidazolidi...)Show SMILES Oc1c(-c2ccccc2)n(-c2cccc(I)c2)c(=S)n1-c1cccc(I)c1 Show InChI InChI=1S/C21H14I2N2OS/c22-15-8-4-10-17(12-15)24-19(14-6-2-1-3-7-14)20(26)25(21(24)27)18-11-5-9-16(23)13-18/h1-13,26H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181458

(5-(4-bromophenyl)-1,3-bis(4-chlorophenyl)imidazoli...)Show SMILES Oc1c(-c2ccc(Br)cc2)n(-c2ccc(Cl)cc2)c(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H13BrCl2N2O2/c22-14-3-1-13(2-4-14)19-20(27)26(18-11-7-16(24)8-12-18)21(28)25(19)17-9-5-15(23)6-10-17/h1-12,27H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 905 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50427809

(CHEMBL2325753)Show SMILES OP(O)(=O)COCC(COCP(O)(O)=O)Cn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C11H18N4O9P2/c16-11-9-10(12-4-13-11)15(5-14-9)1-8(2-23-6-25(17,18)19)3-24-7-26(20,21)22/h4-5,8H,1-3,6-7H2,(H,12,13,16)(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged HGPRT |
J Med Chem 56: 2513-26 (2013)
Article DOI: 10.1021/jm301893b
BindingDB Entry DOI: 10.7270/Q2MW2JGT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181461
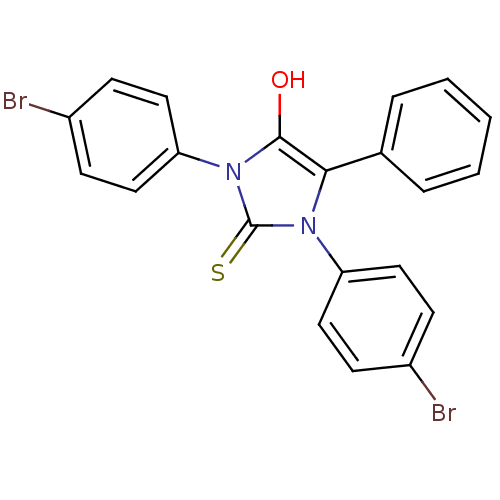
(1,3-bis(4-bromophenyl)-5-phenyl-2-thioxoimidazolid...)Show SMILES Oc1c(-c2ccccc2)n(-c2ccc(Br)cc2)c(=S)n1-c1ccc(Br)cc1 Show InChI InChI=1S/C21H14Br2N2OS/c22-15-6-10-17(11-7-15)24-19(14-4-2-1-3-5-14)20(26)25(21(24)27)18-12-8-16(23)9-13-18/h1-13,26H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089448

(CHEMBL3578113)Show SMILES OCCCN(CCN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O)CCP(O)(O)=O Show InChI InChI=1S/C16H30N6O8P2/c23-9-1-2-20(7-10-31(25,26)27)3-4-21(8-11-32(28,29)30)5-6-22-13-19-14-15(22)17-12-18-16(14)24/h12-13,23H,1-11H2,(H,17,18,24)(H2,25,26,27)(H2,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50181458

(5-(4-bromophenyl)-1,3-bis(4-chlorophenyl)imidazoli...)Show SMILES Oc1c(-c2ccc(Br)cc2)n(-c2ccc(Cl)cc2)c(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H13BrCl2N2O2/c22-14-3-1-13(2-4-14)19-20(27)26(18-11-7-16(24)8-12-18)21(28)25(19)17-9-5-15(23)6-10-17/h1-12,27H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181459
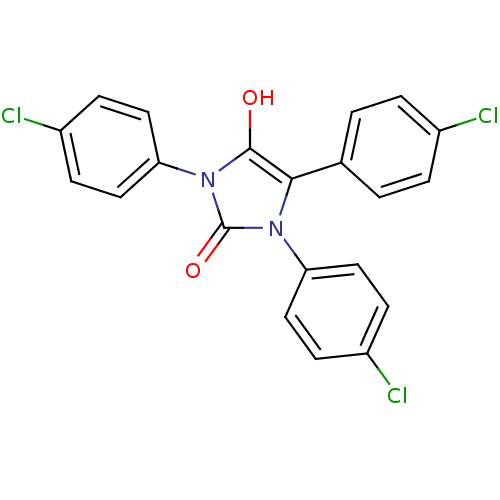
(1,3,5-tris(4-chlorophenyl)imidazolidine-2,4-dione ...)Show SMILES Oc1c(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H13Cl3N2O2/c22-14-3-1-13(2-4-14)19-20(27)26(18-11-7-16(24)8-12-18)21(28)25(19)17-9-5-15(23)6-10-17/h1-12,27H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181466
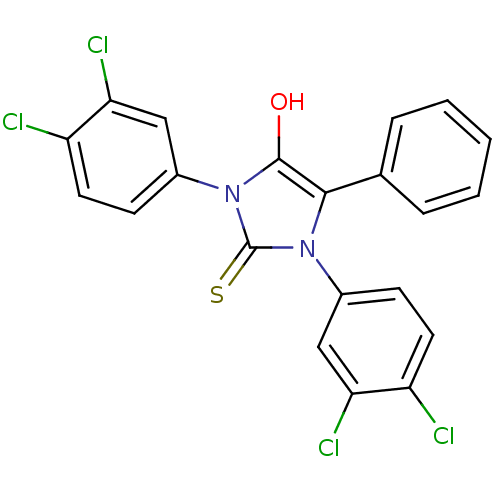
(1,3-bis(4,3-dichlorophenyl)-5-phenyl-2-thioxoimida...)Show SMILES Oc1c(-c2ccccc2)n(-c2ccc(Cl)c(Cl)c2)c(=S)n1-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H12Cl4N2OS/c22-15-8-6-13(10-17(15)24)26-19(12-4-2-1-3-5-12)20(28)27(21(26)29)14-7-9-16(23)18(25)11-14/h1-11,28H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181469
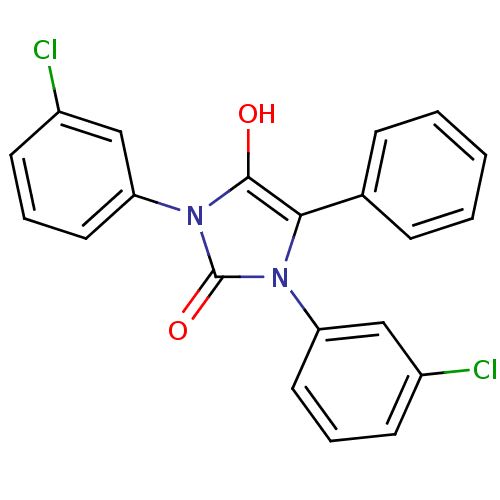
(1,3-bis(3-chlorophenyl)-5-phenylimidazolidine-2,4-...)Show SMILES Oc1c(-c2ccccc2)n(-c2cccc(Cl)c2)c(=O)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C21H14Cl2N2O2/c22-15-8-4-10-17(12-15)24-19(14-6-2-1-3-7-14)20(26)25(21(24)27)18-11-5-9-16(23)13-18/h1-13,26H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181456
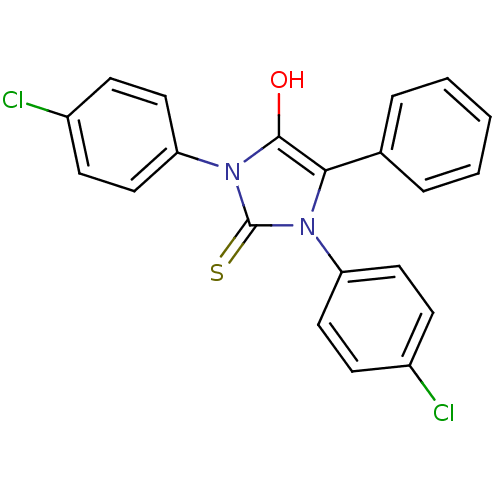
(1,3-bis(4-chlorophenyl)-5-phenyl-2-thioxoimidazoli...)Show SMILES Oc1c(-c2ccccc2)n(-c2ccc(Cl)cc2)c(=S)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H14Cl2N2OS/c22-15-6-10-17(11-7-15)24-19(14-4-2-1-3-5-14)20(26)25(21(24)27)18-12-8-16(23)9-13-18/h1-13,26H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50181459

(1,3,5-tris(4-chlorophenyl)imidazolidine-2,4-dione ...)Show SMILES Oc1c(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H13Cl3N2O2/c22-14-3-1-13(2-4-14)19-20(27)26(18-11-7-16(24)8-12-18)21(28)25(19)17-9-5-15(23)6-10-17/h1-12,27H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181455
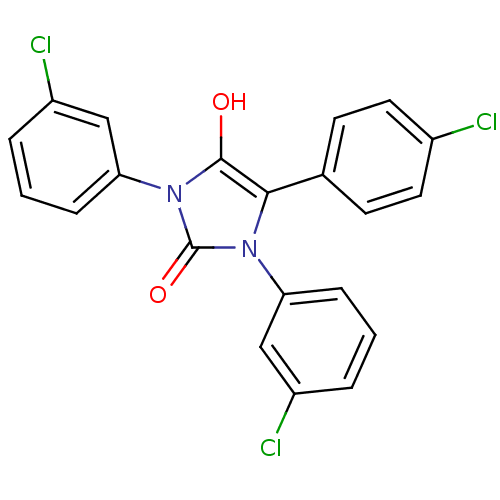
(1,3-bis(3-chlorophenyl)-5-(4-chlorophenyl)imidazol...)Show SMILES Oc1c(-c2ccc(Cl)cc2)n(-c2cccc(Cl)c2)c(=O)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C21H13Cl3N2O2/c22-14-9-7-13(8-10-14)19-20(27)26(18-6-2-4-16(24)12-18)21(28)25(19)17-5-1-3-15(23)11-17/h1-12,27H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089443
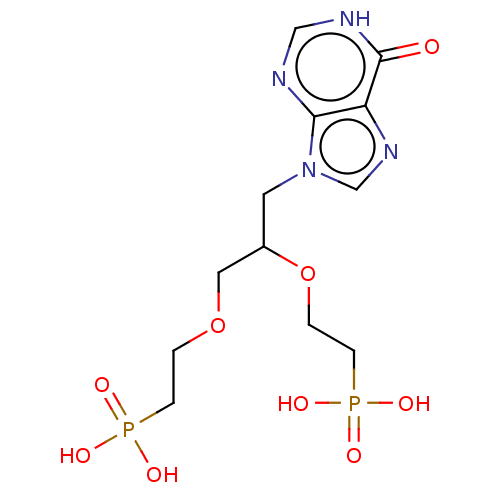
(CHEMBL3578109)Show SMILES OP(O)(=O)CCOCC(Cn1cnc2c1nc[nH]c2=O)OCCP(O)(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181472
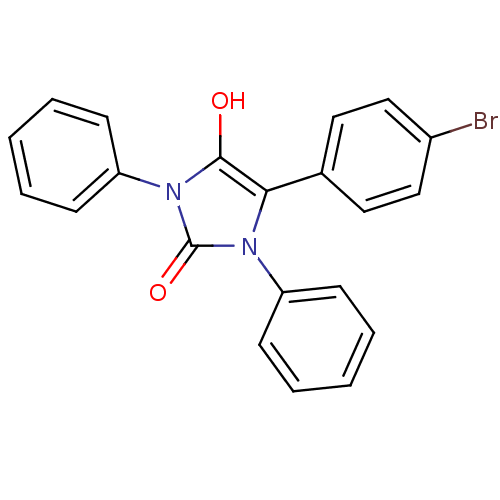
(5-(4-bromophenyl)-1,3-diphenylimidazolidine-2,4-di...)Show SMILES Oc1c(-c2ccc(Br)cc2)n(-c2ccccc2)c(=O)n1-c1ccccc1 Show InChI InChI=1S/C21H15BrN2O2/c22-16-13-11-15(12-14-16)19-20(25)24(18-9-5-2-6-10-18)21(26)23(19)17-7-3-1-4-8-17/h1-14,25H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181470
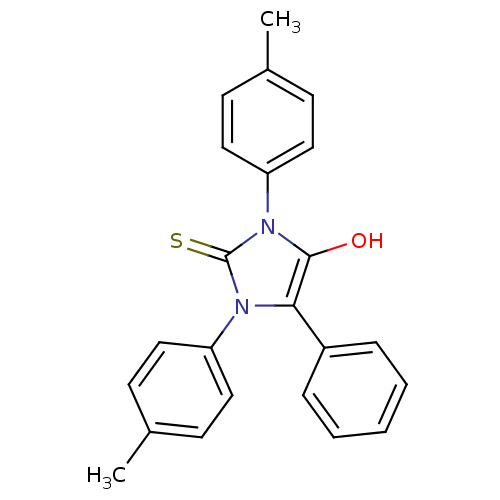
(1,3-bis(4-methylphenyl)-5-phenyl-2-thioxoimidazoli...)Show SMILES Cc1ccc(cc1)-n1c(O)c(-c2ccccc2)n(-c2ccc(C)cc2)c1=S Show InChI InChI=1S/C23H20N2OS/c1-16-8-12-19(13-9-16)24-21(18-6-4-3-5-7-18)22(26)25(23(24)27)20-14-10-17(2)11-15-20/h3-15,26H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM19254

(IMP | Inosine | Inosinic acid | US11185100, TABLE ...)Show SMILES O[C@@H]1[C@@H](COP(O)(O)=O)O[C@H]([C@@H]1O)n1cnc2c1nc[nH]c2=O |r| Show InChI InChI=1S/C10H13N4O8P/c15-6-4(1-21-23(18,19)20)22-10(7(6)16)14-3-13-5-8(14)11-2-12-9(5)17/h2-4,6-7,10,15-16H,1H2,(H,11,12,17)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50010318
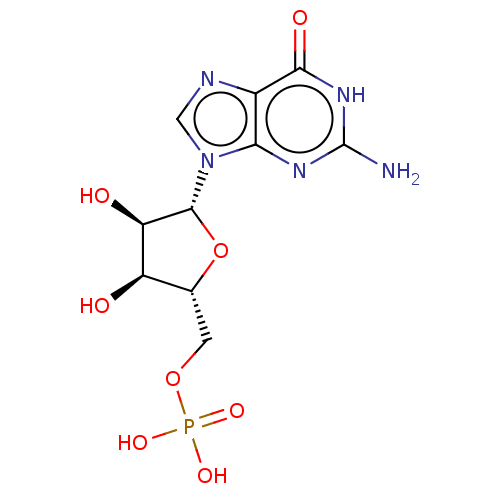
(CHEBI:17345 | CHEMBL283807)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N5O8P/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,16-17H,1H2,(H2,19,20,21)(H3,11,13,14,18)/t3-,5-,6-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181471
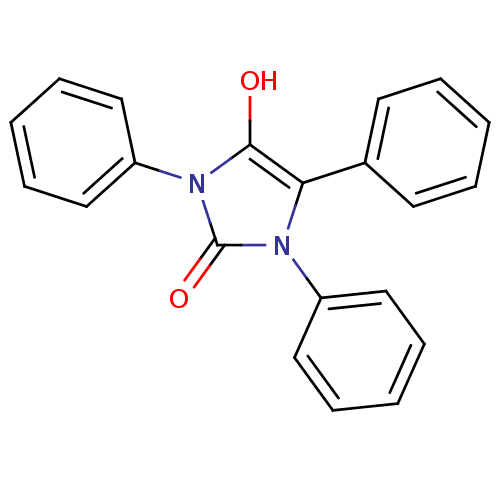
(1,3,5-triphenylimidazolidine-2,4-dione | CHEMBL381...)Show SMILES Oc1c(-c2ccccc2)n(-c2ccccc2)c(=O)n1-c1ccccc1 Show InChI InChI=1S/C21H16N2O2/c24-20-19(16-10-4-1-5-11-16)22(17-12-6-2-7-13-17)21(25)23(20)18-14-8-3-9-15-18/h1-15,24H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181467
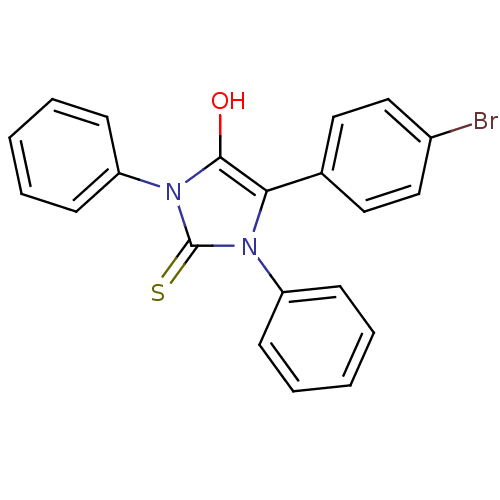
(5-(4-bromophenyl)-1,3-diphenyl-2-thioxoimidazolidi...)Show SMILES Oc1c(-c2ccc(Br)cc2)n(-c2ccccc2)c(=S)n1-c1ccccc1 Show InChI InChI=1S/C21H15BrN2OS/c22-16-13-11-15(12-14-16)19-20(25)24(18-9-5-2-6-10-18)21(26)23(19)17-7-3-1-4-8-17/h1-14,25H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181468
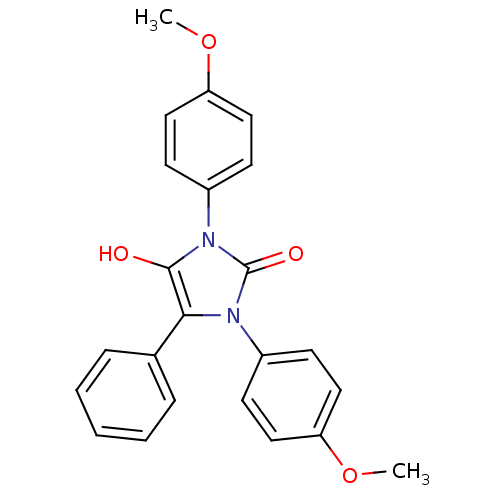
(1,3-bis(4-methoxyphenyl)-5-phenylimidazolidine-2,4...)Show SMILES COc1ccc(cc1)-n1c(O)c(-c2ccccc2)n(-c2ccc(OC)cc2)c1=O Show InChI InChI=1S/C23H20N2O4/c1-28-19-12-8-17(9-13-19)24-21(16-6-4-3-5-7-16)22(26)25(23(24)27)18-10-14-20(29-2)15-11-18/h3-15,26H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181465
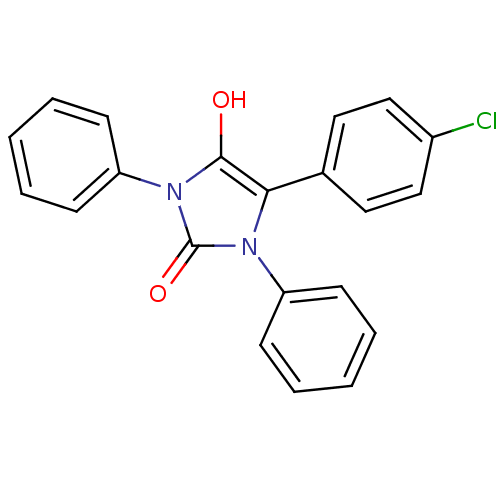
(5-(4-chlorophenyl)-1,3-diphenylimidazolidine-2,4-d...)Show SMILES Oc1c(-c2ccc(Cl)cc2)n(-c2ccccc2)c(=O)n1-c1ccccc1 Show InChI InChI=1S/C21H15ClN2O2/c22-16-13-11-15(12-14-16)19-20(25)24(18-9-5-2-6-10-18)21(26)23(19)17-7-3-1-4-8-17/h1-14,25H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181457
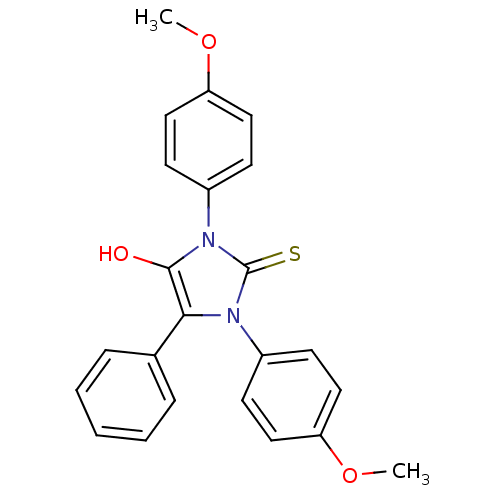
(1,3-bis(4-methoxyphenyl)-5-phenyl-2-thioxoimidazol...)Show SMILES COc1ccc(cc1)-n1c(O)c(-c2ccccc2)n(-c2ccc(OC)cc2)c1=S Show InChI InChI=1S/C23H20N2O3S/c1-27-19-12-8-17(9-13-19)24-21(16-6-4-3-5-7-16)22(26)25(23(24)29)18-10-14-20(28-2)15-11-18/h3-15,26H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50181464
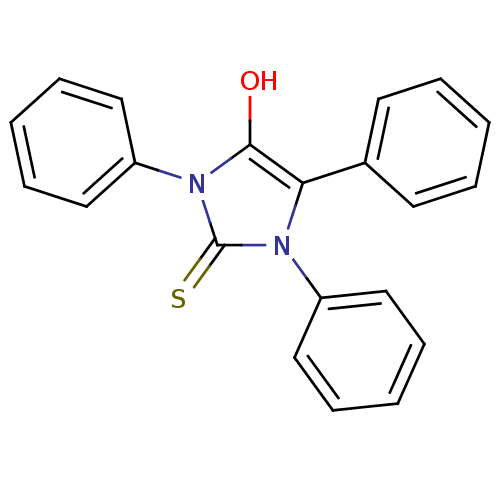
(1,3,5-triphenyl-2-thioxoimidazolidin-4-one | CHEMB...)Show SMILES Oc1c(-c2ccccc2)n(-c2ccccc2)c(=S)n1-c1ccccc1 Show InChI InChI=1S/C21H16N2OS/c24-20-19(16-10-4-1-5-11-16)22(17-12-6-2-7-13-17)21(25)23(20)18-14-8-3-9-15-18/h1-15,24H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50427807
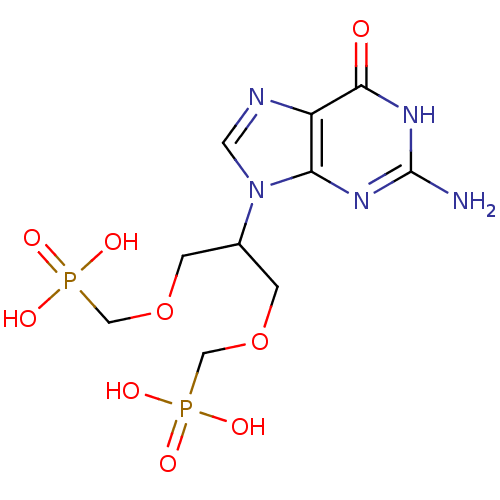
(CHEMBL2325755)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)C(COCP(O)(O)=O)COCP(O)(O)=O Show InChI InChI=1S/C10H17N5O9P2/c11-10-13-8-7(9(16)14-10)12-3-15(8)6(1-23-4-25(17,18)19)2-24-5-26(20,21)22/h3,6H,1-2,4-5H2,(H2,17,18,19)(H2,20,21,22)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged HGPRT |
J Med Chem 56: 2513-26 (2013)
Article DOI: 10.1021/jm301893b
BindingDB Entry DOI: 10.7270/Q2MW2JGT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612447

(CHEMBL5285293) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612445

(CHEMBL5282110) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein
(Human immunodeficiency virus type 1 group M subtyp...) | BDBM25330
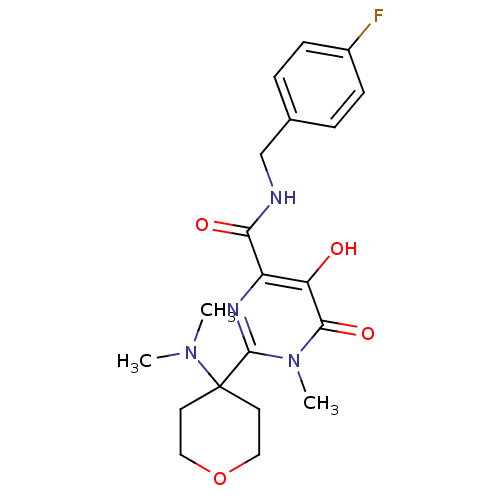
(2-[4-(dimethylamino)oxan-4-yl]-N-[(4-fluorophenyl)...)Show SMILES CN(C)C1(CCOCC1)c1nc(C(=O)NCc2ccc(F)cc2)c(O)c(=O)n1C Show InChI InChI=1S/C20H25FN4O4/c1-24(2)20(8-10-29-11-9-20)19-23-15(16(26)18(28)25(19)3)17(27)22-12-13-4-6-14(21)7-5-13/h4-7,26H,8-12H2,1-3H3,(H,22,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Merck Research Laboratories
| Assay Description
The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... |
J Med Chem 51: 5843-55 (2008)
Article DOI: 10.1021/jm800245z
BindingDB Entry DOI: 10.7270/Q2QJ7FMR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612450
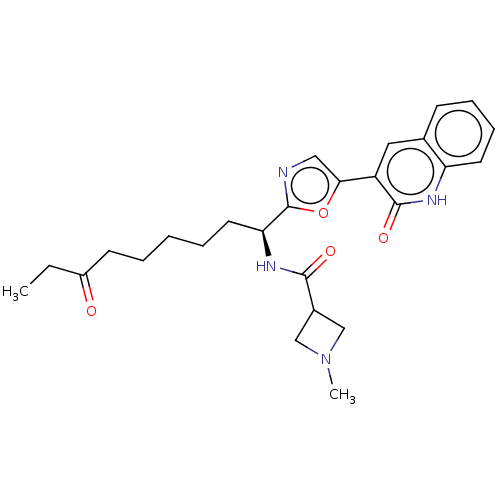
(CHEMBL5279520) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612452
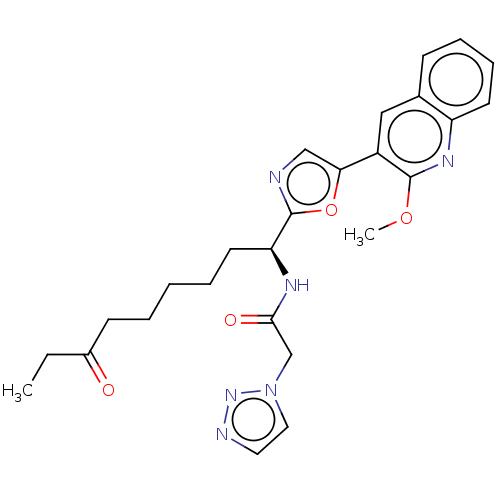
(CHEMBL5285418) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein
(Human immunodeficiency virus type 1 group M subtyp...) | BDBM25329
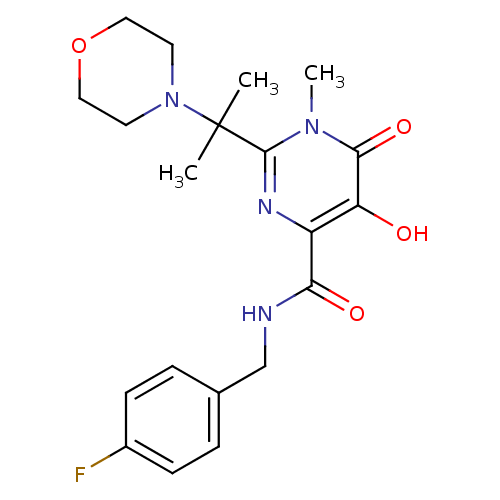
(N-[(4-fluorophenyl)methyl]-5-hydroxy-1-methyl-2-[2...)Show SMILES Cn1c(nc(C(=O)NCc2ccc(F)cc2)c(O)c1=O)C(C)(C)N1CCOCC1 Show InChI InChI=1S/C20H25FN4O4/c1-20(2,25-8-10-29-11-9-25)19-23-15(16(26)18(28)24(19)3)17(27)22-12-13-4-6-14(21)7-5-13/h4-7,26H,8-12H2,1-3H3,(H,22,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Merck Research Laboratories
| Assay Description
The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... |
J Med Chem 51: 5843-55 (2008)
Article DOI: 10.1021/jm800245z
BindingDB Entry DOI: 10.7270/Q2QJ7FMR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612453
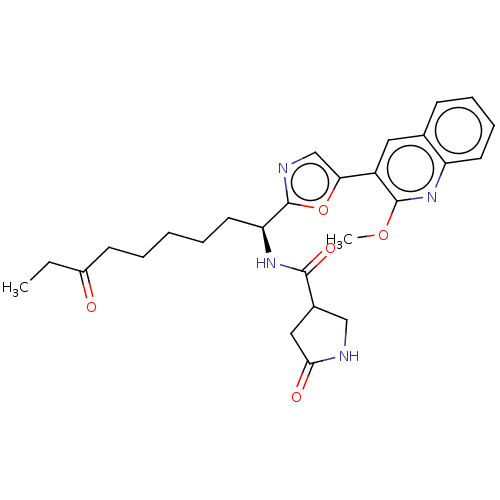
(CHEMBL5285433) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data