

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366125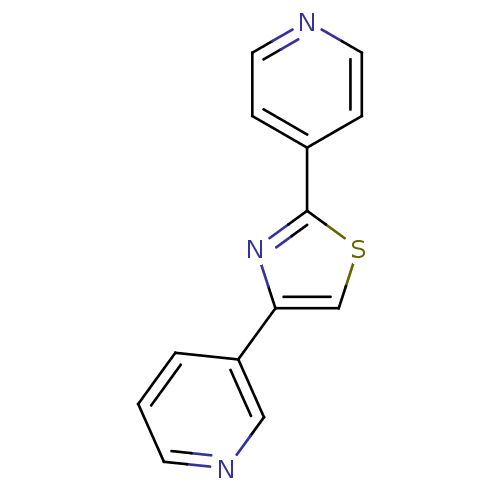 (CHEMBL1957214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10015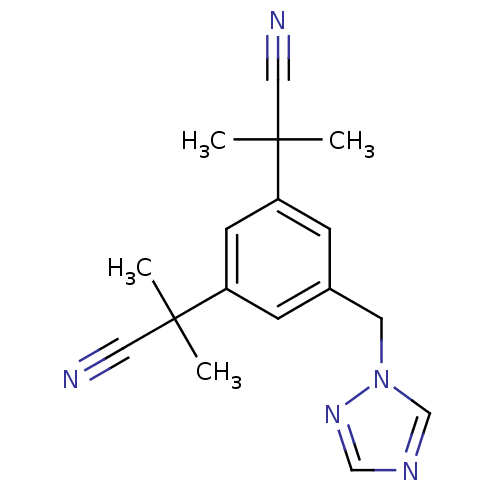 (2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366128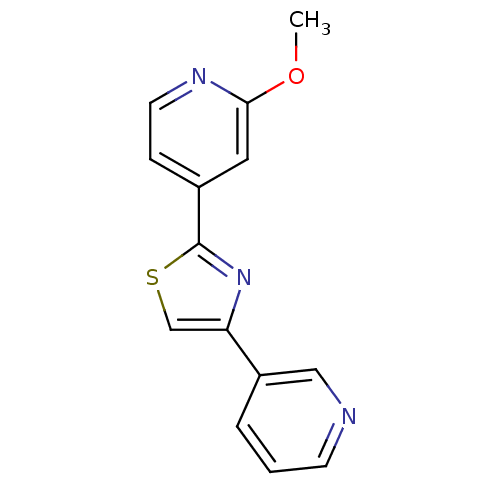 (CHEMBL1957217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of 5-HT2A receptor | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to SERT | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of SERT | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366129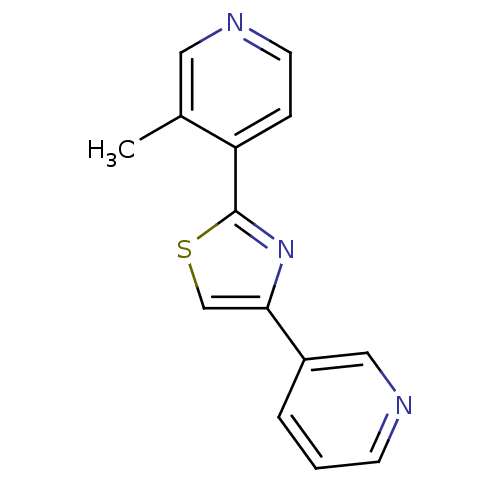 (CHEMBL1957218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50069447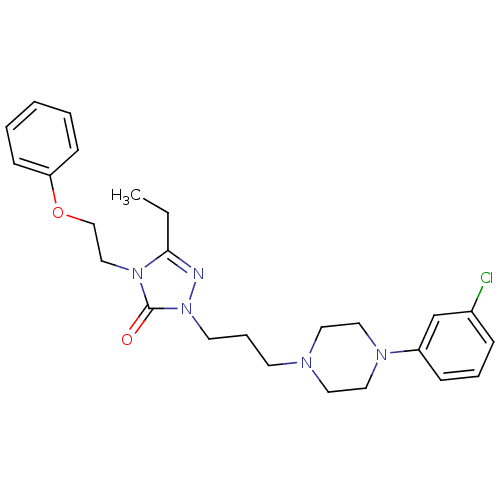 (1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366127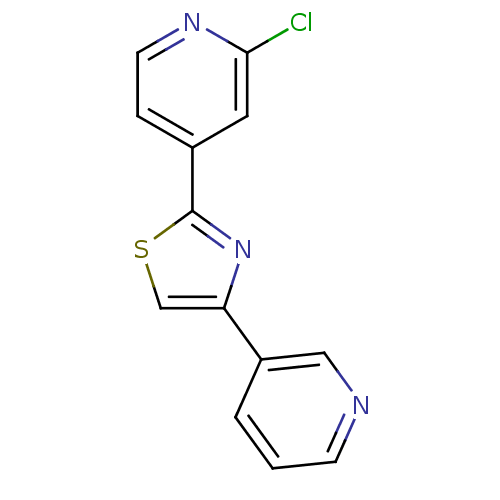 (CHEMBL1957216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313284 ((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of SERT | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313284 ((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to SERT | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366134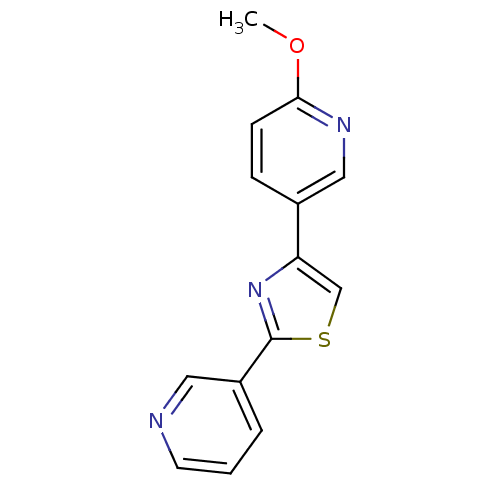 (CHEMBL1957223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366120 (CHEMBL1601919) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366132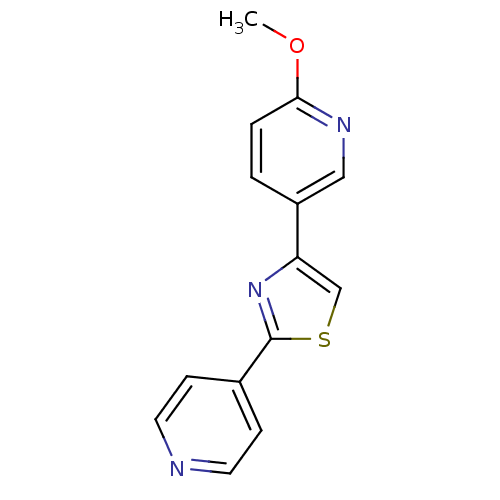 (CHEMBL1957221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313284 ((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of 5-HT2A receptor | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313284 ((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to SERT | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366133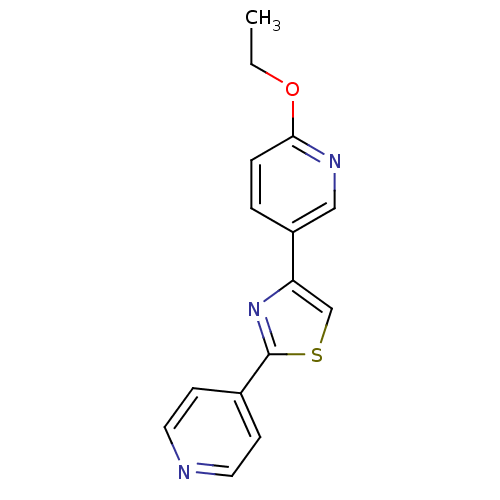 (CHEMBL1957222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366122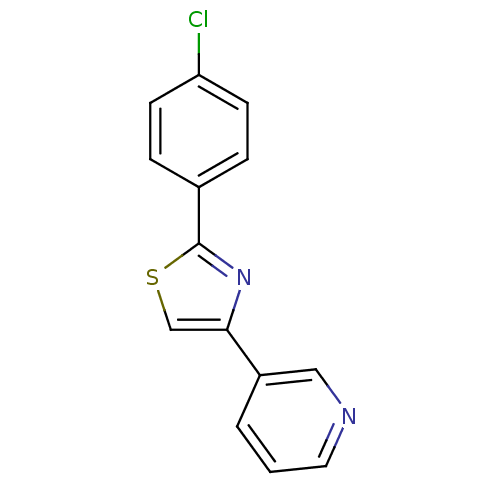 (CHEMBL1957211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366130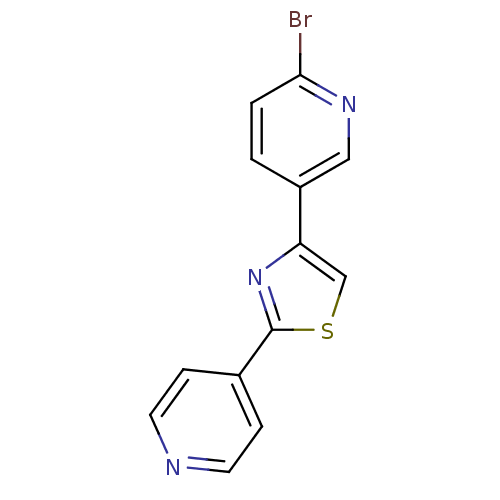 (CHEMBL1957219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50069447 (1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to SERT | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366118 (CHEMBL1957208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366131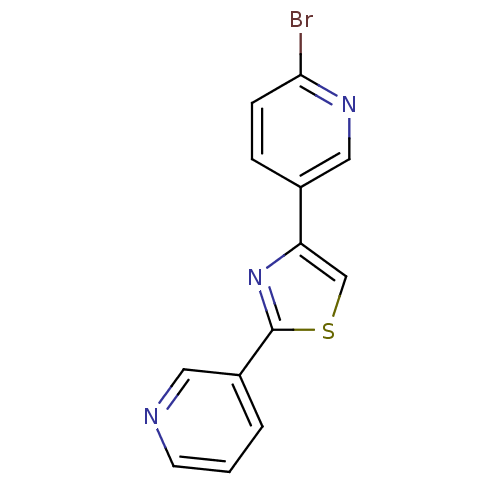 (CHEMBL1957220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93073 (ACS No 172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366123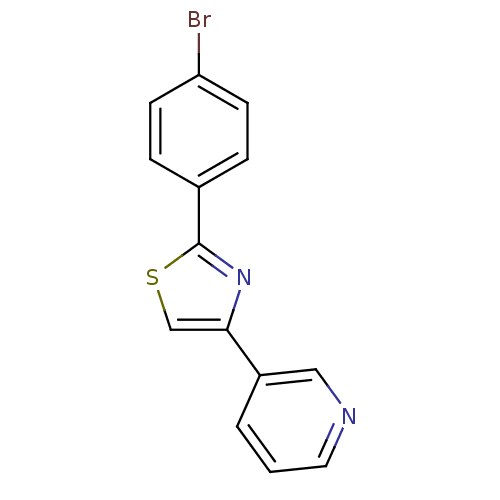 (CHEMBL1957212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366126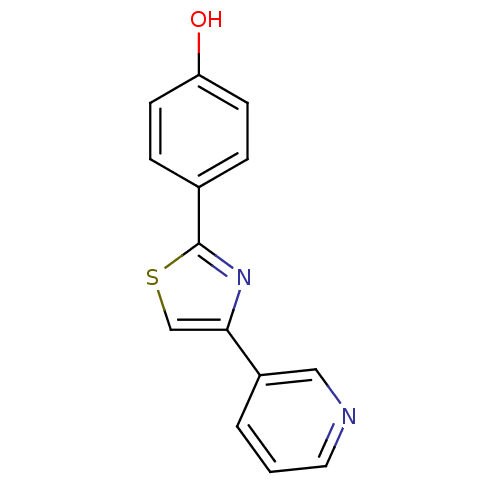 (CHEMBL1957215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive (Escherichia coli (strain K12)) | BDBM50350727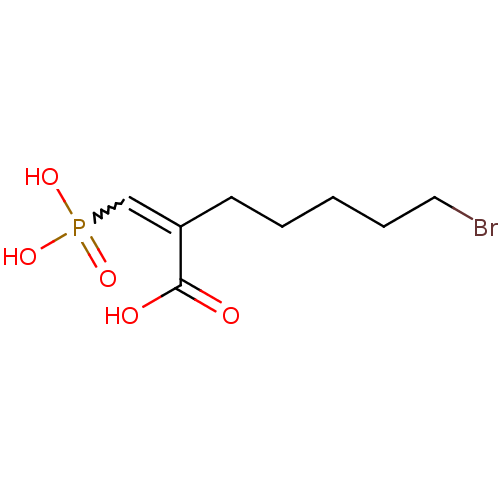 (CHEMBL1818159) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Escherichia coli DAH7P synthase using PEP as substrate by Michaelis-Menten equation analysis using UV-visible s... | Bioorg Med Chem Lett 21: 5092-7 (2011) Article DOI: 10.1016/j.bmcl.2011.03.071 BindingDB Entry DOI: 10.7270/Q2KW5GDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive (Escherichia coli (strain K12)) | BDBM50350723 (CHEMBL1255768 | CHEMBL1818156) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DAH7P synthase | Bioorg Med Chem Lett 21: 5092-7 (2011) Article DOI: 10.1016/j.bmcl.2011.03.071 BindingDB Entry DOI: 10.7270/Q2KW5GDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive (Escherichia coli (strain K12)) | BDBM50350725 (CHEMBL1818157 | CHEMBL1818158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Escherichia coli DAH7P synthase using PEP as substrate by Michaelis-Menten equation analysis using UV-visible s... | Bioorg Med Chem Lett 21: 5092-7 (2011) Article DOI: 10.1016/j.bmcl.2011.03.071 BindingDB Entry DOI: 10.7270/Q2KW5GDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366119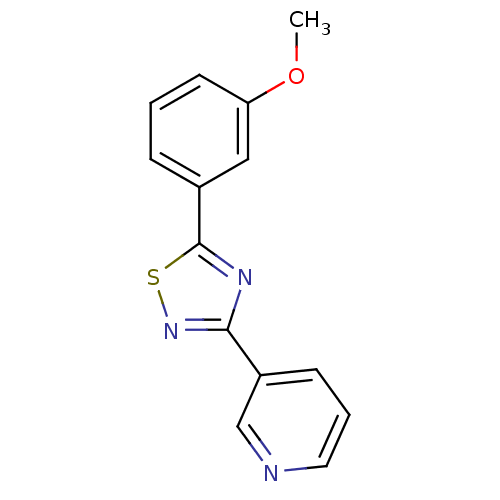 (CHEMBL1957209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93074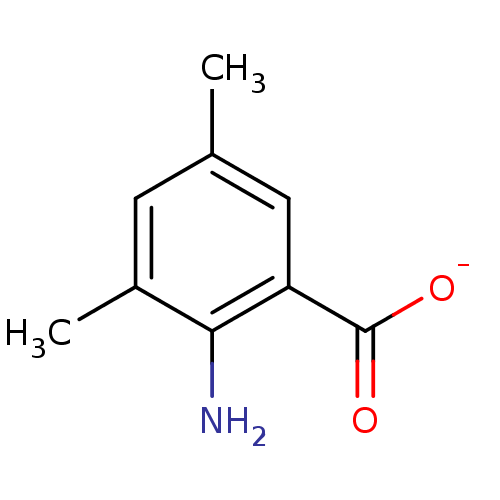 (ACS No 142) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-dehydro-3-deoxyphosphooctonate aldolase (Neisseria meningitidis serogroup B (strain MC58)) | BDBM50362546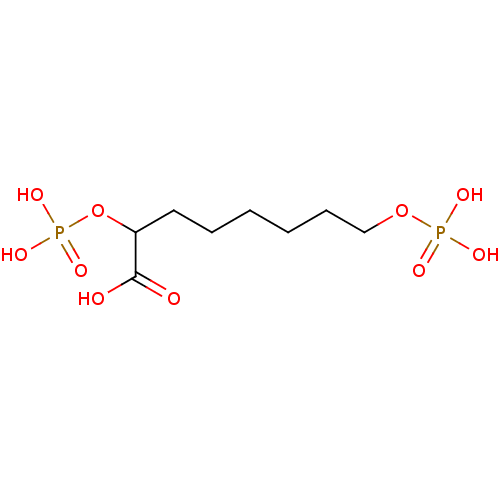 (CHEMBL1938424) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description Competitive inhibition of Neisseria meningitidis serotype B strain MC58 ATCC BAA355 KDO8P synthase expressed in Escherichia coli BL21 (DE3) cells usi... | Bioorg Med Chem Lett 22: 907-11 (2012) Article DOI: 10.1016/j.bmcl.2011.12.025 BindingDB Entry DOI: 10.7270/Q2N87B76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366121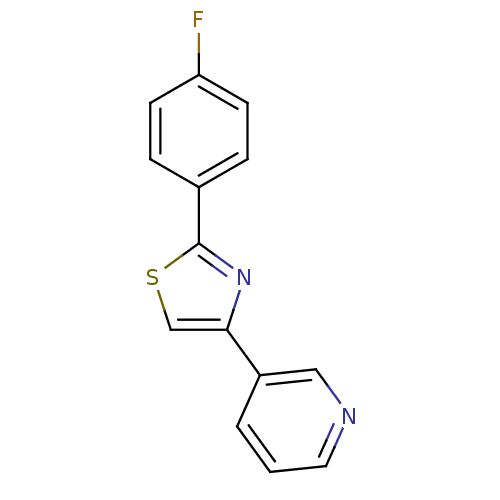 (CHEMBL1957210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366124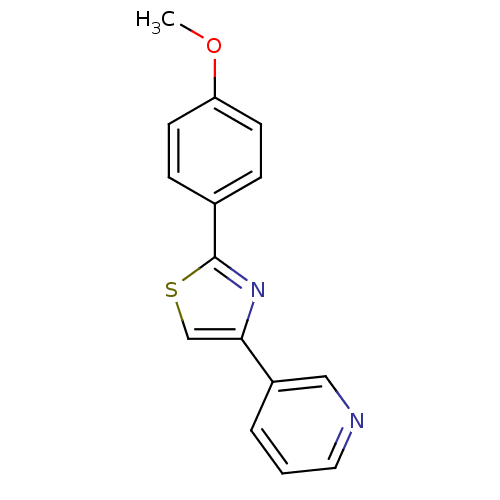 (CHEMBL1957213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93075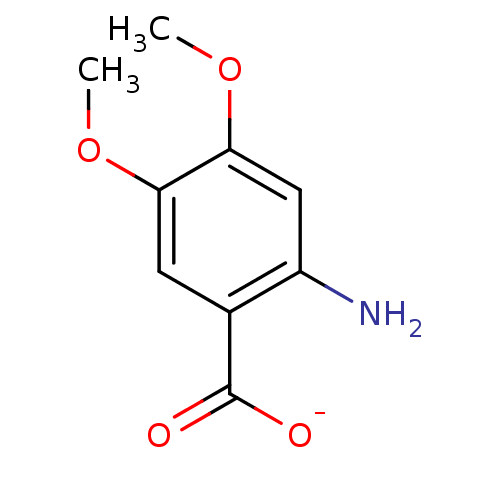 (ACS No 145) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93076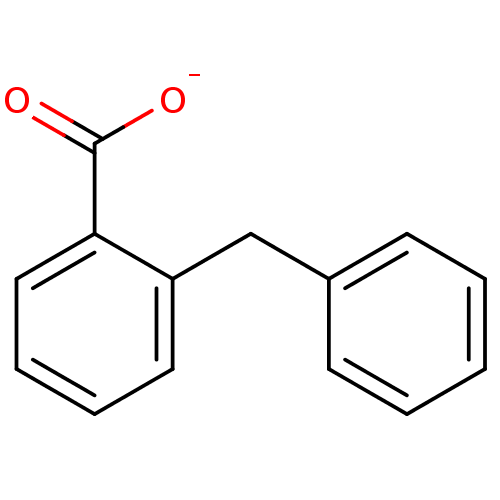 (ACS No 174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93077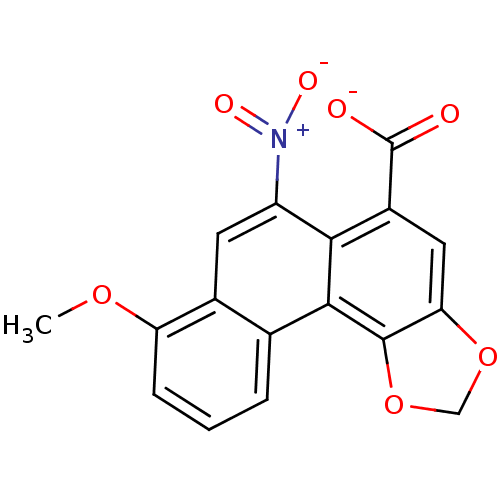 (ACS No 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93078 (ACS No 126) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93079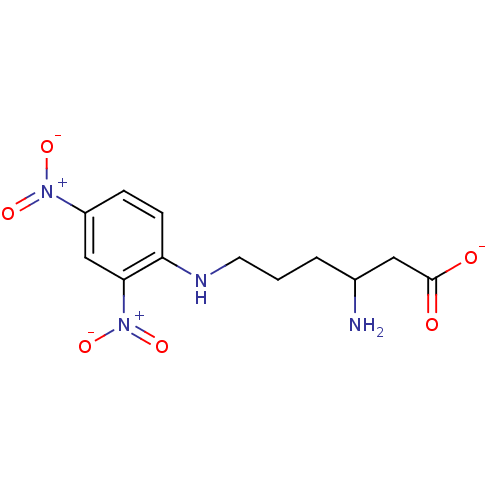 (ACS No 165) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM85513 (ACS No 10 | CAS_54-21-7 | NSC_5900 | Sodium salicy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive (Escherichia coli (strain K12)) | BDBM50350725 (CHEMBL1818157 | CHEMBL1818158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Escherichia coli DAH7P synthase using PEP as substrate by Michaelis-Menten equation analysis using UV-visible s... | Bioorg Med Chem Lett 21: 5092-7 (2011) Article DOI: 10.1016/j.bmcl.2011.03.071 BindingDB Entry DOI: 10.7270/Q2KW5GDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-dehydro-3-deoxyphosphooctonate aldolase (Neisseria meningitidis serogroup B (strain MC58)) | BDBM50281349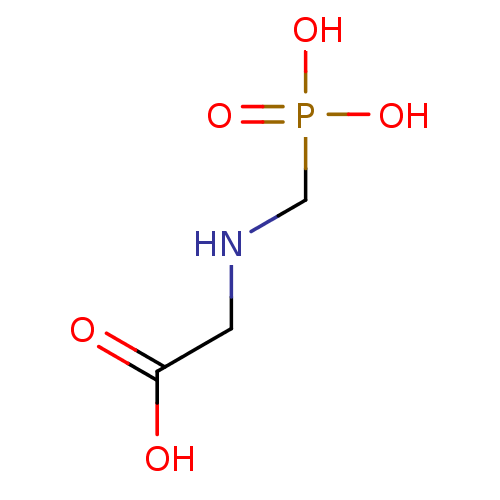 (CHEMBL95764 | N-(phosphonomethyl)glycine | glyphos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description Competitive inhibition of Neisseria meningitidis serotype B strain MC58 ATCC BAA355 KDO8P synthase expressed in Escherichia coli BL21 (DE3) cells usi... | Bioorg Med Chem Lett 22: 907-11 (2012) Article DOI: 10.1016/j.bmcl.2011.12.025 BindingDB Entry DOI: 10.7270/Q2N87B76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive (Escherichia coli (strain K12)) | BDBM50350723 (CHEMBL1255768 | CHEMBL1818156) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DAH7P synthase | Bioorg Med Chem Lett 21: 5092-7 (2011) Article DOI: 10.1016/j.bmcl.2011.03.071 BindingDB Entry DOI: 10.7270/Q2KW5GDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-dehydro-3-deoxyphosphooctonate aldolase (Neisseria meningitidis serogroup B (strain MC58)) | BDBM50362548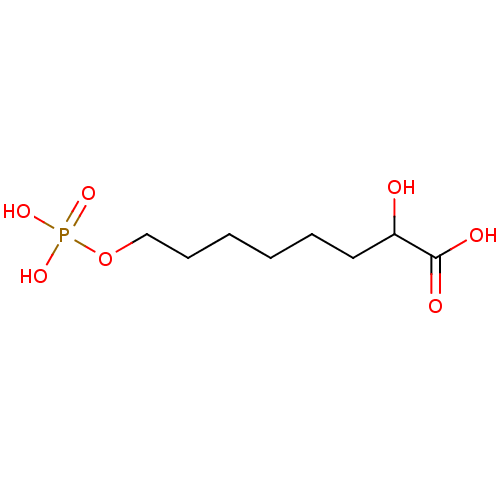 (CHEMBL1941140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description Competitive inhibition of Neisseria meningitidis serotype B strain MC58 ATCC BAA355 KDO8P synthase expressed in Escherichia coli BL21 (DE3) cells usi... | Bioorg Med Chem Lett 22: 907-11 (2012) Article DOI: 10.1016/j.bmcl.2011.12.025 BindingDB Entry DOI: 10.7270/Q2N87B76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-dehydro-3-deoxyphosphooctonate aldolase (Neisseria meningitidis serogroup B (strain MC58)) | BDBM50362544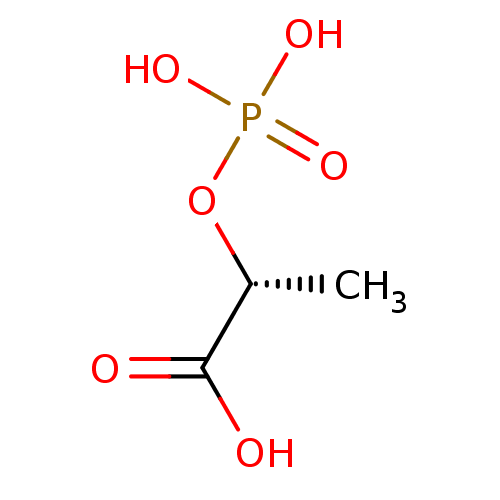 (CHEMBL1941138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 8.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description Competitive inhibition of Neisseria meningitidis serotype B strain MC58 ATCC BAA355 KDO8P synthase expressed in Escherichia coli BL21 (DE3) cells usi... | Bioorg Med Chem Lett 22: 907-11 (2012) Article DOI: 10.1016/j.bmcl.2011.12.025 BindingDB Entry DOI: 10.7270/Q2N87B76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1121 total ) | Next | Last >> |