

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50280516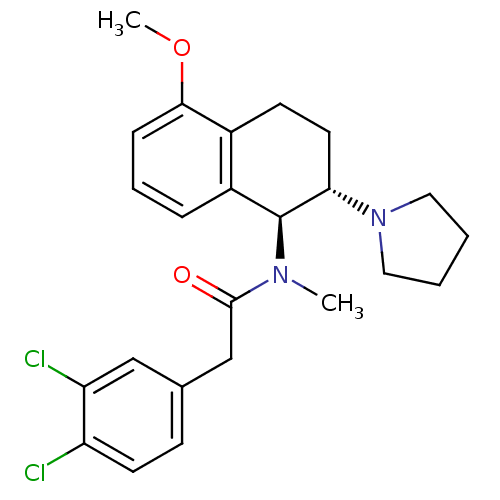 (2-(3,4-Dichloro-phenyl)-N-((1S,2S)-5-methoxy-2-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to kappa opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50280515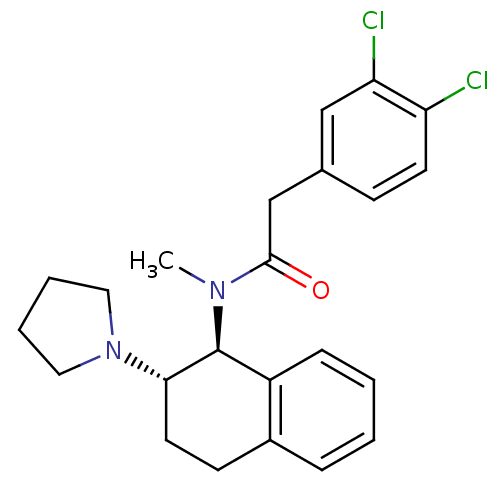 (2-(3,4-Dichloro-phenyl)-N-methyl-N-((1S,2S)-2-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to kappa opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to kappa opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to mu opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to delta opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50280516 (2-(3,4-Dichloro-phenyl)-N-((1S,2S)-5-methoxy-2-pyr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 304 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to mu opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50280515 (2-(3,4-Dichloro-phenyl)-N-methyl-N-((1S,2S)-2-pyrr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to mu opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | 696 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to sigma opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | 825 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to mu opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to kappa opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50280516 (2-(3,4-Dichloro-phenyl)-N-((1S,2S)-5-methoxy-2-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to delta opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50280516 (2-(3,4-Dichloro-phenyl)-N-((1S,2S)-5-methoxy-2-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to sigma opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50280515 (2-(3,4-Dichloro-phenyl)-N-methyl-N-((1S,2S)-2-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to sigma opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50280515 (2-(3,4-Dichloro-phenyl)-N-methyl-N-((1S,2S)-2-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to delta opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to sigma opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to delta opioid receptor | Bioorg Med Chem Lett 2: 715-720 (1992) Article DOI: 10.1016/S0960-894X(00)80398-4 BindingDB Entry DOI: 10.7270/Q27P8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037666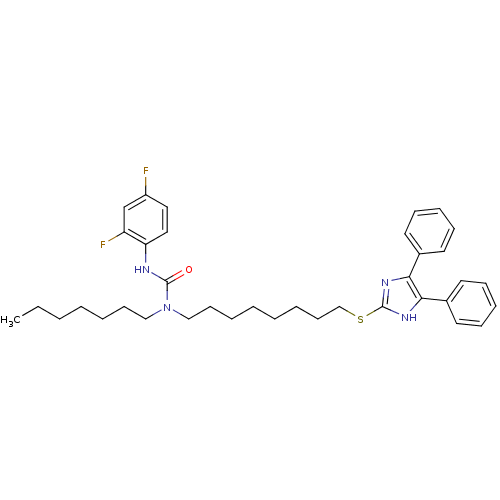 (3-(2,4-Difluoro-phenyl)-1-[8-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037663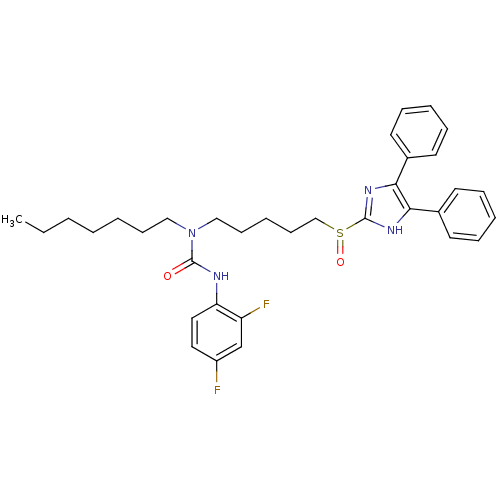 (3-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033969 (3-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of Acyl coenzyme A:cholesterol acyltransferase 1 in vitro using rat liver microsome. | J Med Chem 38: 1067-83 (1995) BindingDB Entry DOI: 10.7270/Q28S4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033969 (3-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033969 (3-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of Acyl coenzyme A:cholesterol acyltransferase 1 in vitro using rat liver microsome. | J Med Chem 38: 1067-83 (1995) BindingDB Entry DOI: 10.7270/Q28S4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037664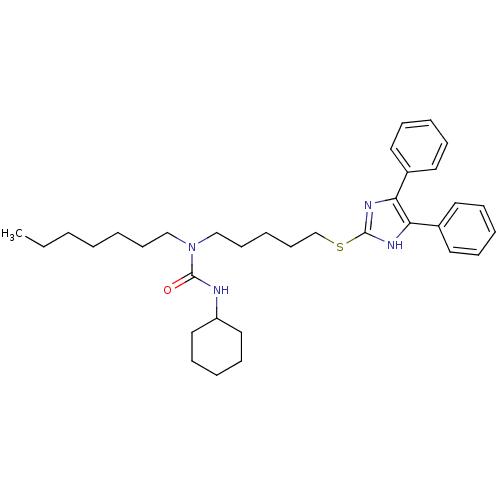 (3-Cyclohexyl-1-[5-(4,5-diphenyl-1H-imidazol-2-ylsu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037638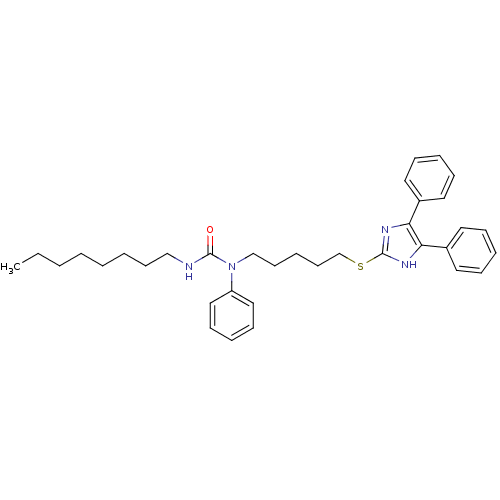 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037622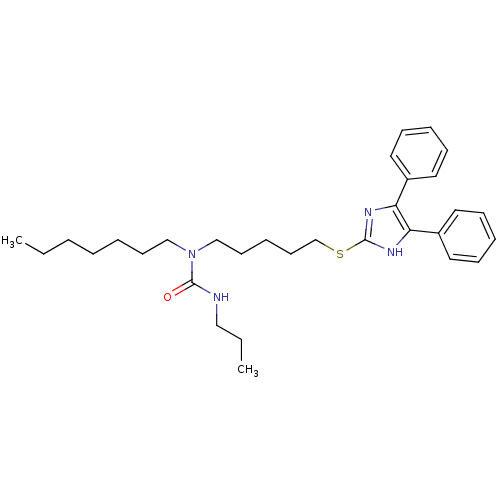 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033960 (CHEMBL11612 | N-{5-[4,5-Bis-(4-methoxy-phenyl)-1H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of Acyl coenzyme A:cholesterol acyltransferase 1 in vitro using rat liver microsome. | J Med Chem 38: 1067-83 (1995) BindingDB Entry DOI: 10.7270/Q28S4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037634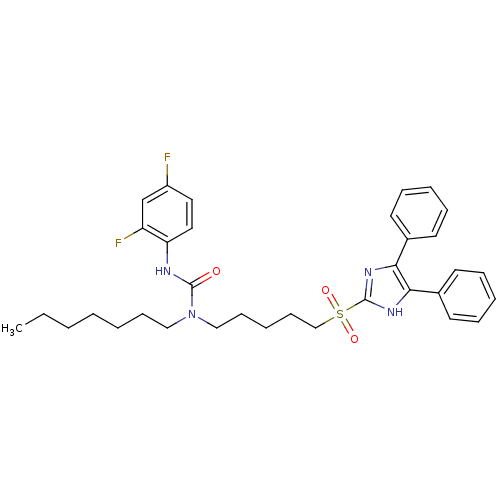 (3-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037625 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037637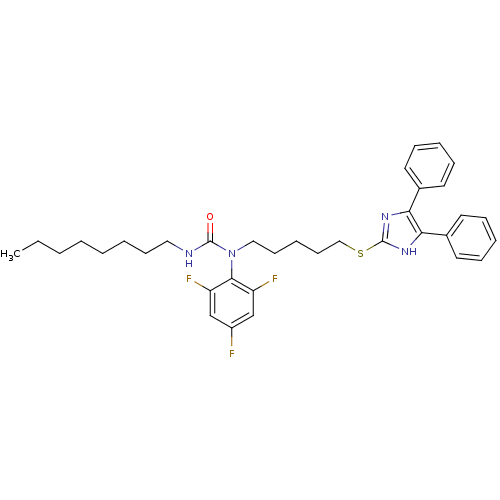 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037636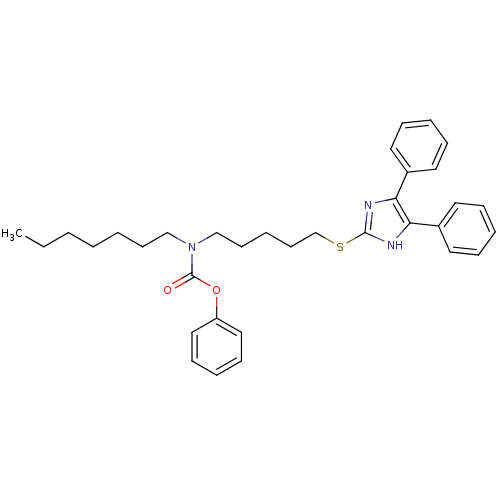 (CHEMBL119759 | [5-(4,5-Diphenyl-1H-imidazol-2-ylsu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033950 (3-(2,4-Difluoro-phenyl)-1-[5-(4,5-diisopropyl-1H-i...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of Acyl coenzyme A:cholesterol acyltransferase 1 in vitro using rat liver microsome. | J Med Chem 38: 1067-83 (1995) BindingDB Entry DOI: 10.7270/Q28S4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033949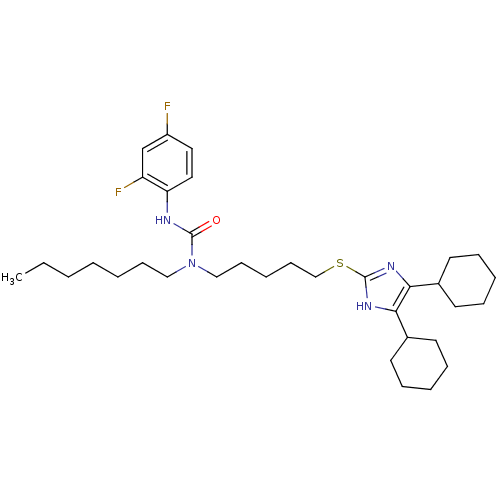 (1-[5-(4,5-Dicyclohexyl-1H-imidazol-2-ylsulfanyl)-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of Acyl coenzyme A:cholesterol acyltransferase 1 in vitro using rat liver microsome. | J Med Chem 38: 1067-83 (1995) BindingDB Entry DOI: 10.7270/Q28S4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037665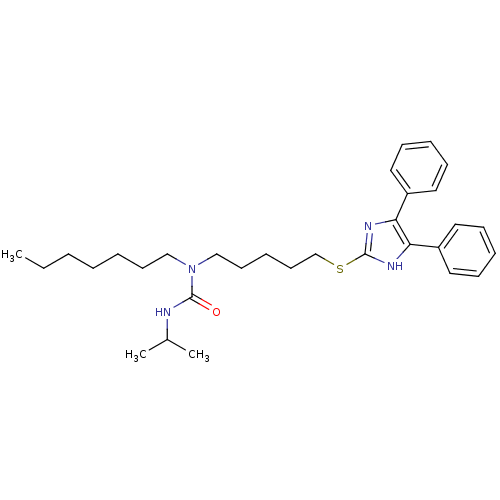 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037642 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037650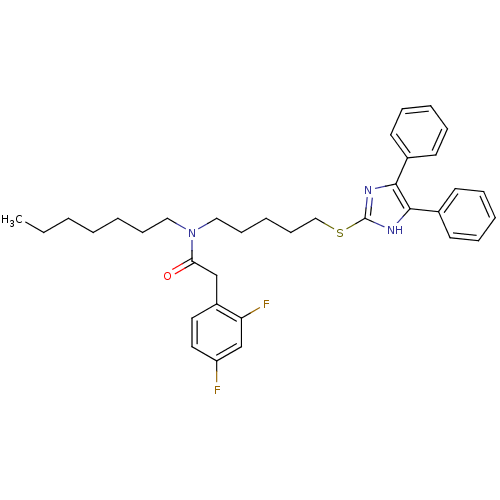 (2-(2,4-Difluoro-phenyl)-N-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037620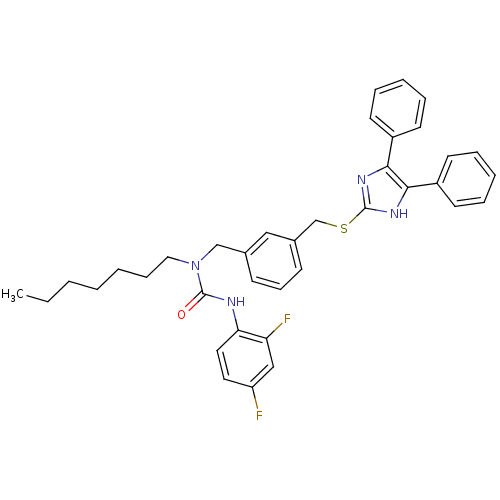 (3-(2,4-Difluoro-phenyl)-1-[3-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037653 (3-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037656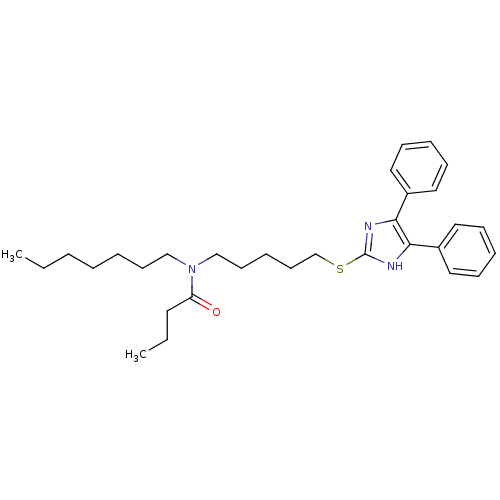 (CHEMBL115686 | N-[5-(4,5-Diphenyl-1H-imidazol-2-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037661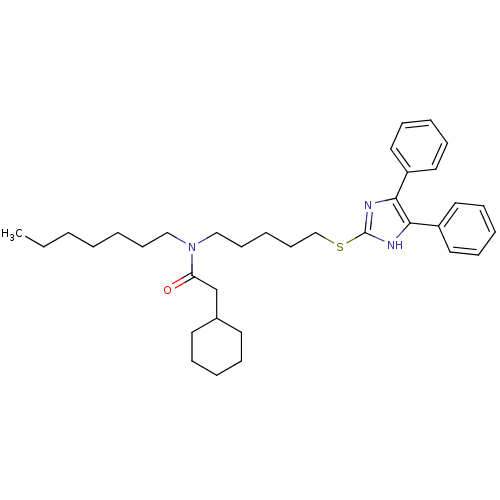 (2-Cyclohexyl-N-[5-(4,5-diphenyl-1H-imidazol-2-ylsu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037648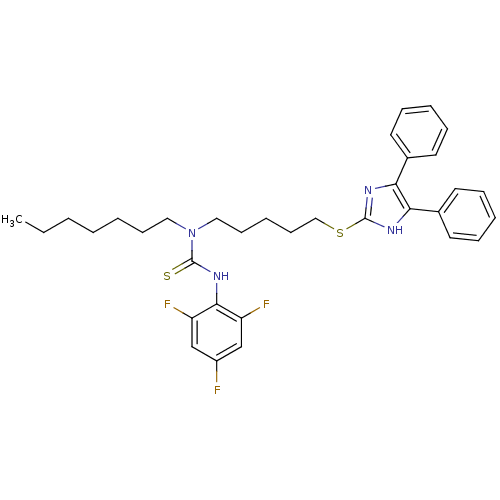 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033966 (CHEMBL11267 | N-[5-[(1H,9H-dibenzo[4,5:8,9] [1,3]d...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of Acyl coenzyme A:cholesterol acyltransferase 1 in vitro using rat liver microsome. | J Med Chem 38: 1067-83 (1995) BindingDB Entry DOI: 10.7270/Q28S4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033971 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of Acyl coenzyme A:cholesterol acyltransferase 1 in vitro using rat liver microsome. | J Med Chem 38: 1067-83 (1995) BindingDB Entry DOI: 10.7270/Q28S4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037624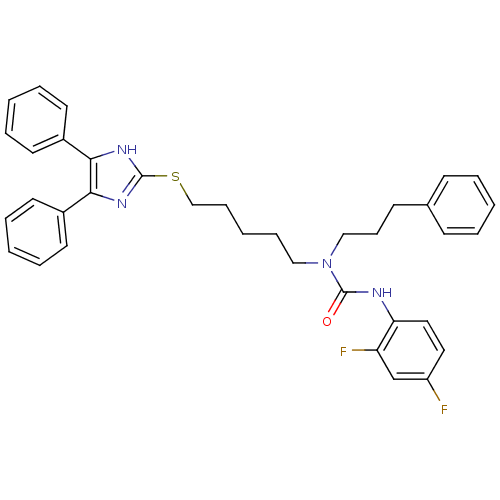 (3-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037623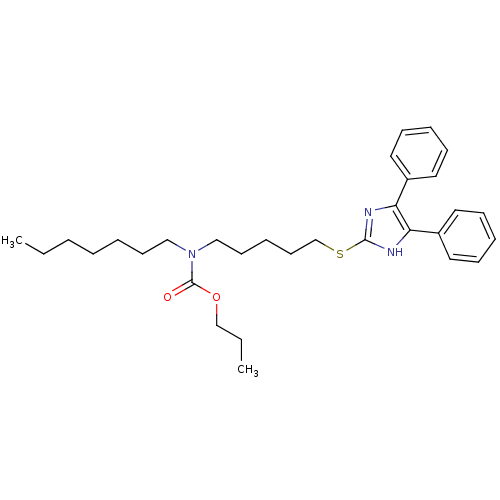 (CHEMBL119271 | [5-(4,5-Diphenyl-1H-imidazol-2-ylsu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033975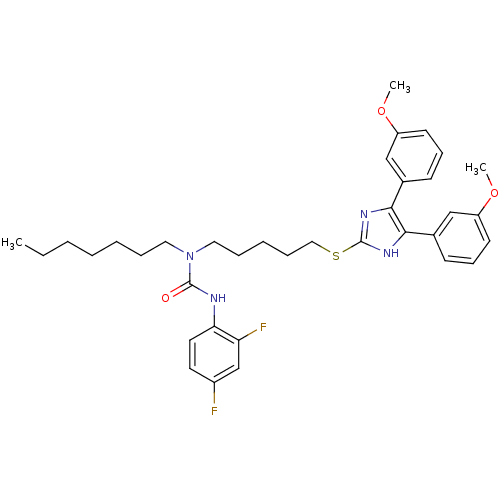 (1-{5-[4,5-Bis-(3-methoxy-phenyl)-1H-imidazol-2-yls...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of Acyl coenzyme A:cholesterol acyltransferase 1 in vitro using rat liver microsome. | J Med Chem 38: 1067-83 (1995) BindingDB Entry DOI: 10.7270/Q28S4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037654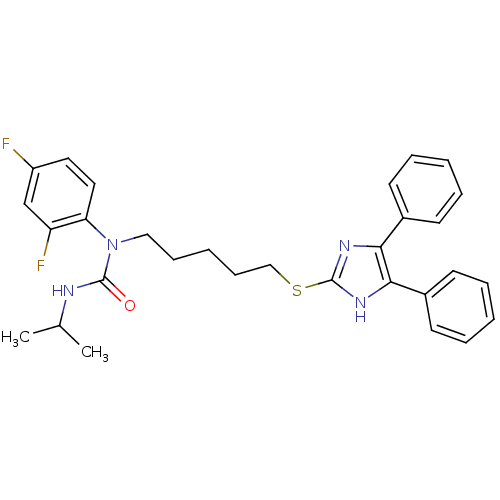 (1-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037628 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033998 (3-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of Acyl coenzyme A:cholesterol acyltransferase 1 in vitro using rat liver microsome. | J Med Chem 38: 1067-83 (1995) BindingDB Entry DOI: 10.7270/Q28S4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50033997 (1-{5-[4,5-Bis-(4-fluoro-phenyl)-1H-imidazol-2-ylsu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of Acyl coenzyme A:cholesterol acyltransferase 1 in vitro using rat liver microsome. | J Med Chem 38: 1067-83 (1995) BindingDB Entry DOI: 10.7270/Q28S4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037615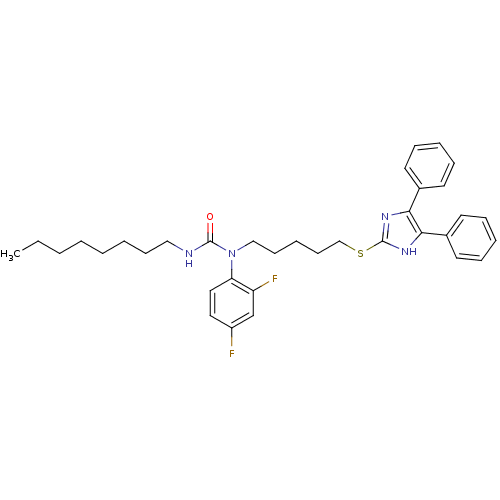 (1-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50037644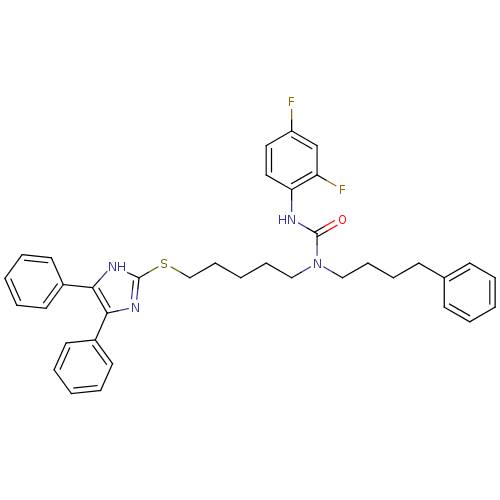 (3-(2,4-Difluoro-phenyl)-1-[5-(4,5-diphenyl-1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes | J Med Chem 37: 3511-22 (1994) BindingDB Entry DOI: 10.7270/Q2MK6BXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 120 total ) | Next | Last >> |