

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stromelysin-3 (Homo sapiens (Human)) | BDBM50138684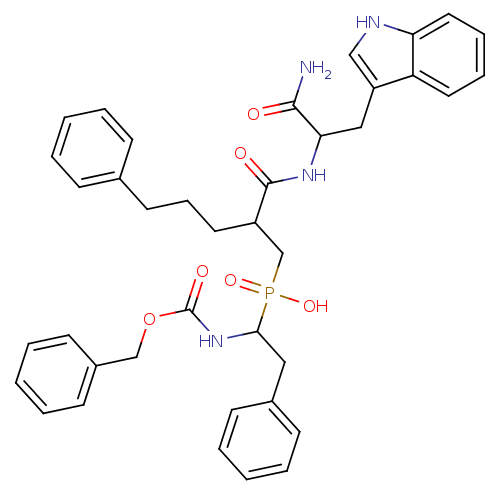 ((1-Benzyloxycarbonylamino-2-phenyl-ethyl)-{2-[1-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP11 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50138684 ((1-Benzyloxycarbonylamino-2-phenyl-ethyl)-{2-[1-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP9 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50180017 (14-methylcarbamoyl-2,8-dioxo-3-phenethyl-1,4,9-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP8 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50138684 ((1-Benzyloxycarbonylamino-2-phenyl-ethyl)-{2-[1-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP2 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50138684 ((1-Benzyloxycarbonylamino-2-phenyl-ethyl)-{2-[1-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP14 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50180017 (14-methylcarbamoyl-2,8-dioxo-3-phenethyl-1,4,9-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP1 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50180017 (14-methylcarbamoyl-2,8-dioxo-3-phenethyl-1,4,9-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP3 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50180017 (14-methylcarbamoyl-2,8-dioxo-3-phenethyl-1,4,9-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP9 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50180017 (14-methylcarbamoyl-2,8-dioxo-3-phenethyl-1,4,9-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP2 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212110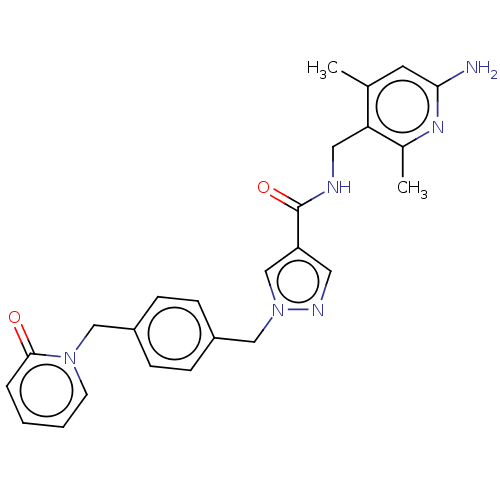 (US9290485, 142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0224 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212116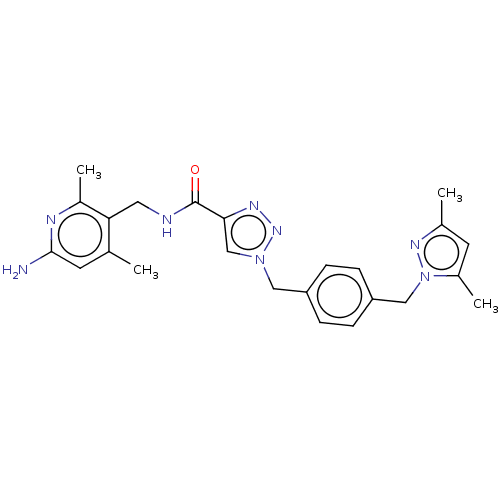 (US9290485, 148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212114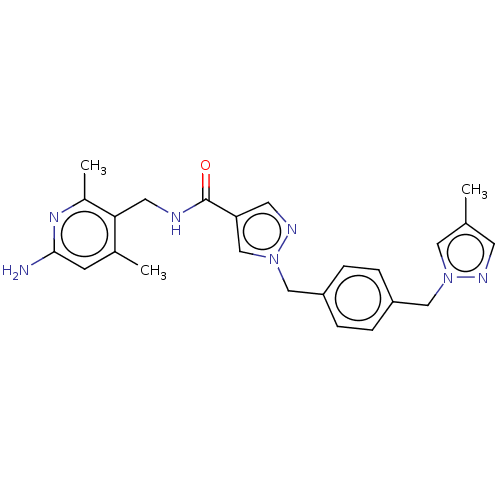 (US9290485, 146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM211977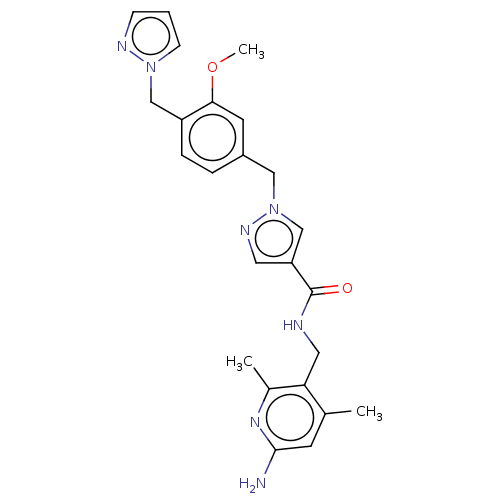 (US9290485, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0912 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212115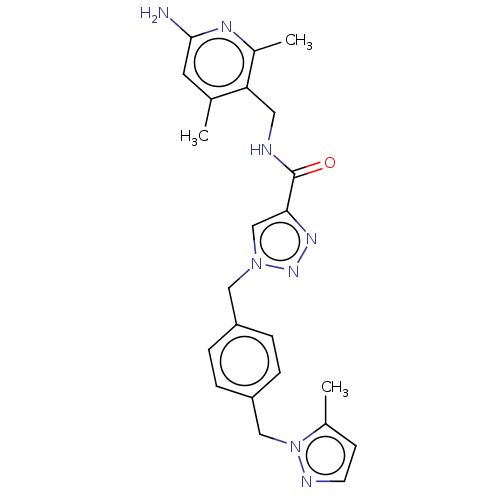 (US9290485, 147) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50104001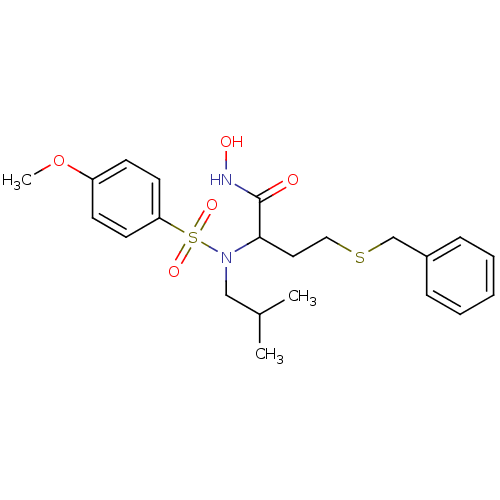 (4-(benzylthio)-N-hydroxy-2-(N-isobutyl-4-methoxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP9 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212136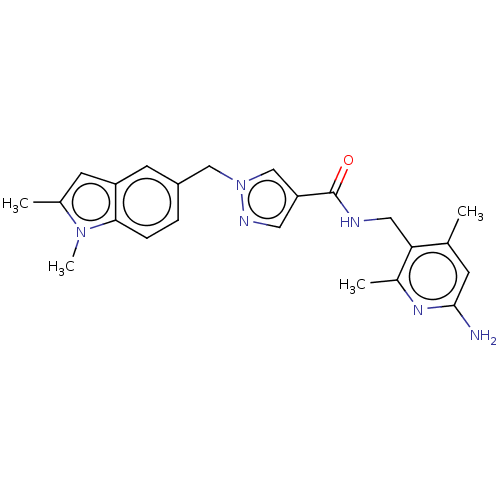 (US9290485, 168) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212004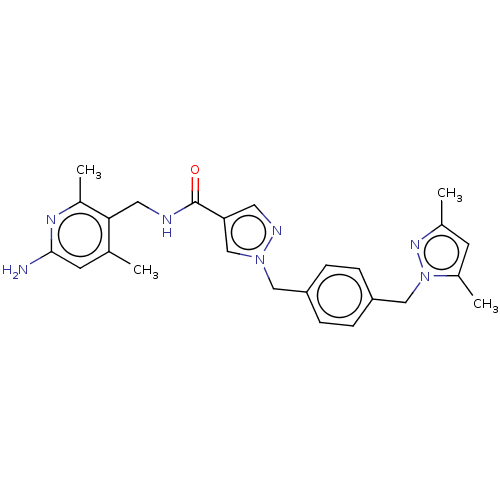 (US9290485, 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.134 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212135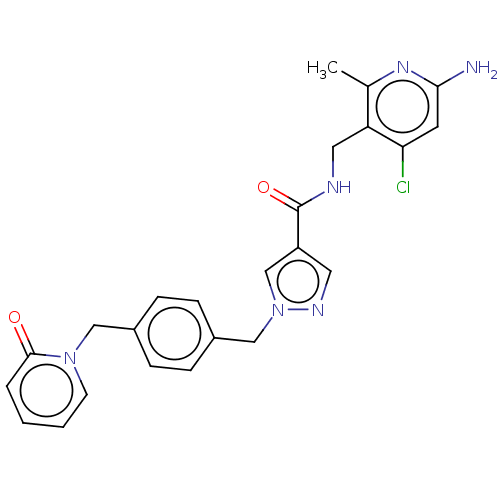 (US9290485, 167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212134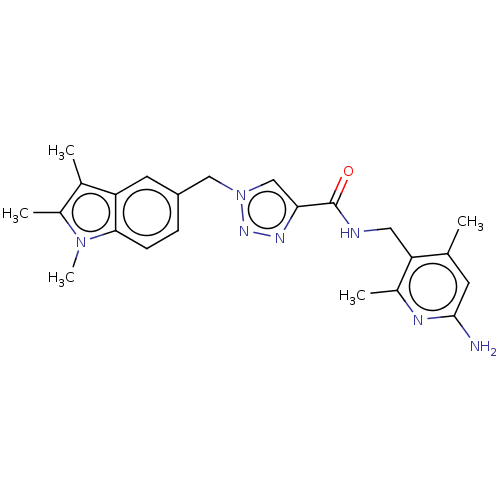 (US9290485, 166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM211974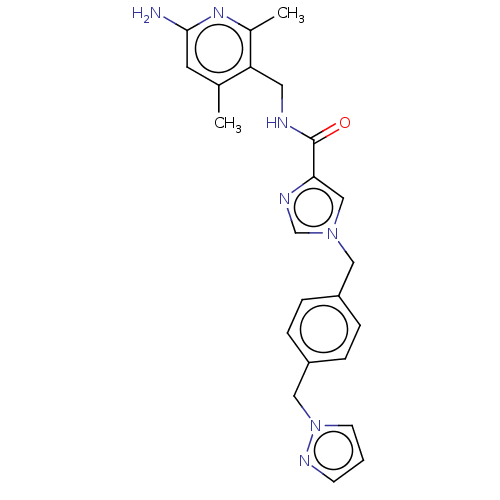 (US9290485, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212113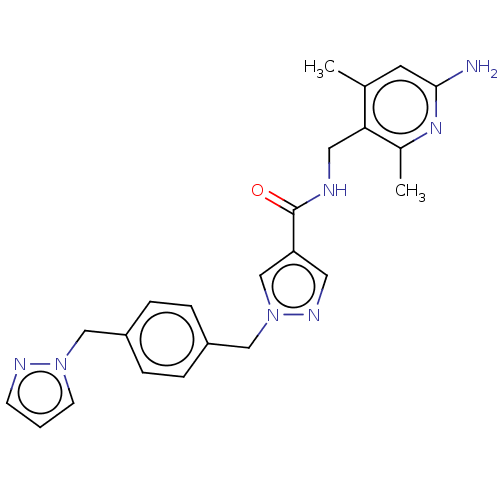 (US9290485, 145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212141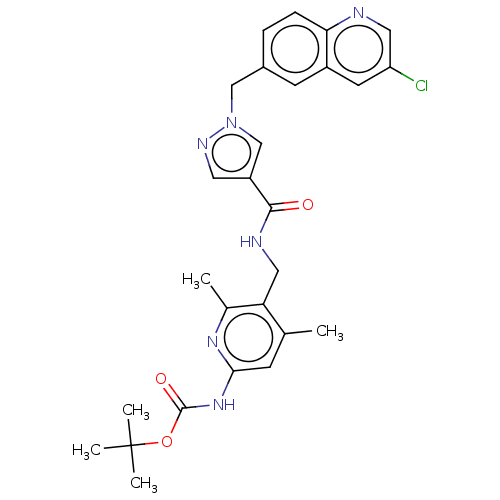 (US9290485, 173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50104001 (4-(benzylthio)-N-hydroxy-2-(N-isobutyl-4-methoxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP2 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50104001 (4-(benzylthio)-N-hydroxy-2-(N-isobutyl-4-methoxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP3 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212124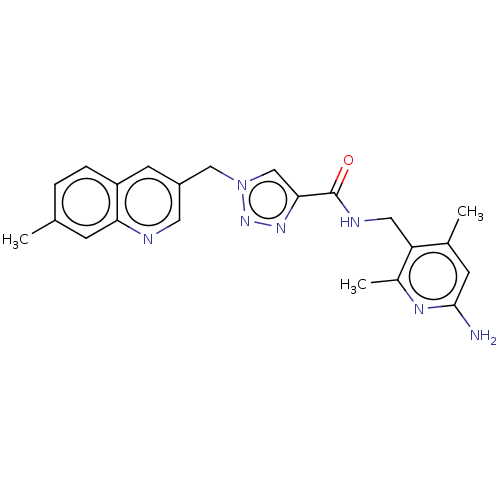 (US9290485, 156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212109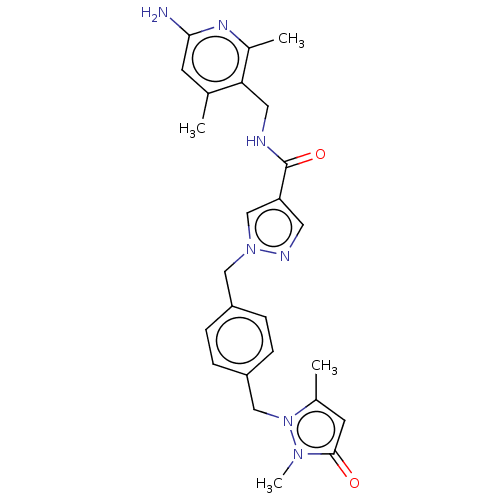 (US9290485, 141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212122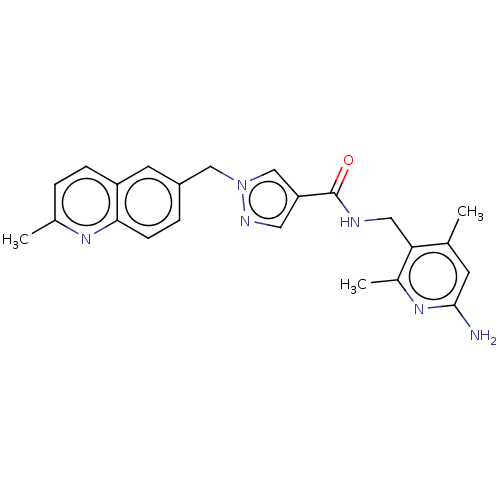 (US9290485, 154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50320214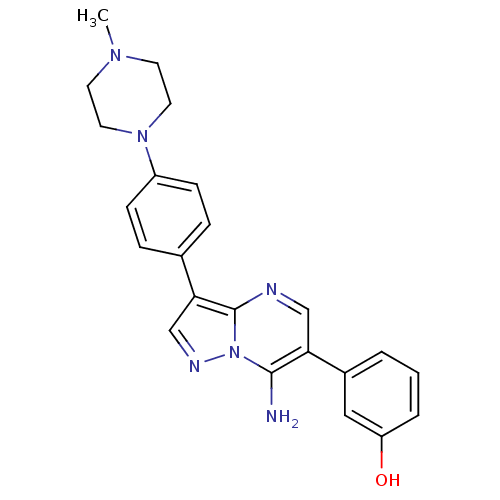 (3-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of SRC | Bioorg Med Chem Lett 20: 3628-31 (2010) Article DOI: 10.1016/j.bmcl.2010.04.112 BindingDB Entry DOI: 10.7270/Q2XP754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212125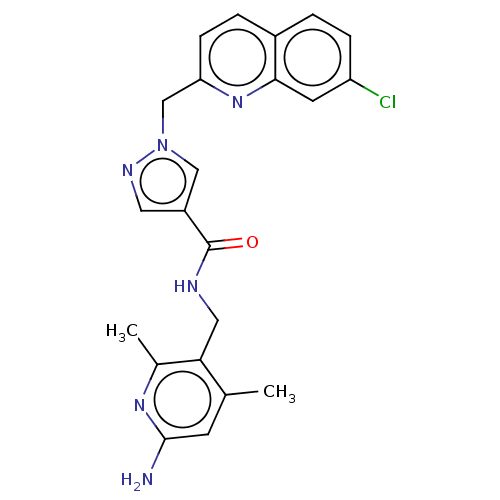 (US9290485, 157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212062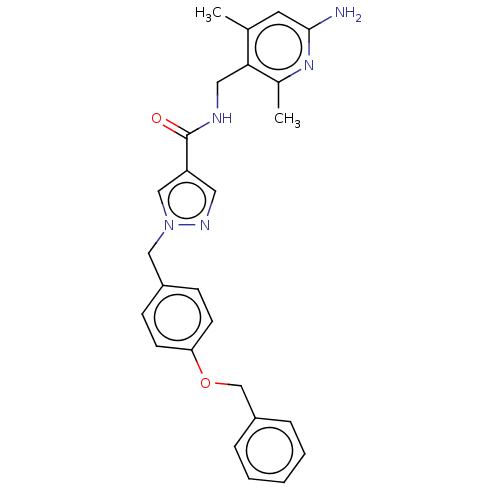 (US9290485, 94) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10487 (1-chloro-6H,7H,8H,9H,10H-cyclohepta[b]quinolin-11-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50138684 ((1-Benzyloxycarbonylamino-2-phenyl-ethyl)-{2-[1-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP8 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212111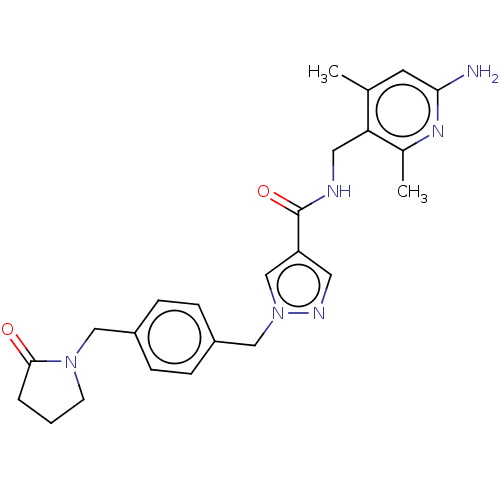 (US9290485, 143) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50180016 ((S)-2-((4-(4-bromobenzamido)phenylsulfonyl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP2 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50180016 ((S)-2-((4-(4-bromobenzamido)phenylsulfonyl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Binding affinity to MMP9 | J Med Chem 49: 51-69 (2006) Article DOI: 10.1021/jm050363f BindingDB Entry DOI: 10.7270/Q20K285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10501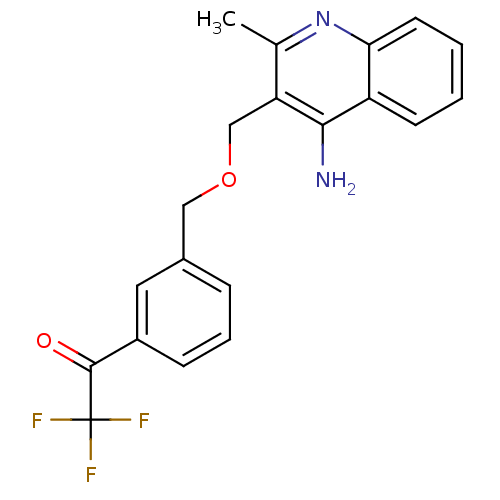 (1-(3-{[(4-amino-2-methylquinolin-3-yl)methoxy]meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10503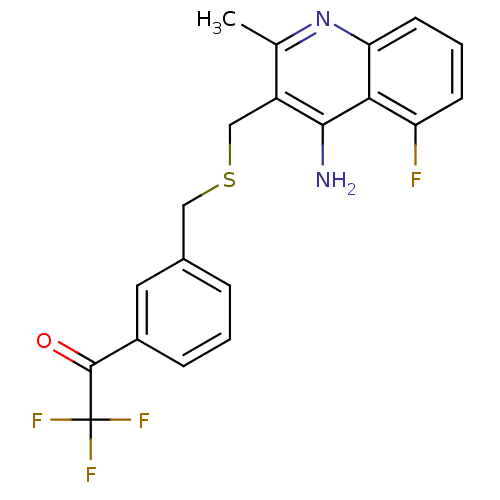 (1-[3-({[(4-amino-5-fluoro-2-methylquinolin-3-yl)me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212137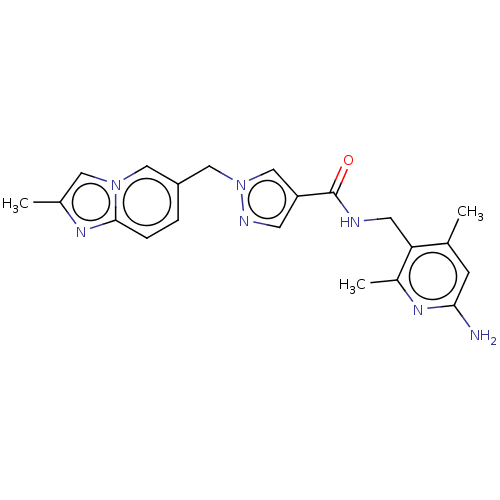 (US9290485, 169) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212123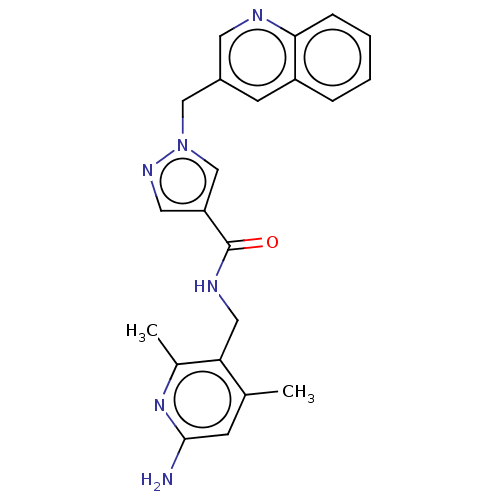 (US9290485, 155) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212043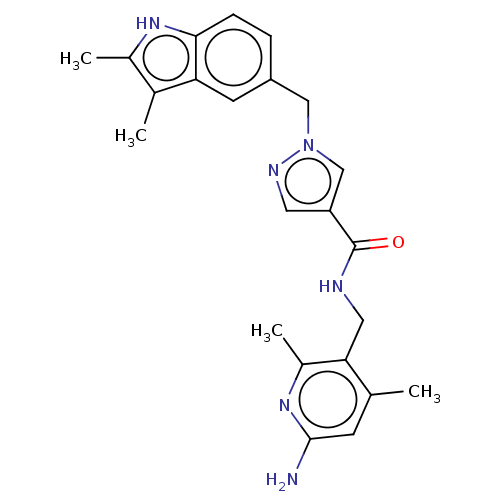 (US9290485, 75) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212083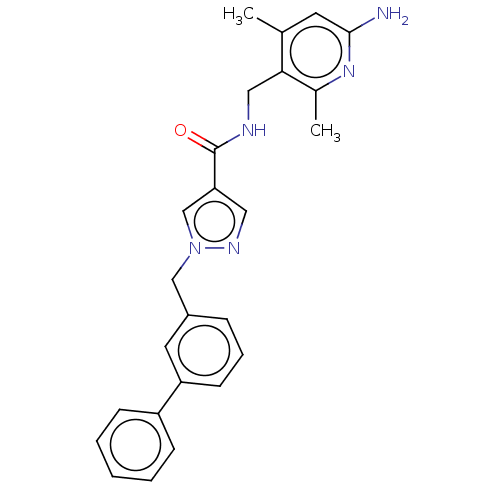 (US9290485, 115) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10499 (1-(3-{[(4-amino-5-fluoro-2-methylquinolin-3-yl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212120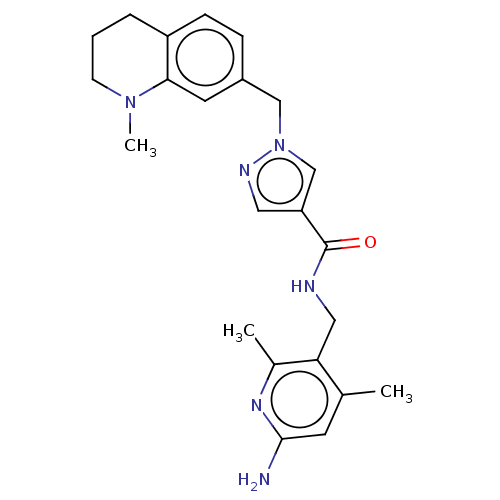 (US9290485, 152) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50320205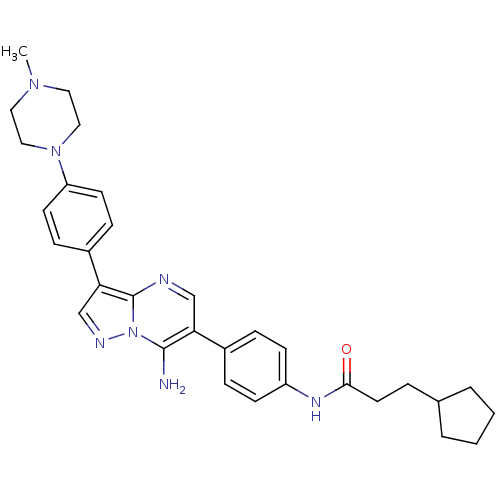 (CHEMBL1083001 | N-(4-(7-amino-3-(4-(4-methylpipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of LCK | Bioorg Med Chem Lett 20: 3628-31 (2010) Article DOI: 10.1016/j.bmcl.2010.04.112 BindingDB Entry DOI: 10.7270/Q2XP754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212126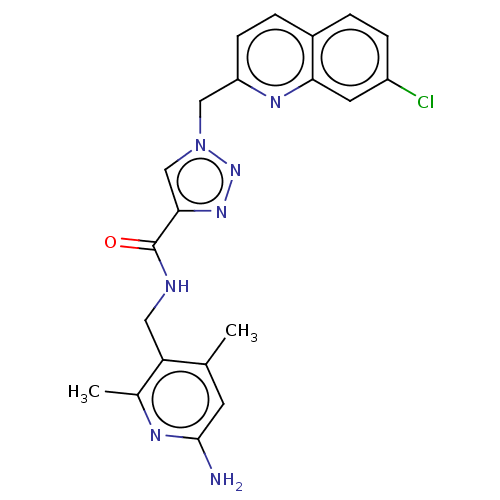 (US9290485, 158) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212129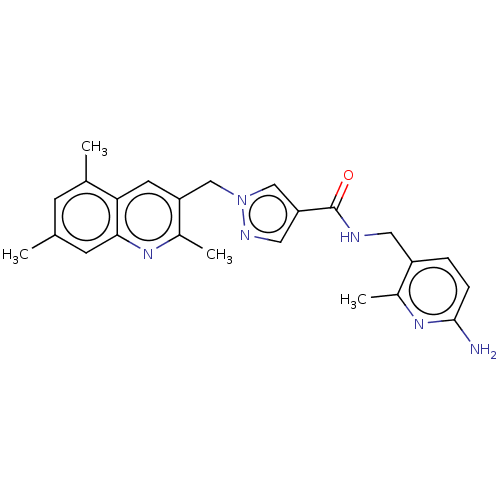 (US9290485, 161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50320225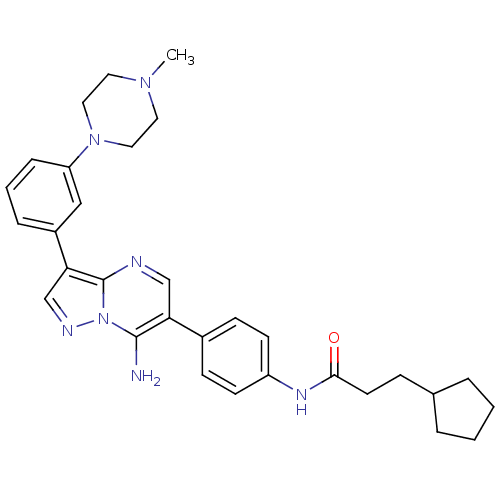 (CHEMBL1085316 | N-(4-(7-amino-3-(3-(4-methylpipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of LCK | Bioorg Med Chem Lett 20: 3628-31 (2010) Article DOI: 10.1016/j.bmcl.2010.04.112 BindingDB Entry DOI: 10.7270/Q2XP754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212108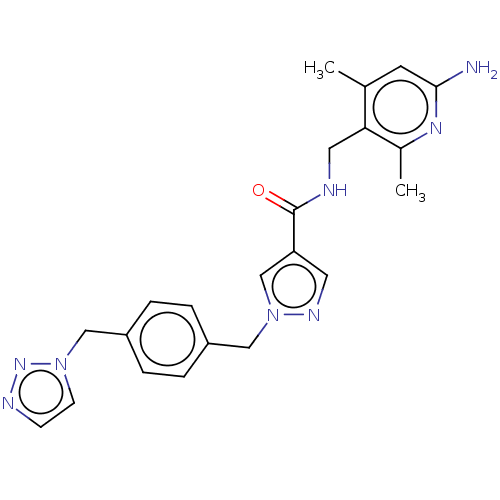 (US9290485, 140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212107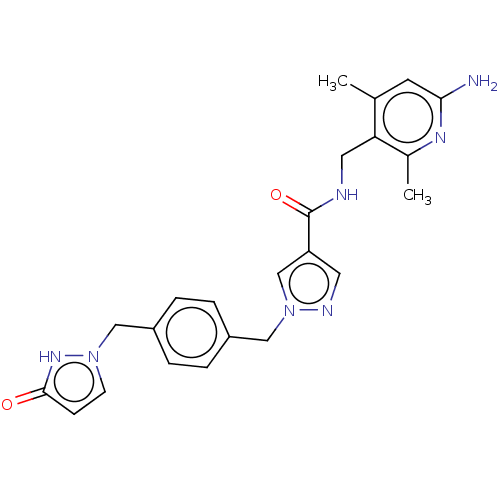 (US9290485, 139) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212130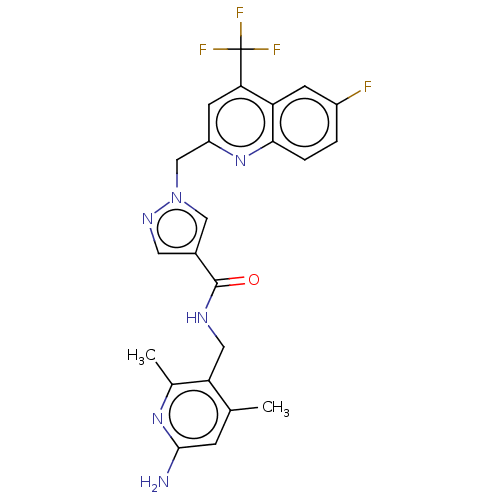 (US9290485, 162) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 438 total ) | Next | Last >> |