 Found 56 hits with Last Name = 'pobanz' and Initial = 'm'
Found 56 hits with Last Name = 'pobanz' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28206
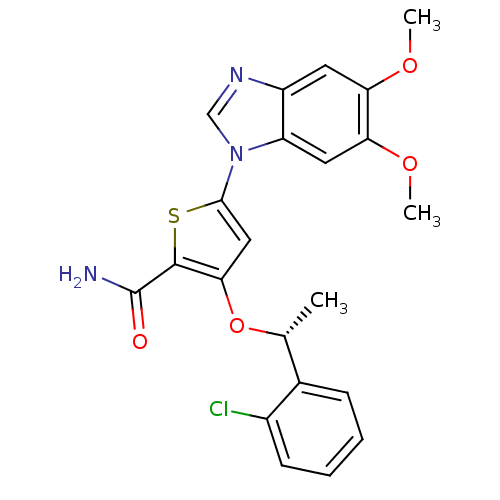
(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...)Show SMILES COc1cc2ncn(-c3cc(O[C@H](C)c4ccccc4Cl)c(s3)C(N)=O)c2cc1OC |r| Show InChI InChI=1S/C22H20ClN3O4S/c1-12(13-6-4-5-7-14(13)23)30-19-10-20(31-21(19)22(24)27)26-11-25-15-8-17(28-2)18(29-3)9-16(15)26/h4-12H,1-3H3,(H2,24,27)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28210
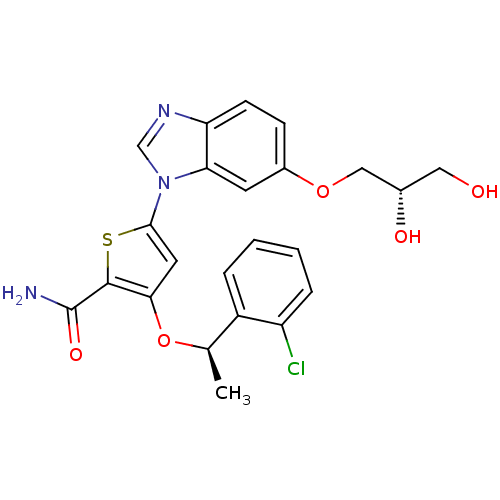
(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2,3-d...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OC[C@@H](O)CO)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C23H22ClN3O5S/c1-13(16-4-2-3-5-17(16)24)32-20-9-21(33-22(20)23(25)30)27-12-26-18-7-6-15(8-19(18)27)31-11-14(29)10-28/h2-9,12-14,28-29H,10-11H2,1H3,(H2,25,30)/t13-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28208

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-methoxy-1H-...)Show SMILES COc1ccc2ncn(-c3cc(O[C@H](C)c4ccccc4Cl)c(s3)C(N)=O)c2c1 |r| Show InChI InChI=1S/C21H18ClN3O3S/c1-12(14-5-3-4-6-15(14)22)28-18-10-19(29-20(18)21(23)26)25-11-24-16-8-7-13(27-2)9-17(16)25/h3-12H,1-2H3,(H2,23,26)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28216

(5-{6-[(1-methylpiperidin-4-yl)oxy]-1H-1,3-benzodia...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OC3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C27H27F3N4O3S/c1-16(19-5-3-4-6-20(19)27(28,29)30)36-23-14-24(38-25(23)26(31)35)34-15-32-21-8-7-18(13-22(21)34)37-17-9-11-33(2)12-10-17/h3-8,13-17H,9-12H2,1-2H3,(H2,31,35)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28178
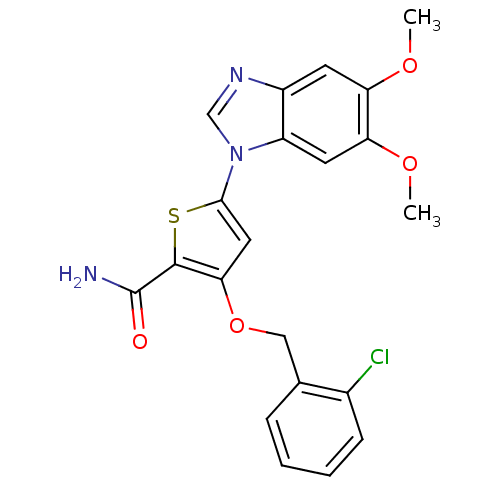
(3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4Cl)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C21H18ClN3O4S/c1-27-16-7-14-15(8-17(16)28-2)25(11-24-14)19-9-18(20(30-19)21(23)26)29-10-12-5-3-4-6-13(12)22/h3-9,11H,10H2,1-2H3,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28217
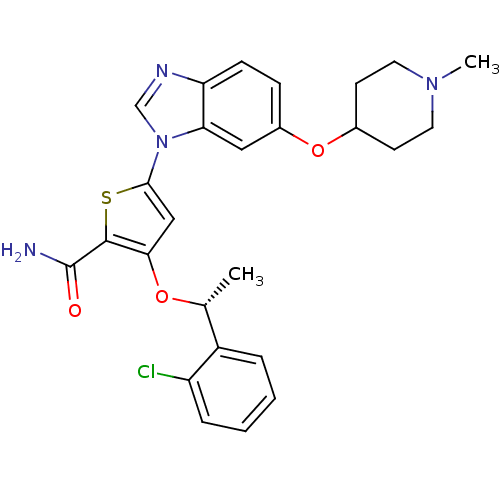
(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OC3CCN(C)CC3)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C26H27ClN4O3S/c1-16(19-5-3-4-6-20(19)27)33-23-14-24(35-25(23)26(28)32)31-15-29-21-8-7-18(13-22(21)31)34-17-9-11-30(2)12-10-17/h3-8,13-17H,9-12H2,1-2H3,(H2,28,32)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28218

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4S)-1-me...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(O[C@H]3CCCN(C)CC3)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C27H29ClN4O3S/c1-17(20-7-3-4-8-21(20)28)34-24-15-25(36-26(24)27(29)33)32-16-30-22-10-9-19(14-23(22)32)35-18-6-5-12-31(2)13-11-18/h3-4,7-10,14-18H,5-6,11-13H2,1-2H3,(H2,29,33)/t17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28209

(5-(6-methoxy-1H-1,3-benzodiazol-1-yl)-3-[(1R)-1-[2...)Show SMILES COc1ccc2ncn(-c3cc(O[C@H](C)c4ccccc4C(F)(F)F)c(s3)C(N)=O)c2c1 |r| Show InChI InChI=1S/C22H18F3N3O3S/c1-12(14-5-3-4-6-15(14)22(23,24)25)31-18-10-19(32-20(18)21(26)29)28-11-27-16-8-7-13(30-2)9-17(16)28/h3-12H,1-2H3,(H2,26,29)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28215
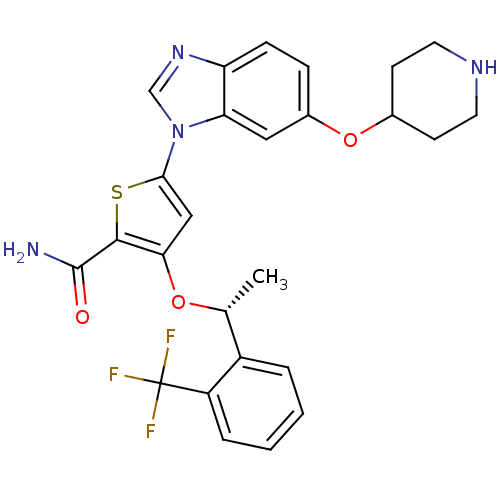
(5-[6-(piperidin-4-yloxy)-1H-1,3-benzodiazol-1-yl]-...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OC3CCNCC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C26H25F3N4O3S/c1-15(18-4-2-3-5-19(18)26(27,28)29)35-22-13-23(37-24(22)25(30)34)33-14-32-20-7-6-17(12-21(20)33)36-16-8-10-31-11-9-16/h2-7,12-16,31H,8-11H2,1H3,(H2,30,34)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28212
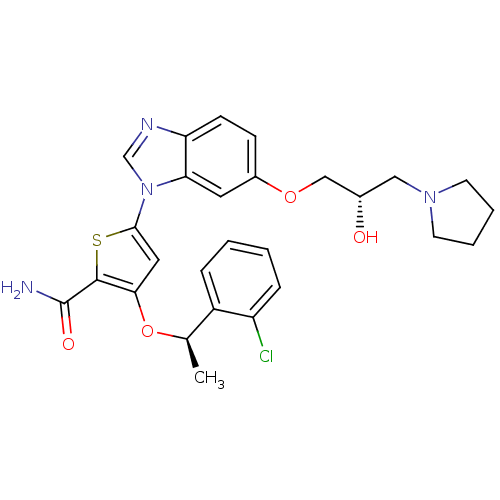
(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2-hyd...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OC[C@@H](O)CN3CCCC3)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C27H29ClN4O4S/c1-17(20-6-2-3-7-21(20)28)36-24-13-25(37-26(24)27(29)34)32-16-30-22-9-8-19(12-23(22)32)35-15-18(33)14-31-10-4-5-11-31/h2-3,6-9,12-13,16-18,33H,4-5,10-11,14-15H2,1H3,(H2,29,34)/t17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28219

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4R)-1-me...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(O[C@@H]3CCCN(C)CC3)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C27H29ClN4O3S/c1-17(20-7-3-4-8-21(20)28)34-24-15-25(36-26(24)27(29)33)32-16-30-22-10-9-19(14-23(22)32)35-18-6-5-12-31(2)13-11-18/h3-4,7-10,14-18H,5-6,11-13H2,1-2H3,(H2,29,33)/t17-,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28211
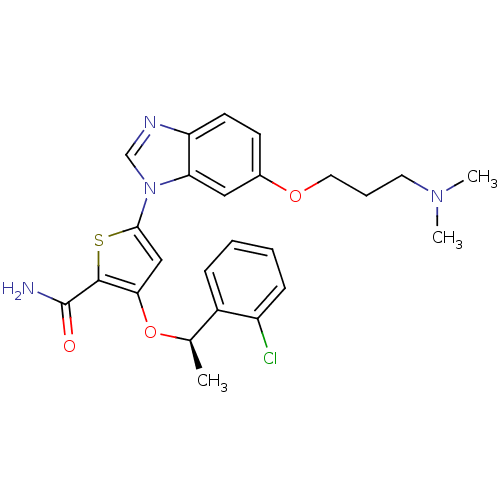
(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[3-(dimethy...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OCCCN(C)C)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C25H27ClN4O3S/c1-16(18-7-4-5-8-19(18)26)33-22-14-23(34-24(22)25(27)31)30-15-28-20-10-9-17(13-21(20)30)32-12-6-11-29(2)3/h4-5,7-10,13-16H,6,11-12H2,1-3H3,(H2,27,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28214

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OCC3CCN(C)CC3)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C27H29ClN4O3S/c1-17(20-5-3-4-6-21(20)28)35-24-14-25(36-26(24)27(29)33)32-16-30-22-8-7-19(13-23(22)32)34-15-18-9-11-31(2)12-10-18/h3-8,13-14,16-18H,9-12,15H2,1-2H3,(H2,29,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28206

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...)Show SMILES COc1cc2ncn(-c3cc(O[C@H](C)c4ccccc4Cl)c(s3)C(N)=O)c2cc1OC |r| Show InChI InChI=1S/C22H20ClN3O4S/c1-12(13-6-4-5-7-14(13)23)30-19-10-20(31-21(19)22(24)27)26-11-25-15-8-17(28-2)18(29-3)9-16(15)26/h4-12H,1-3H3,(H2,24,27)/t12-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28213
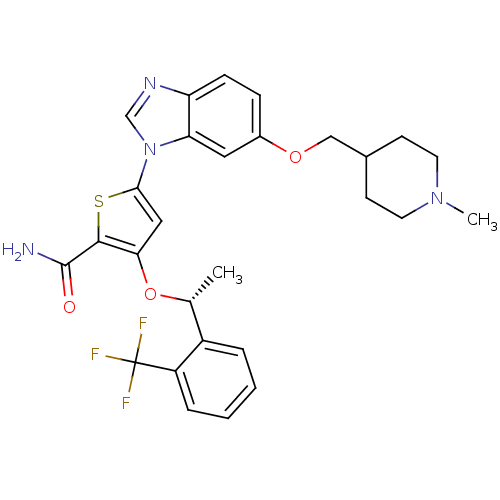
(5-{6-[(1-methylpiperidin-4-yl)methoxy]-1H-1,3-benz...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OCC3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C28H29F3N4O3S/c1-17(20-5-3-4-6-21(20)28(29,30)31)38-24-14-25(39-26(24)27(32)36)35-16-33-22-8-7-19(13-23(22)35)37-15-18-9-11-34(2)12-10-18/h3-8,13-14,16-18H,9-12,15H2,1-2H3,(H2,32,36)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28178

(3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4Cl)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C21H18ClN3O4S/c1-27-16-7-14-15(8-17(16)28-2)25(11-24-14)19-9-18(20(30-19)21(23)26)29-10-12-5-3-4-6-13(12)22/h3-9,11H,10H2,1-2H3,(H2,23,26) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28207

(3-[(1S)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...)Show SMILES COc1cc2ncn(-c3cc(O[C@@H](C)c4ccccc4Cl)c(s3)C(N)=O)c2cc1OC |r| Show InChI InChI=1S/C22H20ClN3O4S/c1-12(13-6-4-5-7-14(13)23)30-19-10-20(31-21(19)22(24)27)26-11-25-15-8-17(28-2)18(29-3)9-16(15)26/h4-12H,1-3H3,(H2,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM50275752

(5-methyl-7-(3-(trifluoromethyl)phenyl)-N-(3,4,5-tr...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4cccc(c4)C(F)(F)F)n3n2)cc(OC)c1OC Show InChI InChI=1S/C22H20F3N5O3/c1-12-16-11-26-21(28-15-9-17(31-2)19(33-4)18(10-15)32-3)29-30(16)20(27-12)13-6-5-7-14(8-13)22(23,24)25/h5-11H,1-4H3,(H,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK3 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275752

(5-methyl-7-(3-(trifluoromethyl)phenyl)-N-(3,4,5-tr...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4cccc(c4)C(F)(F)F)n3n2)cc(OC)c1OC Show InChI InChI=1S/C22H20F3N5O3/c1-12-16-11-26-21(28-15-9-17(31-2)19(33-4)18(10-15)32-3)29-30(16)20(27-12)13-6-5-7-14(8-13)22(23,24)25/h5-11H,1-4H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28208

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-methoxy-1H-...)Show SMILES COc1ccc2ncn(-c3cc(O[C@H](C)c4ccccc4Cl)c(s3)C(N)=O)c2c1 |r| Show InChI InChI=1S/C21H18ClN3O3S/c1-12(14-5-3-4-6-15(14)22)28-18-10-19(29-20(18)21(23)26)25-11-24-16-8-7-13(27-2)9-17(16)25/h3-12H,1-2H3,(H2,23,26)/t12-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275665
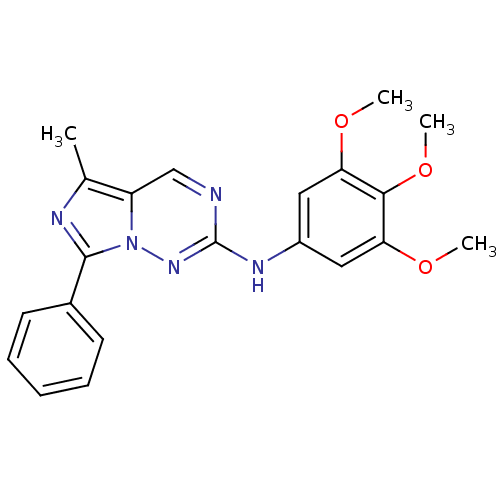
(5-methyl-7-phenyl-N-(3,4,5-trimethoxyphenyl)imidaz...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4ccccc4)n3n2)cc(OC)c1OC Show InChI InChI=1S/C21H21N5O3/c1-13-16-12-22-21(25-26(16)20(23-13)14-8-6-5-7-9-14)24-15-10-17(27-2)19(29-4)18(11-15)28-3/h5-12H,1-4H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28209

(5-(6-methoxy-1H-1,3-benzodiazol-1-yl)-3-[(1R)-1-[2...)Show SMILES COc1ccc2ncn(-c3cc(O[C@H](C)c4ccccc4C(F)(F)F)c(s3)C(N)=O)c2c1 |r| Show InChI InChI=1S/C22H18F3N3O3S/c1-12(14-5-3-4-6-15(14)22(23,24)25)31-18-10-19(32-20(18)21(26)29)28-11-27-16-8-7-13(30-2)9-17(16)28/h3-12H,1-2H3,(H2,26,29)/t12-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28207

(3-[(1S)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...)Show SMILES COc1cc2ncn(-c3cc(O[C@@H](C)c4ccccc4Cl)c(s3)C(N)=O)c2cc1OC |r| Show InChI InChI=1S/C22H20ClN3O4S/c1-12(13-6-4-5-7-14(13)23)30-19-10-20(31-21(19)22(24)27)26-11-25-15-8-17(28-2)18(29-3)9-16(15)26/h4-12H,1-3H3,(H2,24,27)/t12-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28210

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2,3-d...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OC[C@@H](O)CO)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C23H22ClN3O5S/c1-13(16-4-2-3-5-17(16)24)32-20-9-21(33-22(20)23(25)30)27-12-26-18-7-6-15(8-19(18)27)31-11-14(29)10-28/h2-9,12-14,28-29H,10-11H2,1H3,(H2,25,30)/t13-,14+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275702
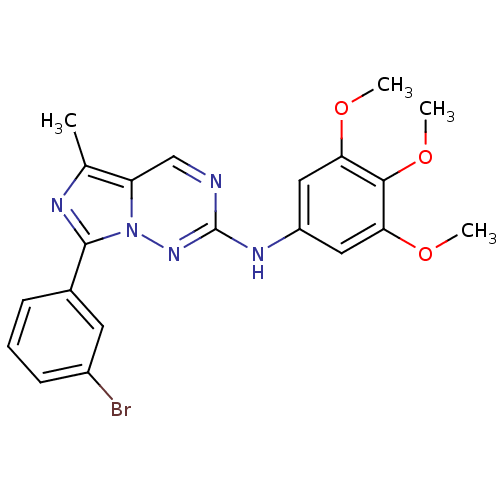
(7-(3-bromophenyl)-5-methyl-N-(3,4,5-trimethoxyphen...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4cccc(Br)c4)n3n2)cc(OC)c1OC Show InChI InChI=1S/C21H20BrN5O3/c1-12-16-11-23-21(25-15-9-17(28-2)19(30-4)18(10-15)29-3)26-27(16)20(24-12)13-6-5-7-14(22)8-13/h5-11H,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275806
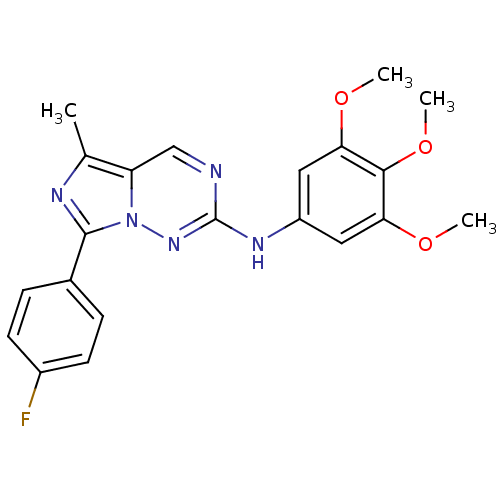
(7-(4-fluorophenyl)-5-methyl-N-(3,4,5-trimethoxyphe...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4ccc(F)cc4)n3n2)cc(OC)c1OC Show InChI InChI=1S/C21H20FN5O3/c1-12-16-11-23-21(25-15-9-17(28-2)19(30-4)18(10-15)29-3)26-27(16)20(24-12)13-5-7-14(22)8-6-13/h5-11H,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275664
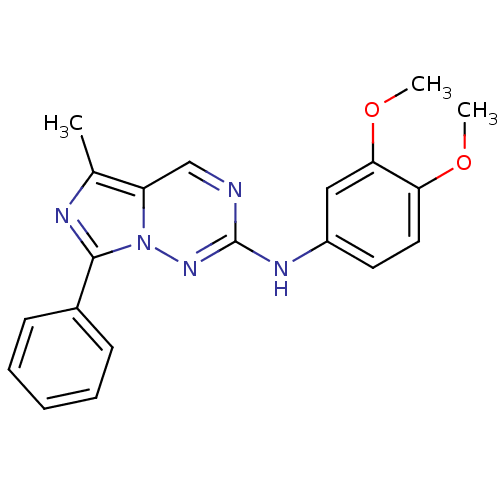
(CHEMBL521204 | N-(3,4-dimethoxyphenyl)-5-methyl-7-...)Show InChI InChI=1S/C20H19N5O2/c1-13-16-12-21-20(23-15-9-10-17(26-2)18(11-15)27-3)24-25(16)19(22-13)14-7-5-4-6-8-14/h4-12H,1-3H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM50275665

(5-methyl-7-phenyl-N-(3,4,5-trimethoxyphenyl)imidaz...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4ccccc4)n3n2)cc(OC)c1OC Show InChI InChI=1S/C21H21N5O3/c1-13-16-12-22-21(25-26(16)20(23-13)14-8-6-5-7-9-14)24-15-10-17(27-2)19(29-4)18(11-15)28-3/h5-12H,1-4H3,(H,24,25) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK3 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50275665

(5-methyl-7-phenyl-N-(3,4,5-trimethoxyphenyl)imidaz...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4ccccc4)n3n2)cc(OC)c1OC Show InChI InChI=1S/C21H21N5O3/c1-13-16-12-22-21(25-26(16)20(23-13)14-8-6-5-7-9-14)24-15-10-17(27-2)19(29-4)18(11-15)28-3/h5-12H,1-4H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin A (unknown origin) |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28218

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4S)-1-me...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(O[C@H]3CCCN(C)CC3)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C27H29ClN4O3S/c1-17(20-7-3-4-8-21(20)28)34-24-15-25(36-26(24)27(29)33)32-16-30-22-10-9-19(14-23(22)32)35-18-6-5-12-31(2)13-11-18/h3-4,7-10,14-18H,5-6,11-13H2,1-2H3,(H2,29,33)/t17-,18+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28219

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4R)-1-me...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(O[C@@H]3CCCN(C)CC3)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C27H29ClN4O3S/c1-17(20-7-3-4-8-21(20)28)34-24-15-25(36-26(24)27(29)33)32-16-30-22-10-9-19(14-23(22)32)35-18-6-5-12-31(2)13-11-18/h3-4,7-10,14-18H,5-6,11-13H2,1-2H3,(H2,29,33)/t17-,18-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28216

(5-{6-[(1-methylpiperidin-4-yl)oxy]-1H-1,3-benzodia...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OC3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C27H27F3N4O3S/c1-16(19-5-3-4-6-20(19)27(28,29)30)36-23-14-24(38-25(23)26(31)35)34-15-32-21-8-7-18(13-22(21)34)37-17-9-11-33(2)12-10-17/h3-8,13-17H,9-12H2,1-2H3,(H2,31,35)/t16-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275626
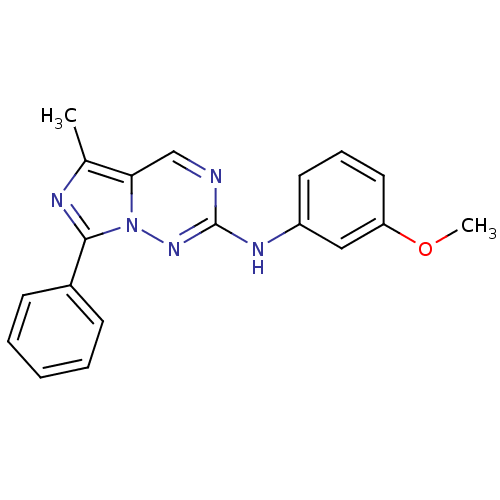
(CHEMBL487977 | N-(3-methoxyphenyl)-5-methyl-7-phen...)Show InChI InChI=1S/C19H17N5O/c1-13-17-12-20-19(22-15-9-6-10-16(11-15)25-2)23-24(17)18(21-13)14-7-4-3-5-8-14/h3-12H,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28215

(5-[6-(piperidin-4-yloxy)-1H-1,3-benzodiazol-1-yl]-...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OC3CCNCC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C26H25F3N4O3S/c1-15(18-4-2-3-5-19(18)26(27,28)29)35-22-13-23(37-24(22)25(30)34)33-14-32-20-7-6-17(12-21(20)33)36-16-8-10-31-11-9-16/h2-7,12-16,31H,8-11H2,1H3,(H2,30,34)/t15-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275628
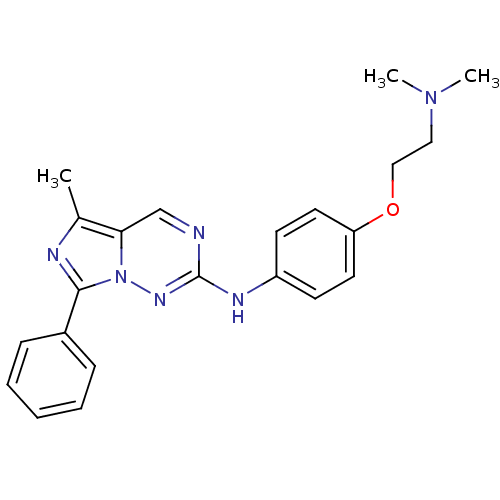
(CHEMBL520692 | N-(4-(2-(dimethylamino)ethoxy)pheny...)Show SMILES CN(C)CCOc1ccc(Nc2ncc3c(C)nc(-c4ccccc4)n3n2)cc1 Show InChI InChI=1S/C22H24N6O/c1-16-20-15-23-22(26-28(20)21(24-16)17-7-5-4-6-8-17)25-18-9-11-19(12-10-18)29-14-13-27(2)3/h4-12,15H,13-14H2,1-3H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275754
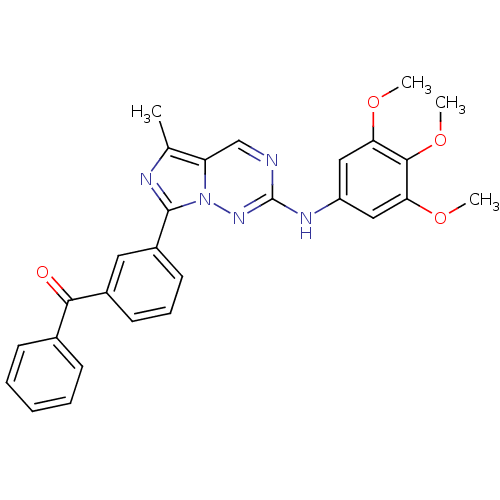
((3-(5-methyl-2-(3,4,5-trimethoxyphenylamino)imidaz...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4cccc(c4)C(=O)c4ccccc4)n3n2)cc(OC)c1OC Show InChI InChI=1S/C28H25N5O4/c1-17-22-16-29-28(31-21-14-23(35-2)26(37-4)24(15-21)36-3)32-33(22)27(30-17)20-12-8-11-19(13-20)25(34)18-9-6-5-7-10-18/h5-16H,1-4H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28211

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[3-(dimethy...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OCCCN(C)C)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C25H27ClN4O3S/c1-16(18-7-4-5-8-19(18)26)33-22-14-23(34-24(22)25(27)31)30-15-28-20-10-9-17(13-21(20)30)32-12-6-11-29(2)3/h4-5,7-10,13-16H,6,11-12H2,1-3H3,(H2,27,31)/t16-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275755
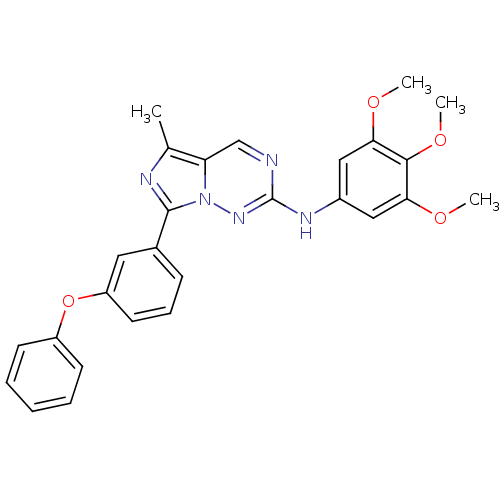
(5-methyl-7-(3-phenoxyphenyl)-N-(3,4,5-trimethoxyph...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4cccc(Oc5ccccc5)c4)n3n2)cc(OC)c1OC Show InChI InChI=1S/C27H25N5O4/c1-17-22-16-28-27(30-19-14-23(33-2)25(35-4)24(15-19)34-3)31-32(22)26(29-17)18-9-8-12-21(13-18)36-20-10-6-5-7-11-20/h5-16H,1-4H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28213

(5-{6-[(1-methylpiperidin-4-yl)methoxy]-1H-1,3-benz...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OCC3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C28H29F3N4O3S/c1-17(20-5-3-4-6-21(20)28(29,30)31)38-24-14-25(39-26(24)27(32)36)35-16-33-22-8-7-19(13-23(22)35)37-15-18-9-11-34(2)12-10-18/h3-8,13-14,16-18H,9-12,15H2,1-2H3,(H2,32,36)/t17-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28212

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2-hyd...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OC[C@@H](O)CN3CCCC3)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C27H29ClN4O4S/c1-17(20-6-2-3-7-21(20)28)36-24-13-25(37-26(24)27(29)34)32-16-30-22-9-8-19(12-23(22)32)35-15-18(33)14-31-10-4-5-11-31/h2-3,6-9,12-13,16-18,33H,4-5,10-11,14-15H2,1H3,(H2,29,34)/t17-,18+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28217

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OC3CCN(C)CC3)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C26H27ClN4O3S/c1-16(19-5-3-4-6-20(19)27)33-23-14-24(35-25(23)26(28)32)31-15-29-21-8-7-18(13-22(21)31)34-17-9-11-30(2)12-10-17/h3-8,13-17H,9-12H2,1-2H3,(H2,28,32)/t16-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275627
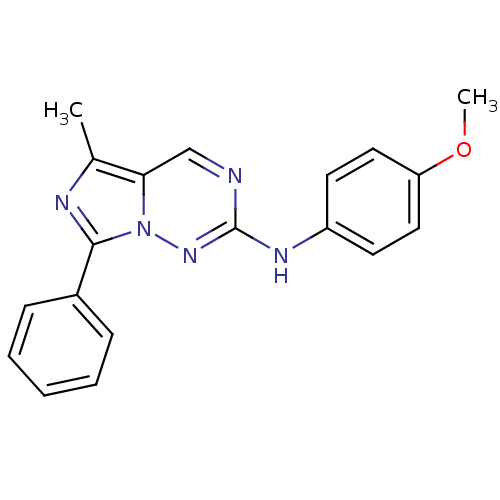
(CHEMBL519257 | N-(4-methoxyphenyl)-5-methyl-7-phen...)Show InChI InChI=1S/C19H17N5O/c1-13-17-12-20-19(22-15-8-10-16(25-2)11-9-15)23-24(17)18(21-13)14-6-4-3-5-7-14/h3-12H,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275753

(5-methyl-7-(4-(trifluoromethyl)phenyl)-N-(3,4,5-tr...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4ccc(cc4)C(F)(F)F)n3n2)cc(OC)c1OC Show InChI InChI=1S/C22H20F3N5O3/c1-12-16-11-26-21(28-15-9-17(31-2)19(33-4)18(10-15)32-3)29-30(16)20(27-12)13-5-7-14(8-6-13)22(23,24)25/h5-11H,1-4H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM28214

(3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(OCC3CCN(C)CC3)cc12)c1ccccc1Cl |r| Show InChI InChI=1S/C27H29ClN4O3S/c1-17(20-5-3-4-6-21(20)28)35-24-14-25(36-26(24)27(29)33)32-16-30-22-8-7-19(13-23(22)32)34-15-18-9-11-31(2)12-10-18/h3-8,13-14,16-18H,9-12,15H2,1-2H3,(H2,29,33)/t17-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1694-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.094
BindingDB Entry DOI: 10.7270/Q2319T6X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275625
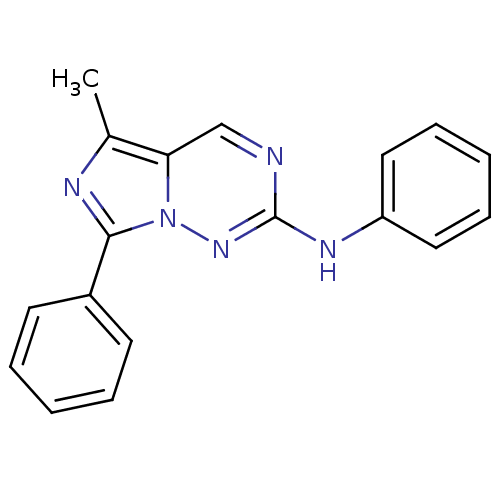
(5-methyl-N,7-diphenylimidazo[1,5-f][1,2,4]triazin-...)Show InChI InChI=1S/C18H15N5/c1-13-16-12-19-18(21-15-10-6-3-7-11-15)22-23(16)17(20-13)14-8-4-2-5-9-14/h2-12H,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275589

(CHEMBL519225 | N-benzyl-5-methyl-7-phenylimidazo[1...)Show InChI InChI=1S/C19H17N5/c1-14-17-13-21-19(20-12-15-8-4-2-5-9-15)23-24(17)18(22-14)16-10-6-3-7-11-16/h2-11,13H,12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275700
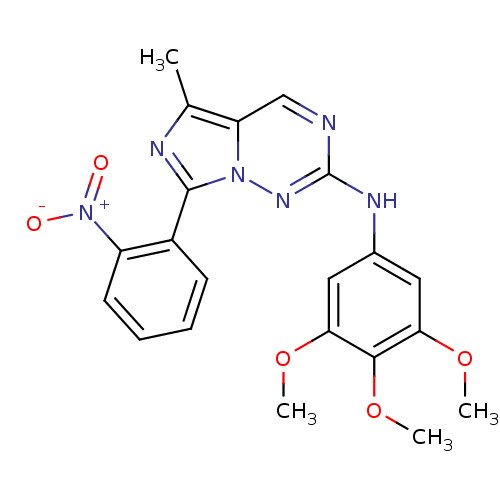
(5-methyl-7-(2-nitrophenyl)-N-(3,4,5-trimethoxyphen...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4ccccc4[N+]([O-])=O)n3n2)cc(OC)c1OC Show InChI InChI=1S/C21H20N6O5/c1-12-16-11-22-21(24-13-9-17(30-2)19(32-4)18(10-13)31-3)25-26(16)20(23-12)14-7-5-6-8-15(14)27(28)29/h5-11H,1-4H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275701
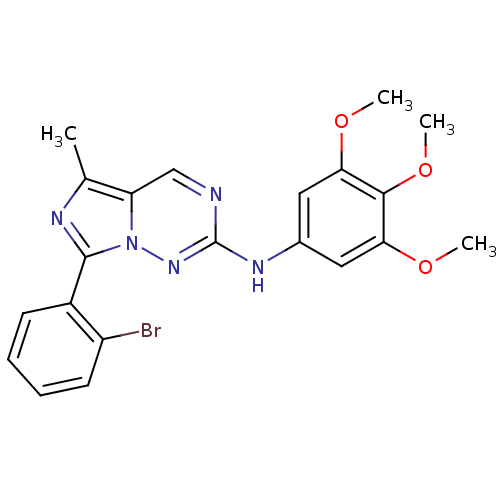
(7-(2-bromophenyl)-5-methyl-N-(3,4,5-trimethoxyphen...)Show SMILES COc1cc(Nc2ncc3c(C)nc(-c4ccccc4Br)n3n2)cc(OC)c1OC Show InChI InChI=1S/C21H20BrN5O3/c1-12-16-11-23-21(25-13-9-17(28-2)19(30-4)18(10-13)29-3)26-27(16)20(24-12)14-7-5-6-8-15(14)22/h5-11H,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275663
(4-(5-methyl-7-phenylimidazo[1,5-f][1,2,4]triazin-2...)Show SMILES Cc1nc(-c2ccccc2)n2nc(Nc3ccc(cc3)S(N)(=O)=O)ncc12 Show InChI InChI=1S/C18H16N6O2S/c1-12-16-11-20-18(22-14-7-9-15(10-8-14)27(19,25)26)23-24(16)17(21-12)13-5-3-2-4-6-13/h2-11H,1H3,(H,22,23)(H2,19,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50275662
(5-methyl-N-(4-nitrophenyl)-7-phenylimidazo[1,5-f][...)Show SMILES Cc1nc(-c2ccccc2)n2nc(Nc3ccc(cc3)[N+]([O-])=O)ncc12 Show InChI InChI=1S/C18H14N6O2/c1-12-16-11-19-18(21-14-7-9-15(10-8-14)24(25)26)22-23(16)17(20-12)13-5-3-2-4-6-13/h2-11H,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) expressed in baculovirus infected Trichoplusia ni cells |
Bioorg Med Chem Lett 18: 6214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.100
BindingDB Entry DOI: 10.7270/Q22Z15DQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data