 Found 48 hits with Last Name = 'polakowski' and Initial = 't'
Found 48 hits with Last Name = 'polakowski' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192056

(Ac-Phe-[Orn-Pro-cha-Trp-Nva] | CHEMBL412031)Show SMILES CCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O Show InChI InChI=1S/C46H62N8O7/c1-3-14-35-41(56)47-23-12-21-36(51-42(57)37(49-29(2)55)25-30-15-6-4-7-16-30)46(61)54-24-13-22-40(54)45(60)53-38(26-31-17-8-5-9-18-31)43(58)52-39(44(59)50-35)27-32-28-48-34-20-11-10-19-33(32)34/h4,6-7,10-11,15-16,19-20,28,31,35-40,48H,3,5,8-9,12-14,17-18,21-27H2,1-2H3,(H,47,56)(H,49,55)(H,50,59)(H,51,57)(H,52,58)(H,53,60)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192070
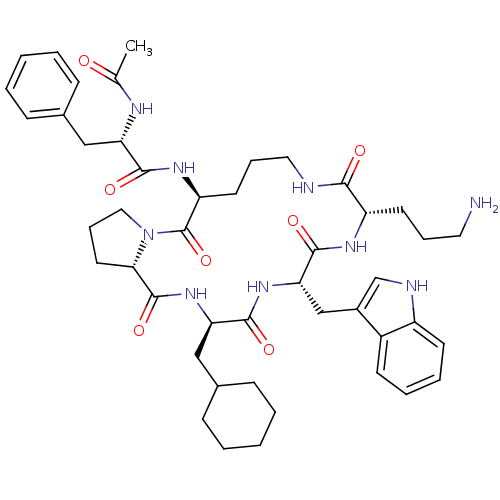
(Ac-Phe-[Orn-Pro-cha-Trp-Orn] | CHEMBL405647)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C46H63N9O7/c1-29(56)50-37(25-30-13-4-2-5-14-30)42(58)52-36-20-11-23-48-41(57)35(19-10-22-47)51-44(60)39(27-32-28-49-34-18-9-8-17-33(32)34)53-43(59)38(26-31-15-6-3-7-16-31)54-45(61)40-21-12-24-55(40)46(36)62/h2,4-5,8-9,13-14,17-18,28,31,35-40,49H,3,6-7,10-12,15-16,19-27,47H2,1H3,(H,48,57)(H,50,56)(H,51,60)(H,52,58)(H,53,59)(H,54,61)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192059
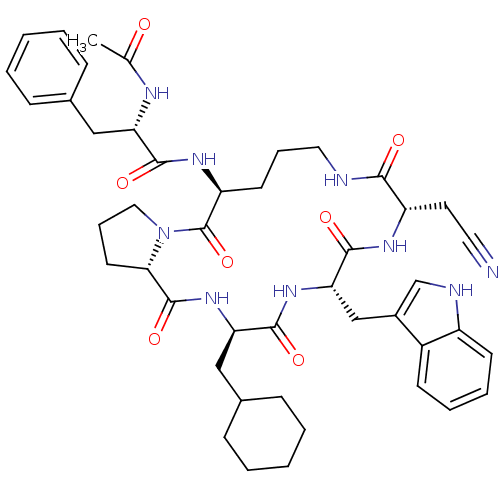
(Ac-Ala-[Orn-Pro-cha-Trp-Eag] | CHEMBL214024)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CC#N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C45H57N9O7/c1-28(55)49-36(24-29-12-4-2-5-13-29)41(57)51-35-18-10-22-47-40(56)34(20-21-46)50-43(59)38(26-31-27-48-33-17-9-8-16-32(31)33)52-42(58)37(25-30-14-6-3-7-15-30)53-44(60)39-19-11-23-54(39)45(35)61/h2,4-5,8-9,12-13,16-17,27,30,34-39,48H,3,6-7,10-11,14-15,18-20,22-26H2,1H3,(H,47,56)(H,49,55)(H,50,59)(H,51,57)(H,52,58)(H,53,60)/t34-,35-,36-,37+,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192043
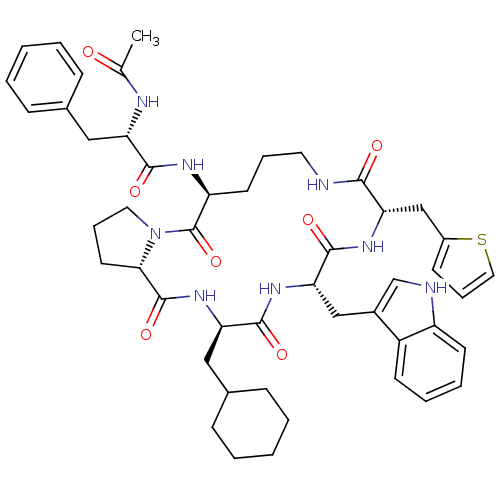
(Ac-Phe-[Orn-Pro-cha-Trp-Thi] | CHEMBL425469)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](Cc2cccs2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C48H60N8O7S/c1-30(57)51-38(25-31-13-4-2-5-14-31)44(59)52-37-20-10-22-49-43(58)41(28-34-17-12-24-64-34)54-46(61)40(27-33-29-50-36-19-9-8-18-35(33)36)53-45(60)39(26-32-15-6-3-7-16-32)55-47(62)42-21-11-23-56(42)48(37)63/h2,4-5,8-9,12-14,17-19,24,29,32,37-42,50H,3,6-7,10-11,15-16,20-23,25-28H2,1H3,(H,49,58)(H,51,57)(H,52,59)(H,53,60)(H,54,61)(H,55,62)/t37-,38-,39+,40-,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192051
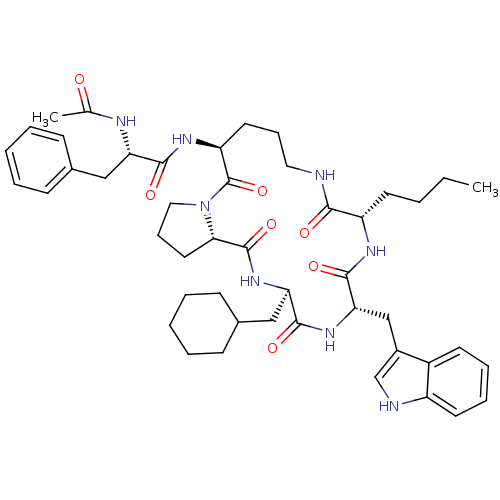
(Ac-Phe-[Orn-Pro-cha-Trp-Nle] | CHEMBL374575)Show SMILES CCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O Show InChI InChI=1S/C47H64N8O7/c1-3-4-20-36-42(57)48-24-13-22-37(52-43(58)38(50-30(2)56)26-31-15-7-5-8-16-31)47(62)55-25-14-23-41(55)46(61)54-39(27-32-17-9-6-10-18-32)44(59)53-40(45(60)51-36)28-33-29-49-35-21-12-11-19-34(33)35/h5,7-8,11-12,15-16,19,21,29,32,36-41,49H,3-4,6,9-10,13-14,17-18,20,22-28H2,1-2H3,(H,48,57)(H,50,56)(H,51,60)(H,52,58)(H,53,59)(H,54,61)/t36-,37-,38-,39+,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192061
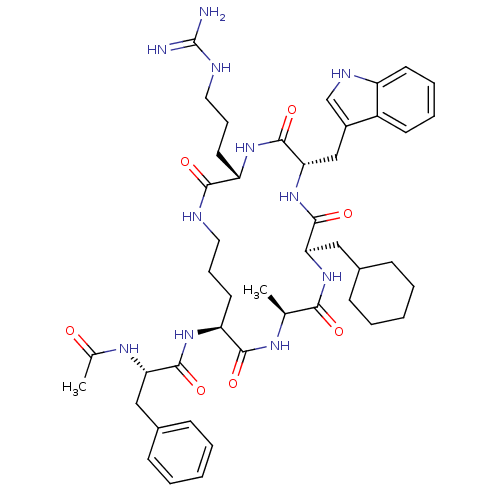
(Ac-Phe-[Orn-Ala-cha-Trp-Arg] | CHEMBL405646)Show SMILES C[C@@H]1NC(=O)[C@H](CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O Show InChI InChI=1S/C45H63N11O7/c1-27-39(58)55-37(24-30-15-7-4-8-16-30)43(62)56-38(25-31-26-50-33-18-10-9-17-32(31)33)44(63)53-34(19-12-22-49-45(46)47)40(59)48-21-11-20-35(41(60)51-27)54-42(61)36(52-28(2)57)23-29-13-5-3-6-14-29/h3,5-6,9-10,13-14,17-18,26-27,30,34-38,50H,4,7-8,11-12,15-16,19-25H2,1-2H3,(H,48,59)(H,51,60)(H,52,57)(H,53,63)(H,54,61)(H,55,58)(H,56,62)(H4,46,47,49)/t27-,34-,35-,36-,37+,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192062
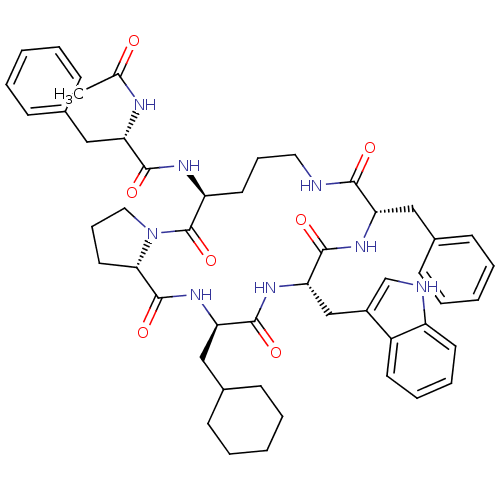
(Ac-Phe-[Orn-Pro-cha-Trp-Phe] | CHEMBL375443)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C50H62N8O7/c1-32(59)53-41(28-34-17-7-3-8-18-34)46(61)54-39-23-13-25-51-45(60)40(27-33-15-5-2-6-16-33)55-48(63)43(30-36-31-52-38-22-12-11-21-37(36)38)56-47(62)42(29-35-19-9-4-10-20-35)57-49(64)44-24-14-26-58(44)50(39)65/h2-3,5-8,11-12,15-18,21-22,31,35,39-44,52H,4,9-10,13-14,19-20,23-30H2,1H3,(H,51,60)(H,53,59)(H,54,61)(H,55,63)(H,56,62)(H,57,64)/t39-,40-,41-,42+,43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192044
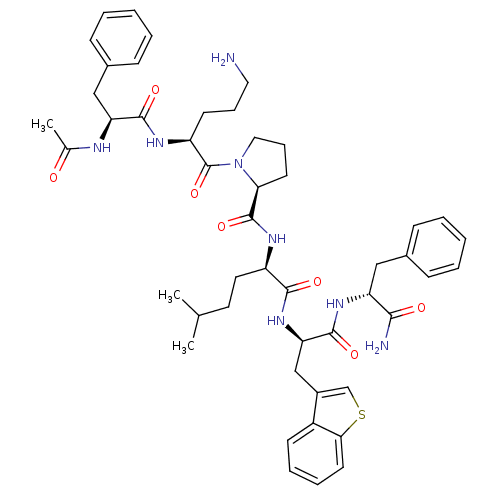
(Ac-Phe-Orn-Pro-hle-Bta-Phe-NH2 | CHEMBL262177)Show SMILES CC(C)CC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@H](Cc1csc2ccccc12)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H62N8O7S/c1-30(2)22-23-36(44(59)55-40(28-34-29-64-42-21-11-10-18-35(34)42)46(61)54-38(43(50)58)26-32-14-6-4-7-15-32)52-47(62)41-20-13-25-56(41)48(63)37(19-12-24-49)53-45(60)39(51-31(3)57)27-33-16-8-5-9-17-33/h4-11,14-18,21,29-30,36-41H,12-13,19-20,22-28,49H2,1-3H3,(H2,50,58)(H,51,57)(H,52,62)(H,53,60)(H,54,61)(H,55,59)/t36-,37+,38-,39+,40-,41+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445
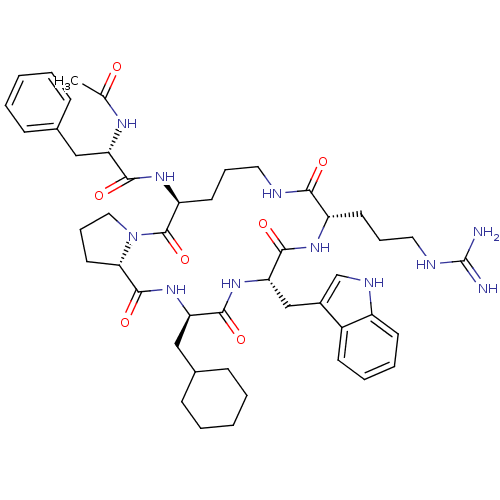
((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192057
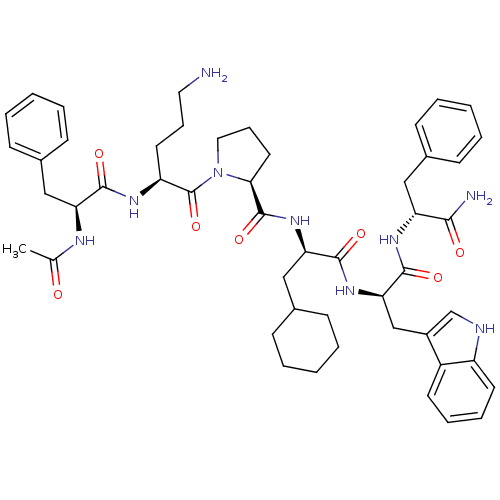
(Ac-Phe-Orn-Pro-cha-Trp-Phe-NH2 | CHEMBL376905)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN)C(=O)N1CCC[C@H]1C(=O)N[C@H](CC1CCCCC1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H65N9O7/c1-32(60)54-41(28-34-17-7-3-8-18-34)46(62)55-39(23-13-25-51)50(66)59-26-14-24-44(59)49(65)58-42(29-35-19-9-4-10-20-35)47(63)57-43(30-36-31-53-38-22-12-11-21-37(36)38)48(64)56-40(45(52)61)27-33-15-5-2-6-16-33/h2-3,5-8,11-12,15-18,21-22,31,35,39-44,53H,4,9-10,13-14,19-20,23-30,51H2,1H3,(H2,52,61)(H,54,60)(H,55,62)(H,56,64)(H,57,63)(H,58,65)/t39-,40+,41-,42+,43+,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR expressed in human PMN cells assessed as inhibition of glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192066
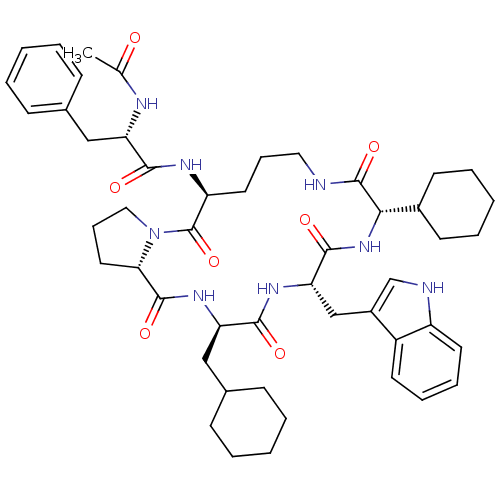
(Ac-Phe-[Orn-Pro-cha-Trp-Chg] | CHEMBL374717)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O)C1CCCCC1 Show InChI InChI=1S/C49H66N8O7/c1-31(58)52-39(27-32-15-5-2-6-16-32)44(59)53-38-23-13-25-50-48(63)43(34-19-9-4-10-20-34)56-46(61)41(29-35-30-51-37-22-12-11-21-36(35)37)54-45(60)40(28-33-17-7-3-8-18-33)55-47(62)42-24-14-26-57(42)49(38)64/h2,5-6,11-12,15-16,21-22,30,33-34,38-43,51H,3-4,7-10,13-14,17-20,23-29H2,1H3,(H,50,63)(H,52,58)(H,53,59)(H,54,60)(H,55,62)(H,56,61)/t38-,39-,40+,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192041

(CHEMBL2371928 | Hoo-Phe-Orn-Pro-hle-Pff-Phe-NH2)Show SMILES CC(C)CC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CC(=O)NC(=O)N1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H63FN10O9/c1-29(2)17-22-34(43(63)56-38(27-32-18-20-33(50)21-19-32)45(65)55-36(42(52)62)25-30-11-5-3-6-12-30)53-47(67)40-16-10-24-60(40)48(68)35(15-9-23-51)54-44(64)37(26-31-13-7-4-8-14-31)57-46(66)39-28-41(61)59-49(69)58-39/h3-8,11-14,18-21,29,34-40H,9-10,15-17,22-28,51H2,1-2H3,(H2,52,62)(H,53,67)(H,54,64)(H,55,65)(H,56,63)(H,57,66)(H2,58,59,61,69)/t34-,35-,36+,37-,38-,39-,40?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192041

(CHEMBL2371928 | Hoo-Phe-Orn-Pro-hle-Pff-Phe-NH2)Show SMILES CC(C)CC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CC(=O)NC(=O)N1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H63FN10O9/c1-29(2)17-22-34(43(63)56-38(27-32-18-20-33(50)21-19-32)45(65)55-36(42(52)62)25-30-11-5-3-6-12-30)53-47(67)40-16-10-24-60(40)48(68)35(15-9-23-51)54-44(64)37(26-31-13-7-4-8-14-31)57-46(66)39-28-41(61)59-49(69)58-39/h3-8,11-14,18-21,29,34-40H,9-10,15-17,22-28,51H2,1-2H3,(H2,52,62)(H,53,67)(H,54,64)(H,55,65)(H,56,63)(H,57,66)(H2,58,59,61,69)/t34-,35-,36+,37-,38-,39-,40?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR expressed in human PMN cells assessed as inhibition of glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192038
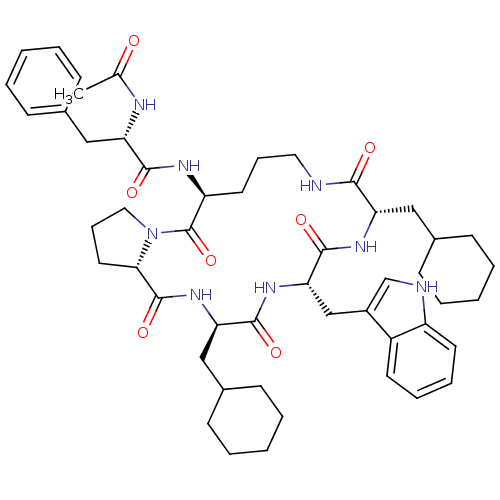
(Ac-Phe-[Orn-Pro-cha-Trp-Cha] | CHEMBL425289)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CC2CCCCC2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C50H68N8O7/c1-32(59)53-41(28-34-17-7-3-8-18-34)46(61)54-39-23-13-25-51-45(60)40(27-33-15-5-2-6-16-33)55-48(63)43(30-36-31-52-38-22-12-11-21-37(36)38)56-47(62)42(29-35-19-9-4-10-20-35)57-49(64)44-24-14-26-58(44)50(39)65/h3,7-8,11-12,17-18,21-22,31,33,35,39-44,52H,2,4-6,9-10,13-16,19-20,23-30H2,1H3,(H,51,60)(H,53,59)(H,54,61)(H,55,63)(H,56,62)(H,57,64)/t39-,40-,41-,42+,43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192053
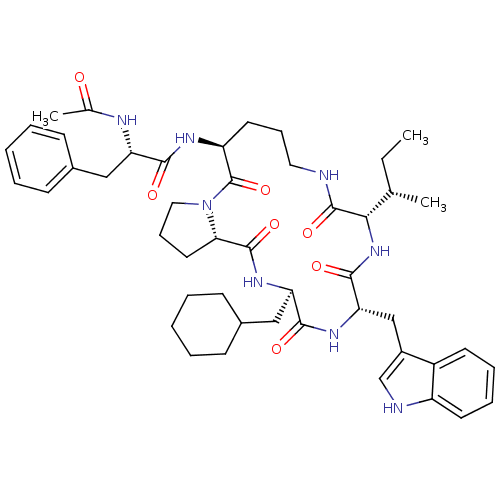
(Ac-Phe-[Orn-Pro-cha-Trp-Ile] | CHEMBL375137)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O Show InChI InChI=1S/C47H64N8O7/c1-4-29(2)41-46(61)48-23-13-21-36(51-42(57)37(50-30(3)56)25-31-15-7-5-8-16-31)47(62)55-24-14-22-40(55)45(60)53-38(26-32-17-9-6-10-18-32)43(58)52-39(44(59)54-41)27-33-28-49-35-20-12-11-19-34(33)35/h5,7-8,11-12,15-16,19-20,28-29,32,36-41,49H,4,6,9-10,13-14,17-18,21-27H2,1-3H3,(H,48,61)(H,50,56)(H,51,57)(H,52,58)(H,53,60)(H,54,59)/t29-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192054
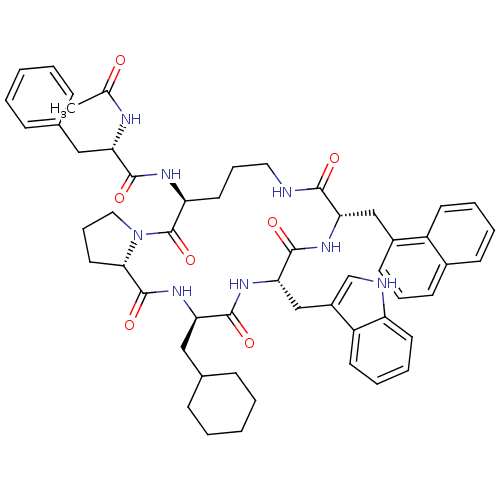
(Ac-Phe-[Orn-Pro-cha-Trp-1Ni] | CHEMBL374542)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C54H64N8O7/c1-34(63)57-44(29-35-15-4-2-5-16-35)50(65)58-43-25-13-27-55-49(64)46(31-38-21-12-20-37-19-8-9-22-40(37)38)59-52(67)47(32-39-33-56-42-24-11-10-23-41(39)42)60-51(66)45(30-36-17-6-3-7-18-36)61-53(68)48-26-14-28-62(48)54(43)69/h2,4-5,8-12,15-16,19-24,33,36,43-48,56H,3,6-7,13-14,17-18,25-32H2,1H3,(H,55,64)(H,57,63)(H,58,65)(H,59,67)(H,60,66)(H,61,68)/t43-,44-,45+,46-,47-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192037

(Ac-Phe-[Orn-Pro-cha-Trp-Leu] | CHEMBL413916)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O Show InChI InChI=1S/C47H64N8O7/c1-29(2)24-37-42(57)48-22-12-20-36(51-43(58)38(50-30(3)56)25-31-14-6-4-7-15-31)47(62)55-23-13-21-41(55)46(61)54-39(26-32-16-8-5-9-17-32)44(59)53-40(45(60)52-37)27-33-28-49-35-19-11-10-18-34(33)35/h4,6-7,10-11,14-15,18-19,28-29,32,36-41,49H,5,8-9,12-13,16-17,20-27H2,1-3H3,(H,48,57)(H,50,56)(H,51,58)(H,52,60)(H,53,59)(H,54,61)/t36-,37-,38-,39+,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192036
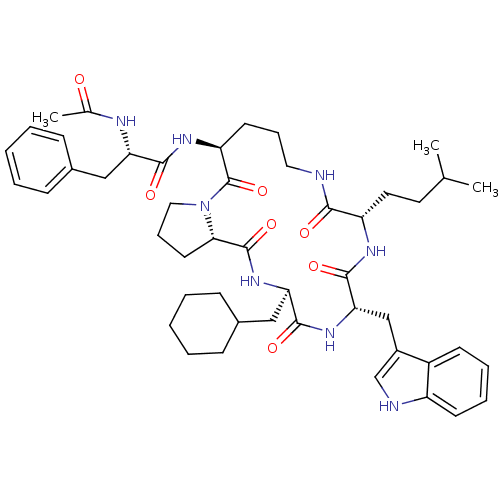
(Ac-Phe-[Orn-Pro-cha-Trp-Hle] | CHEMBL435921)Show SMILES CC(C)CC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O Show InChI InChI=1S/C48H66N8O7/c1-30(2)22-23-37-43(58)49-24-12-20-38(53-44(59)39(51-31(3)57)26-32-14-6-4-7-15-32)48(63)56-25-13-21-42(56)47(62)55-40(27-33-16-8-5-9-17-33)45(60)54-41(46(61)52-37)28-34-29-50-36-19-11-10-18-35(34)36/h4,6-7,10-11,14-15,18-19,29-30,33,37-42,50H,5,8-9,12-13,16-17,20-28H2,1-3H3,(H,49,58)(H,51,57)(H,52,61)(H,53,59)(H,54,60)(H,55,62)/t37-,38-,39-,40+,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192065

(Ac-Phe-Orn-Pro-hle-Mcf-Phe-NH2 | CHEMBL437988)Show SMILES CC(C)CC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@H](Cc1cccc(Cl)c1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C46H61ClN8O7/c1-29(2)21-22-35(42(58)54-39(28-33-17-10-18-34(47)25-33)44(60)53-37(41(49)57)26-31-13-6-4-7-14-31)51-45(61)40-20-12-24-55(40)46(62)36(19-11-23-48)52-43(59)38(50-30(3)56)27-32-15-8-5-9-16-32/h4-10,13-18,25,29,35-40H,11-12,19-24,26-28,48H2,1-3H3,(H2,49,57)(H,50,56)(H,51,61)(H,52,59)(H,53,60)(H,54,58)/t35-,36+,37-,38+,39-,40+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192047
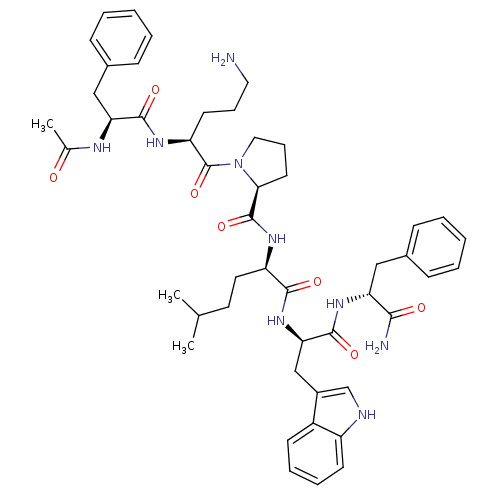
(Ac-Phe-Orn-Pro-hle-Trp-Phe-NH2 | CHEMBL409045)Show SMILES CC(C)CC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H63N9O7/c1-30(2)22-23-37(44(60)56-41(28-34-29-51-36-19-11-10-18-35(34)36)46(62)55-39(43(50)59)26-32-14-6-4-7-15-32)53-47(63)42-21-13-25-57(42)48(64)38(20-12-24-49)54-45(61)40(52-31(3)58)27-33-16-8-5-9-17-33/h4-11,14-19,29-30,37-42,51H,12-13,20-28,49H2,1-3H3,(H2,50,59)(H,52,58)(H,53,63)(H,54,61)(H,55,62)(H,56,60)/t37-,38+,39-,40+,41-,42+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192050
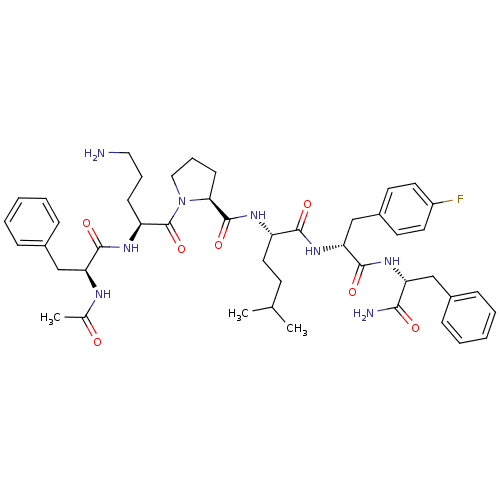
(Ac-Phe-Orn-Pro-hle-Pff-Phe-NH2 | CHEMBL262181)Show SMILES CC(C)CC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C46H61FN8O7/c1-29(2)18-23-35(42(58)54-39(28-33-19-21-34(47)22-20-33)44(60)53-37(41(49)57)26-31-12-6-4-7-13-31)51-45(61)40-17-11-25-55(40)46(62)36(16-10-24-48)52-43(59)38(50-30(3)56)27-32-14-8-5-9-15-32/h4-9,12-15,19-22,29,35-40H,10-11,16-18,23-28,48H2,1-3H3,(H2,49,57)(H,50,56)(H,51,61)(H,52,59)(H,53,60)(H,54,58)/t35-,36-,37+,38-,39+,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192052
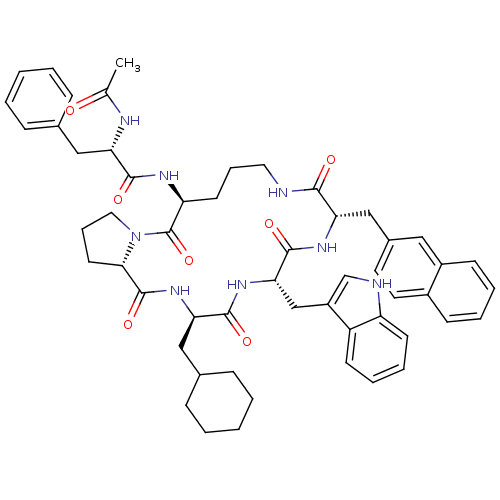
(Ac-Phe-[Orn-Pro-cha-Trp-2Ni] | CHEMBL374694)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C54H64N8O7/c1-34(63)57-45(29-35-14-4-2-5-15-35)50(65)58-43-22-12-26-55-49(64)44(31-37-24-25-38-18-8-9-19-39(38)28-37)59-52(67)47(32-40-33-56-42-21-11-10-20-41(40)42)60-51(66)46(30-36-16-6-3-7-17-36)61-53(68)48-23-13-27-62(48)54(43)69/h2,4-5,8-11,14-15,18-21,24-25,28,33,36,43-48,56H,3,6-7,12-13,16-17,22-23,26-27,29-32H2,1H3,(H,55,64)(H,57,63)(H,58,65)(H,59,67)(H,60,66)(H,61,68)/t43-,44-,45-,46+,47-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to NK2 receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of C5a binding to human C5aR expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192058
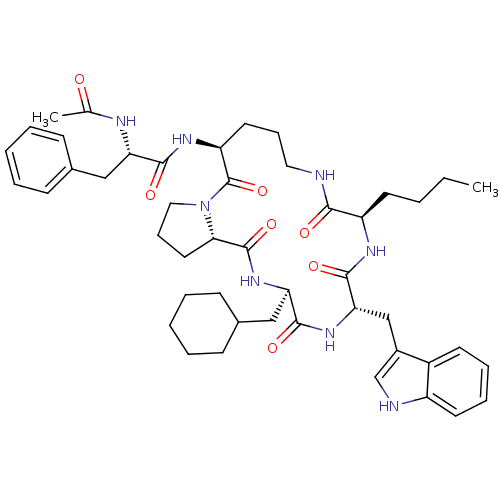
(Ac-Phe-[Orn-Pro-cha-Trp-nle] | CHEMBL407439)Show SMILES CCCC[C@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O Show InChI InChI=1S/C47H64N8O7/c1-3-4-20-36-42(57)48-24-13-22-37(52-43(58)38(50-30(2)56)26-31-15-7-5-8-16-31)47(62)55-25-14-23-41(55)46(61)54-39(27-32-17-9-6-10-18-32)44(59)53-40(45(60)51-36)28-33-29-49-35-21-12-11-19-34(33)35/h5,7-8,11-12,15-16,19,21,29,32,36-41,49H,3-4,6,9-10,13-14,17-18,20,22-28H2,1-2H3,(H,48,57)(H,50,56)(H,51,60)(H,52,58)(H,53,59)(H,54,61)/t36-,37+,38+,39-,40+,41+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192041

(CHEMBL2371928 | Hoo-Phe-Orn-Pro-hle-Pff-Phe-NH2)Show SMILES CC(C)CC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CC(=O)NC(=O)N1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H63FN10O9/c1-29(2)17-22-34(43(63)56-38(27-32-18-20-33(50)21-19-32)45(65)55-36(42(52)62)25-30-11-5-3-6-12-30)53-47(67)40-16-10-24-60(40)48(68)35(15-9-23-51)54-44(64)37(26-31-13-7-4-8-14-31)57-46(66)39-28-41(61)59-49(69)58-39/h3-8,11-14,18-21,29,34-40H,9-10,15-17,22-28,51H2,1-2H3,(H2,52,62)(H,53,67)(H,54,64)(H,55,65)(H,56,63)(H,57,66)(H2,58,59,61,69)/t34-,35-,36+,37-,38-,39-,40?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of C5a binding to human C5aR expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192045
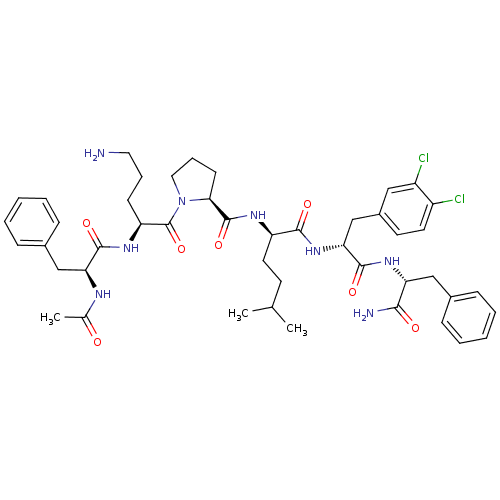
(Ac-Phe-Orn-Pro-hle-Dcf-Phe-NH2 | CHEMBL262178)Show SMILES CC(C)CC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@H](Cc1ccc(Cl)c(Cl)c1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C46H60Cl2N8O7/c1-28(2)18-21-35(42(59)55-39(27-32-19-20-33(47)34(48)24-32)44(61)54-37(41(50)58)25-30-12-6-4-7-13-30)52-45(62)40-17-11-23-56(40)46(63)36(16-10-22-49)53-43(60)38(51-29(3)57)26-31-14-8-5-9-15-31/h4-9,12-15,19-20,24,28,35-40H,10-11,16-18,21-23,25-27,49H2,1-3H3,(H2,50,58)(H,51,57)(H,52,62)(H,53,60)(H,54,61)(H,55,59)/t35-,36+,37-,38+,39-,40+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192064
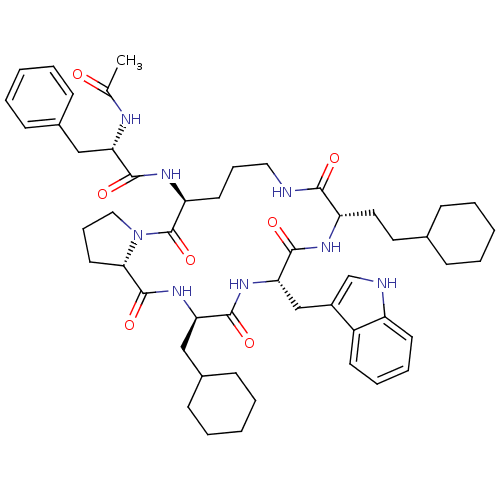
(Ac-Phe-[Orn-Pro-cha-Trp-Hch] | CHEMBL219386)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCC2CCCCC2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C51H70N8O7/c1-33(60)54-42(29-35-17-7-3-8-18-35)47(62)56-41-23-13-27-52-46(61)40(26-25-34-15-5-2-6-16-34)55-49(64)44(31-37-32-53-39-22-12-11-21-38(37)39)57-48(63)43(30-36-19-9-4-10-20-36)58-50(65)45-24-14-28-59(45)51(41)66/h3,7-8,11-12,17-18,21-22,32,34,36,40-45,53H,2,4-6,9-10,13-16,19-20,23-31H2,1H3,(H,52,61)(H,54,60)(H,55,64)(H,56,62)(H,57,63)(H,58,65)/t40-,41-,42-,43+,44-,45-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192063

(Ac-Phe-Orn-Pro-cha-Trp-Arg-NH2 | CHEMBL373600)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN)C(=O)N1CCC[C@H]1C(=O)N[C@H](CC1CCCCC1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](CCCN=C(N)N)C(N)=O |wU:30.31,wD:26.28,4.3,55.59,15.15,41.43,(13.44,-22.24,;14.22,-23.57,;13.44,-24.92,;15.76,-23.57,;16.53,-22.24,;18.08,-22.24,;18.84,-23.57,;20.4,-23.58,;21.16,-24.91,;20.39,-26.25,;18.84,-26.24,;18.08,-24.91,;15.77,-20.91,;14.22,-20.91,;16.53,-19.58,;15.78,-18.24,;16.54,-16.9,;18.09,-16.9,;18.85,-15.56,;20.4,-15.57,;14.23,-18.24,;13.45,-19.57,;13.23,-16.5,;14.02,-15.02,;12.81,-14,;11.5,-14.8,;11.85,-16.3,;11.85,-17.84,;13.18,-18.61,;10.52,-18.61,;9.19,-17.84,;9.19,-16.3,;7.86,-15.53,;7.85,-13.98,;6.53,-13.21,;5.19,-13.98,;5.18,-15.52,;6.52,-16.3,;7.86,-18.61,;6.53,-17.84,;7.86,-20.15,;6.53,-20.93,;6.53,-22.47,;7.81,-23.3,;9.22,-22.75,;10.21,-23.94,;9.41,-25.25,;9.84,-26.72,;8.76,-27.85,;7.28,-27.47,;6.84,-26,;7.91,-24.88,;5.19,-20.15,;5.19,-18.61,;3.85,-20.92,;2.52,-20.15,;1.17,-20.92,;-.16,-20.15,;-1.49,-20.92,;-2.82,-20.15,;-4.16,-20.92,;-5.5,-20.15,;-4.17,-22.46,;2.52,-18.61,;3.85,-17.83,;1.17,-17.83,)| Show InChI InChI=1S/C47H68N12O7/c1-29(60)54-37(25-30-13-4-2-5-14-30)42(62)56-36(19-10-22-48)46(66)59-24-12-21-40(59)45(65)58-38(26-31-15-6-3-7-16-31)43(63)57-39(27-32-28-53-34-18-9-8-17-33(32)34)44(64)55-35(41(49)61)20-11-23-52-47(50)51/h2,4-5,8-9,13-14,17-18,28,31,35-40,53H,3,6-7,10-12,15-16,19-27,48H2,1H3,(H2,49,61)(H,54,60)(H,55,64)(H,56,62)(H,57,63)(H,58,65)(H4,50,51,52)/t35-,36+,37+,38-,39-,40+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192060

(Ac-Phe-[Orn-Pro-cha-Trp-Ocg] | CHEMBL374494)Show SMILES CCCCCCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O Show InChI InChI=1S/C51H72N8O7/c1-3-4-5-6-7-14-25-40-46(61)52-28-17-26-41(56-47(62)42(54-34(2)60)30-35-19-10-8-11-20-35)51(66)59-29-18-27-45(59)50(65)58-43(31-36-21-12-9-13-22-36)48(63)57-44(49(64)55-40)32-37-33-53-39-24-16-15-23-38(37)39/h8,10-11,15-16,19-20,23-24,33,36,40-45,53H,3-7,9,12-14,17-18,21-22,25-32H2,1-2H3,(H,52,61)(H,54,60)(H,55,64)(H,56,62)(H,57,63)(H,58,65)/t40-,41-,42-,43+,44-,45-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192046
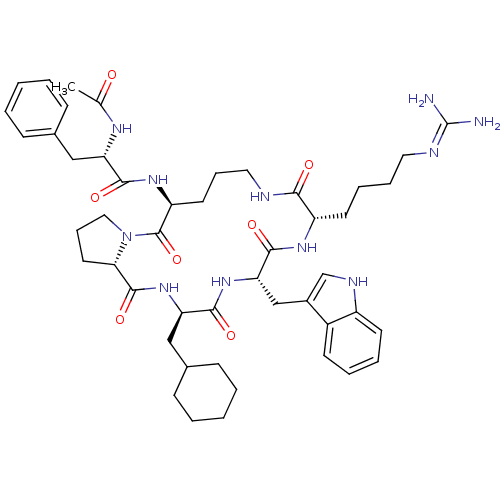
(Ac-Phe-[Orn-Pro-cha-Trp-Har] | CHEMBL415150)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O |wU:59.67,34.35,22.23,4.3,wD:48.51,15.15,(-3.46,-34.69,;-2.69,-33.36,;-3.46,-32.02,;-1.17,-33.36,;-.4,-32.02,;-1.17,-30.69,;-.4,-29.36,;1.14,-29.36,;1.92,-28.03,;1.14,-26.69,;-.41,-26.7,;-1.16,-28.04,;1.14,-32.02,;1.91,-33.35,;1.91,-30.69,;3.45,-30.69,;5.11,-31.8,;4.49,-33.54,;5.67,-34.52,;7.27,-33.56,;8.66,-35.05,;8.92,-36.57,;10.1,-34.52,;10.87,-35.86,;10.1,-37.2,;10.87,-38.53,;10.1,-39.86,;10.87,-41.19,;12.41,-41.19,;13.18,-42.53,;13.18,-39.86,;10.48,-32.68,;12.05,-32.21,;13.49,-32.72,;12.31,-30.69,;13.85,-30.69,;14.62,-29.36,;14,-27.95,;15.14,-26.92,;16.48,-27.69,;17.94,-27.21,;19.09,-28.25,;18.76,-29.75,;17.3,-30.22,;16.15,-29.19,;10.76,-29.48,;11.28,-27.84,;12.46,-26.85,;10.1,-26.85,;10.87,-25.52,;10.1,-24.19,;10.87,-22.85,;10.1,-21.52,;8.56,-21.52,;7.79,-22.85,;8.56,-24.19,;8.54,-27.32,;7.12,-26.33,;6.85,-24.8,;5.67,-26.85,;4.32,-26.07,;3.4,-27.2,;3.87,-28.66,;5.41,-28.66,;3.72,-29.17,;2.28,-28.65,)| Show InChI InChI=1S/C48H67N11O7/c1-30(60)54-38(26-31-14-4-2-5-15-31)43(62)56-37-21-12-24-51-42(61)36(20-10-11-23-52-48(49)50)55-45(64)40(28-33-29-53-35-19-9-8-18-34(33)35)57-44(63)39(27-32-16-6-3-7-17-32)58-46(65)41-22-13-25-59(41)47(37)66/h2,4-5,8-9,14-15,18-19,29,32,36-41,53H,3,6-7,10-13,16-17,20-28H2,1H3,(H,51,61)(H,54,60)(H,55,64)(H,56,62)(H,57,63)(H,58,65)(H4,49,50,52)/t36-,37-,38-,39+,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to MC4 receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192042

(Ac-Phe-Orn-Pro-cha-Trp-Nle-NH2 | CHEMBL374120)Show SMILES CCCC[C@@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CC1CCCCC1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(N)=O Show InChI InChI=1S/C47H67N9O7/c1-3-4-20-36(42(49)58)52-45(61)40(28-33-29-50-35-21-12-11-19-34(33)35)54-44(60)39(27-32-17-9-6-10-18-32)55-46(62)41-23-14-25-56(41)47(63)37(22-13-24-48)53-43(59)38(51-30(2)57)26-31-15-7-5-8-16-31/h5,7-8,11-12,15-16,19,21,29,32,36-41,50H,3-4,6,9-10,13-14,17-18,20,22-28,48H2,1-2H3,(H2,49,58)(H,51,57)(H,52,61)(H,53,59)(H,54,60)(H,55,62)/t36-,37+,38+,39-,40-,41+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 463 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192068

(Ac-Phe-[Orn-Pro-cha-Trp-Cit] | CHEMBL219236)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H64N10O8/c1-29(58)52-37(25-30-13-4-2-5-14-30)42(60)54-36-20-11-22-49-41(59)35(19-10-23-50-47(48)65)53-44(62)39(27-32-28-51-34-18-9-8-17-33(32)34)55-43(61)38(26-31-15-6-3-7-16-31)56-45(63)40-21-12-24-57(40)46(36)64/h2,4-5,8-9,13-14,17-18,28,31,35-40,51H,3,6-7,10-12,15-16,19-27H2,1H3,(H,49,59)(H,52,58)(H,53,62)(H,54,60)(H,55,61)(H,56,63)(H3,48,50,65)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192040

(Ac-Phe-Orn-Pro-cha-Trp-Arg-OH | CHEMBL409977)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN)C(=O)N1CCC[C@H]1C(=O)N[C@H](CC1CCCCC1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](CCCN=C(N)N)C(O)=O |wU:30.31,wD:26.28,4.3,55.59,15.15,41.43,(16.41,-6.42,;17.18,-7.76,;16.41,-9.1,;18.72,-7.76,;19.49,-6.43,;21.03,-6.43,;21.8,-7.77,;23.35,-7.76,;24.11,-9.1,;23.35,-10.43,;21.81,-10.43,;21.03,-9.1,;18.72,-5.1,;17.18,-5.1,;19.49,-3.76,;18.74,-2.41,;19.5,-1.08,;21.05,-1.08,;21.81,.26,;23.36,.25,;17.19,-2.41,;16.42,-3.75,;16.19,-.68,;16.97,.8,;15.77,1.82,;14.46,1.02,;14.82,-.48,;14.82,-2.02,;16.15,-2.79,;13.48,-2.79,;12.15,-2.02,;12.15,-.48,;10.82,.29,;10.81,1.84,;9.49,2.61,;8.16,1.84,;8.15,.3,;9.49,-.48,;10.82,-2.79,;9.49,-2.02,;10.82,-4.33,;9.49,-5.1,;9.49,-6.64,;10.78,-7.48,;12.18,-6.93,;13.17,-8.11,;12.37,-9.43,;12.8,-10.9,;11.72,-12.03,;10.24,-11.64,;9.8,-10.18,;10.87,-9.06,;8.16,-4.33,;8.16,-2.79,;6.82,-5.09,;5.49,-4.32,;4.15,-5.09,;2.81,-4.32,;1.48,-5.09,;.15,-4.32,;-1.18,-5.1,;-2.53,-4.33,;-1.2,-6.64,;5.49,-2.78,;6.82,-2,;4.15,-2.01,)| Show InChI InChI=1S/C47H67N11O8/c1-29(59)53-37(25-30-13-4-2-5-14-30)41(60)54-35(19-10-22-48)45(64)58-24-12-21-40(58)44(63)57-38(26-31-15-6-3-7-16-31)42(61)56-39(27-32-28-52-34-18-9-8-17-33(32)34)43(62)55-36(46(65)66)20-11-23-51-47(49)50/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27,48H2,1H3,(H,53,59)(H,54,60)(H,55,62)(H,56,61)(H,57,63)(H,65,66)(H4,49,50,51)/t35-,36+,37-,38+,39+,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 535 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192049

(Ac-Phe-[Orn-Pro-cha-Trp-Arg(NO2)] | CHEMBL435927)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N[N+]([O-])=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O |w:29.30| Show InChI InChI=1S/C47H64N12O9/c1-29(60)52-37(25-30-13-4-2-5-14-30)42(62)54-36-20-11-22-49-41(61)35(19-10-23-50-47(48)57-59(67)68)53-44(64)39(27-32-28-51-34-18-9-8-17-33(32)34)55-43(63)38(26-31-15-6-3-7-16-31)56-45(65)40-21-12-24-58(40)46(36)66/h2,4-5,8-9,13-14,17-18,28,31,35-40,51H,3,6-7,10-12,15-16,19-27H2,1H3,(H,49,61)(H,52,60)(H,53,64)(H,54,62)(H,55,63)(H,56,65)(H3,48,50,57)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50192041

(CHEMBL2371928 | Hoo-Phe-Orn-Pro-hle-Pff-Phe-NH2)Show SMILES CC(C)CC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CC(=O)NC(=O)N1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H63FN10O9/c1-29(2)17-22-34(43(63)56-38(27-32-18-20-33(50)21-19-32)45(65)55-36(42(52)62)25-30-11-5-3-6-12-30)53-47(67)40-16-10-24-60(40)48(68)35(15-9-23-51)54-44(64)37(26-31-13-7-4-8-14-31)57-46(66)39-28-41(61)59-49(69)58-39/h3-8,11-14,18-21,29,34-40H,9-10,15-17,22-28,51H2,1-2H3,(H2,52,62)(H,53,67)(H,54,64)(H,55,65)(H,56,63)(H,57,66)(H2,58,59,61,69)/t34-,35-,36+,37-,38-,39-,40?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to NK2 receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to V1a receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50192041

(CHEMBL2371928 | Hoo-Phe-Orn-Pro-hle-Pff-Phe-NH2)Show SMILES CC(C)CC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CC(=O)NC(=O)N1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H63FN10O9/c1-29(2)17-22-34(43(63)56-38(27-32-18-20-33(50)21-19-32)45(65)55-36(42(52)62)25-30-11-5-3-6-12-30)53-47(67)40-16-10-24-60(40)48(68)35(15-9-23-51)54-44(64)37(26-31-13-7-4-8-14-31)57-46(66)39-28-41(61)59-49(69)58-39/h3-8,11-14,18-21,29,34-40H,9-10,15-17,22-28,51H2,1-2H3,(H2,52,62)(H,53,67)(H,54,64)(H,55,65)(H,56,63)(H,57,66)(H2,58,59,61,69)/t34-,35-,36+,37-,38-,39-,40?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to V1a receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to ORL1 receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192039

(Ac-Phe-[Orn-Pro-cha-Trp-Ala] | CHEMBL214456)Show SMILES C[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O Show InChI InChI=1S/C44H58N8O7/c1-27-39(54)45-21-11-19-34(49-41(56)35(48-28(2)53)23-29-13-5-3-6-14-29)44(59)52-22-12-20-38(52)43(58)51-36(24-30-15-7-4-8-16-30)42(57)50-37(40(55)47-27)25-31-26-46-33-18-10-9-17-32(31)33/h3,5-6,9-10,13-14,17-18,26-27,30,34-38,46H,4,7-8,11-12,15-16,19-25H2,1-2H3,(H,45,54)(H,47,55)(H,48,53)(H,49,56)(H,50,57)(H,51,58)/t27-,34-,35-,36+,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192069
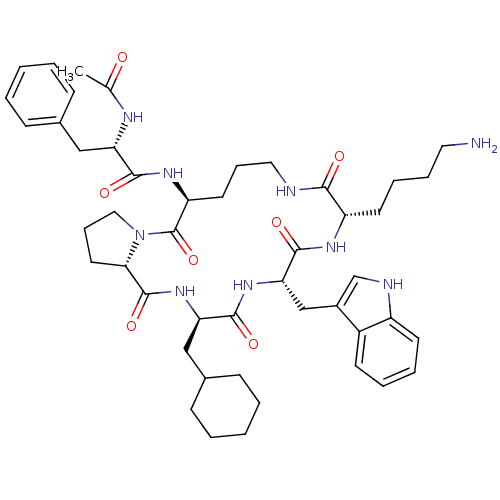
(Ac-Phe-[Orn-Pro-cha-Trp-Lys] | CHEMBL412008)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N9O7/c1-30(57)51-38(26-31-14-4-2-5-15-31)43(59)53-37-21-12-24-49-42(58)36(20-10-11-23-48)52-45(61)40(28-33-29-50-35-19-9-8-18-34(33)35)54-44(60)39(27-32-16-6-3-7-17-32)55-46(62)41-22-13-25-56(41)47(37)63/h2,4-5,8-9,14-15,18-19,29,32,36-41,50H,3,6-7,10-13,16-17,20-28,48H2,1H3,(H,49,58)(H,51,57)(H,52,61)(H,53,59)(H,54,60)(H,55,62)/t36-,37-,38-,39+,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50192041

(CHEMBL2371928 | Hoo-Phe-Orn-Pro-hle-Pff-Phe-NH2)Show SMILES CC(C)CC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CC(=O)NC(=O)N1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H63FN10O9/c1-29(2)17-22-34(43(63)56-38(27-32-18-20-33(50)21-19-32)45(65)55-36(42(52)62)25-30-11-5-3-6-12-30)53-47(67)40-16-10-24-60(40)48(68)35(15-9-23-51)54-44(64)37(26-31-13-7-4-8-14-31)57-46(66)39-28-41(61)59-49(69)58-39/h3-8,11-14,18-21,29,34-40H,9-10,15-17,22-28,51H2,1-2H3,(H2,52,62)(H,53,67)(H,54,64)(H,55,65)(H,56,63)(H,57,66)(H2,58,59,61,69)/t34-,35-,36+,37-,38-,39-,40?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to MC4 receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192048
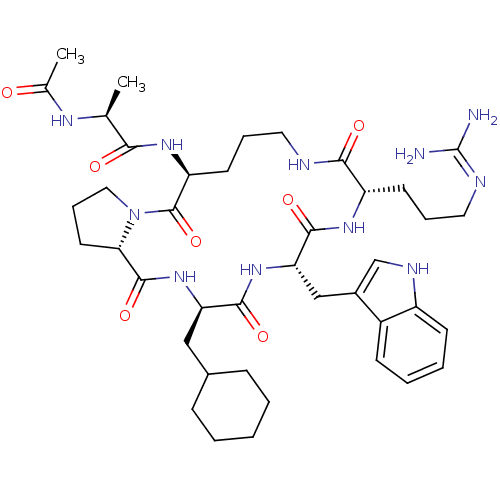
(Ac-Ala-[Orn-Pro-cha-Trp-Arg] | CHEMBL267491)Show SMILES C[C@H](NC(C)=O)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O |wU:27.27,16.16,1.0,52.59,wD:9.8,41.43,(-6.04,-28.46,;-5.27,-29.8,;-6.04,-31.13,;-7.58,-31.13,;-8.35,-32.47,;-8.35,-29.8,;-3.73,-29.8,;-2.96,-31.13,;-2.96,-28.46,;-1.42,-28.46,;.11,-29.25,;-.38,-31.32,;.79,-32.3,;2.67,-31.18,;3.78,-32.83,;4.04,-34.35,;5.23,-32.3,;6,-33.64,;5.23,-34.98,;6,-36.31,;5.13,-37.58,;5.81,-38.97,;4.94,-40.24,;7.34,-39.08,;5.58,-30.65,;7.18,-29.98,;8.62,-30.5,;7.44,-28.46,;8.98,-28.46,;9.75,-27.13,;9.13,-25.72,;10.27,-24.69,;11.61,-25.46,;13.07,-24.99,;14.22,-26.02,;13.9,-27.52,;12.43,-28,;11.28,-26.97,;5.76,-27.33,;6.41,-25.61,;7.59,-24.62,;5.23,-24.62,;6,-23.29,;5.23,-21.96,;6,-20.62,;5.23,-19.29,;3.69,-19.29,;2.92,-20.62,;3.69,-21.96,;3.66,-25.29,;2.24,-24.1,;1.97,-22.57,;.79,-24.62,;-.46,-24.05,;-1.39,-25.06,;-.72,-26.26,;.63,-25.99,;-1.15,-26.95,;-2.6,-26.42,)| Show InChI InChI=1S/C41H61N11O7/c1-24(47-25(2)53)35(54)49-31-16-9-18-44-36(55)30(15-8-19-45-41(42)43)48-38(57)33(22-27-23-46-29-14-7-6-13-28(27)29)50-37(56)32(21-26-11-4-3-5-12-26)51-39(58)34-17-10-20-52(34)40(31)59/h6-7,13-14,23-24,26,30-34,46H,3-5,8-12,15-22H2,1-2H3,(H,44,55)(H,47,53)(H,48,57)(H,49,54)(H,50,56)(H,51,58)(H4,42,43,45)/t24-,30-,31-,32+,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50192041

(CHEMBL2371928 | Hoo-Phe-Orn-Pro-hle-Pff-Phe-NH2)Show SMILES CC(C)CC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CC(=O)NC(=O)N1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H63FN10O9/c1-29(2)17-22-34(43(63)56-38(27-32-18-20-33(50)21-19-32)45(65)55-36(42(52)62)25-30-11-5-3-6-12-30)53-47(67)40-16-10-24-60(40)48(68)35(15-9-23-51)54-44(64)37(26-31-13-7-4-8-14-31)57-46(66)39-28-41(61)59-49(69)58-39/h3-8,11-14,18-21,29,34-40H,9-10,15-17,22-28,51H2,1-2H3,(H2,52,62)(H,53,67)(H,54,64)(H,55,65)(H,56,63)(H,57,66)(H2,58,59,61,69)/t34-,35-,36+,37-,38-,39-,40?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to ORL1 receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192067
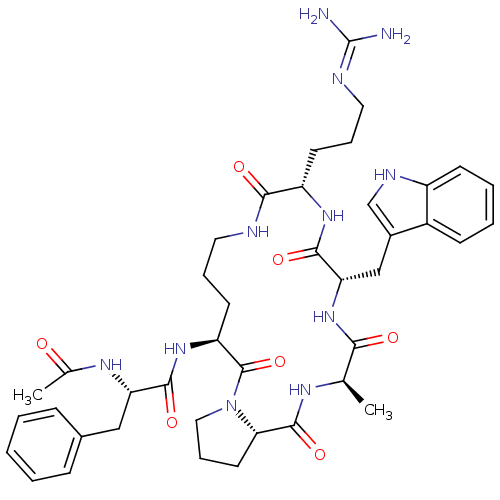
(Ac-Phe-[Orn-Pro-ala-Trp-Arg] | CHEMBL407285)Show SMILES C[C@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O |wU:30.31,19.20,47.59,5.9,wD:1.0,12.47,(5.97,-23.8,;5.2,-25.12,;3.7,-25.68,;2.22,-24.59,;1.96,-23.08,;.78,-25.12,;-.33,-24.47,;-1.57,-25.38,;-1.1,-26.85,;.44,-26.85,;-1.17,-27.44,;-2.61,-26.92,;-1.44,-28.96,;.32,-30.11,;-.4,-31.81,;.77,-32.79,;2.53,-31.98,;3.76,-33.32,;4.03,-34.84,;5.2,-32.79,;5.97,-34.13,;5.2,-35.47,;5.97,-36.8,;4.84,-37.84,;6.19,-38.57,;4.89,-40.05,;7.29,-38.18,;5.56,-30.99,;7.16,-30.48,;8.6,-30.99,;7.41,-28.96,;8.95,-28.96,;9.72,-27.63,;9.1,-26.22,;10.25,-25.18,;11.58,-25.96,;13.05,-25.48,;14.19,-26.52,;13.87,-28.02,;12.41,-28.49,;11.26,-27.47,;6.63,-27.63,;6.39,-26.11,;7.57,-25.12,;-2.95,-28.66,;-3.24,-30.17,;-2.47,-31.51,;-4.78,-30.17,;-5.55,-28.83,;-4.78,-27.51,;-3.24,-27.51,;-2.47,-26.18,;-3.24,-24.84,;-4.8,-24.85,;-5.55,-26.19,;-5.55,-31.51,;-7.09,-31.51,;-7.86,-32.84,;-7.86,-30.17,)| Show InChI InChI=1S/C41H55N11O7/c1-24-35(54)51-33(22-27-23-46-29-14-7-6-13-28(27)29)38(57)49-30(15-8-19-45-41(42)43)36(55)44-18-9-16-31(40(59)52-20-10-17-34(52)39(58)47-24)50-37(56)32(48-25(2)53)21-26-11-4-3-5-12-26/h3-7,11-14,23-24,30-34,46H,8-10,15-22H2,1-2H3,(H,44,55)(H,47,58)(H,48,53)(H,49,57)(H,50,56)(H,51,54)(H4,42,43,45)/t24-,30+,31+,32+,33+,34+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50192055
(Ac-Phe-[Orn-Pro-cha-Ala-Arg] | CHEMBL385012)Show SMILES C[C@@H]1NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(=O)[C@H](CCCNC(N)=N)NC1=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O Show InChI InChI=1S/C39H60N10O7/c1-24-33(51)46-28(16-9-20-43-39(40)41)34(52)42-19-10-17-29(47-36(54)30(45-25(2)50)22-26-12-5-3-6-13-26)38(56)49-21-11-18-32(49)37(55)48-31(35(53)44-24)23-27-14-7-4-8-15-27/h3,5-6,12-13,24,27-32H,4,7-11,14-23H2,1-2H3,(H,42,52)(H,44,53)(H,45,50)(H,46,51)(H,47,54)(H,48,55)(H4,40,41,43)/t24-,28-,29-,30-,31+,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data















































