

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM84868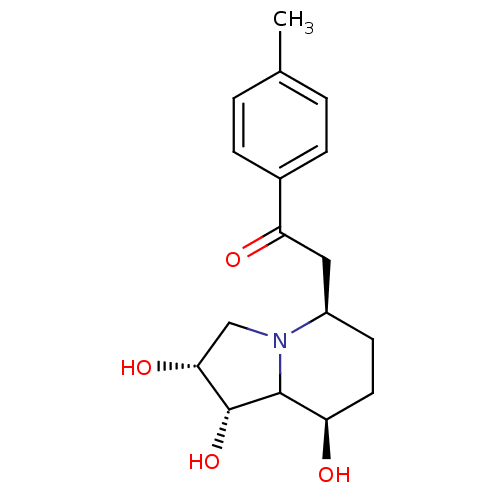 (Swainsonine derivative, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | -48.9 | 29 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto | Assay Description Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... | Chembiochem 11: 673-80 (2010) Article DOI: 10.1002/cbic.200900750 BindingDB Entry DOI: 10.7270/Q2WS8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM84869 (Swainsonine derivative, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | -48.9 | 29 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto | Assay Description Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... | Chembiochem 11: 673-80 (2010) Article DOI: 10.1002/cbic.200900750 BindingDB Entry DOI: 10.7270/Q2WS8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM84867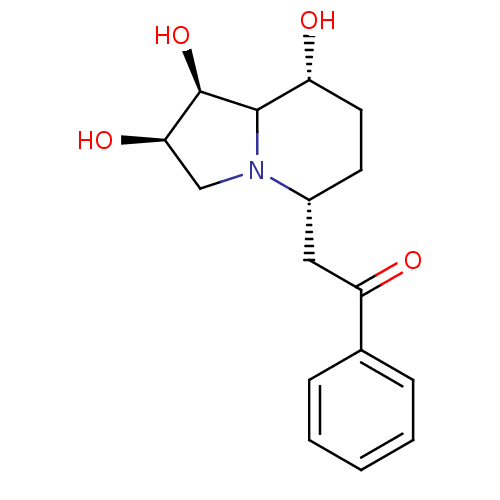 (Swainsonine derivative, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80 | -48.8 | 30 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto | Assay Description Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... | Chembiochem 11: 673-80 (2010) Article DOI: 10.1002/cbic.200900750 BindingDB Entry DOI: 10.7270/Q2WS8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM50168995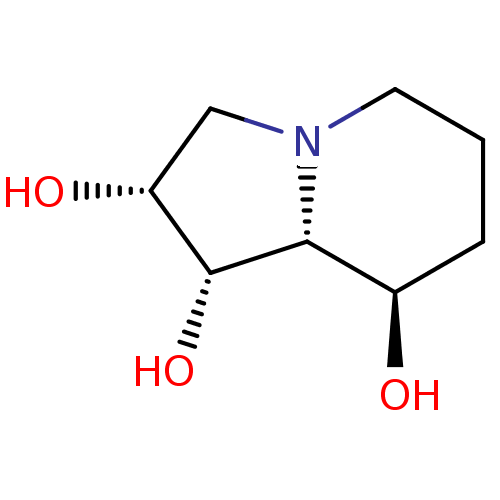 ((-)-swainsonine | (1S,2R,8R,8aR)-Octahydro-indoliz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | -48.6 | 37 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto | Assay Description Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... | Chembiochem 11: 673-80 (2010) Article DOI: 10.1002/cbic.200900750 BindingDB Entry DOI: 10.7270/Q2WS8RRM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50327504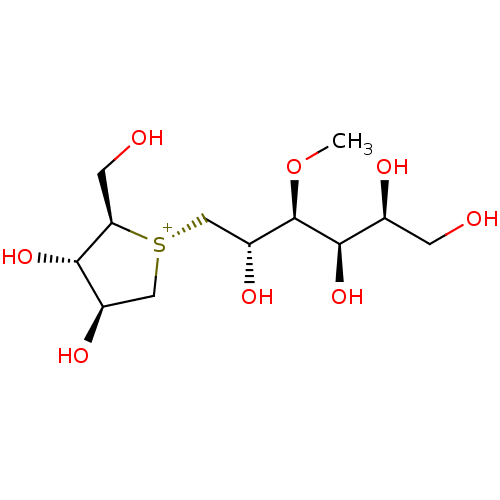 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327503 ((1R,2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-1-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem Lett 20: 5686-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.020 BindingDB Entry DOI: 10.7270/Q2WQ0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330961 ((2R,3S,4S)-1-((2S,3S,4R,5R,6S)-2,3,4,5,6,7-hexahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330954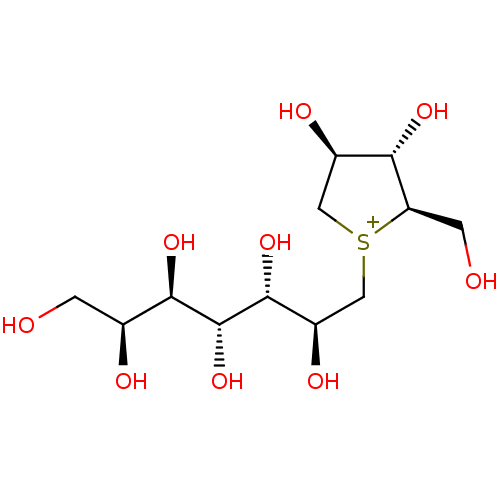 (CHEMBL1276973 | de-O-sulfonated kotalanol) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM50078117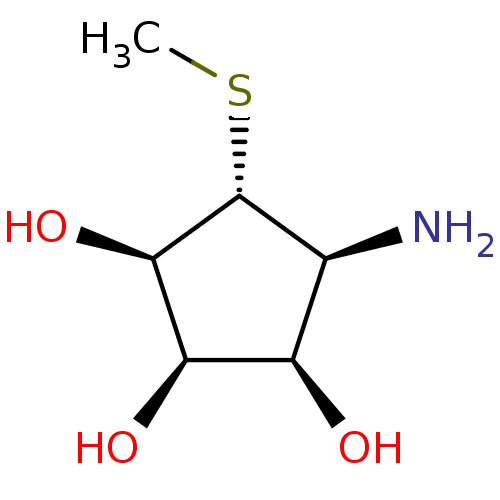 ((1R,2R,3R,4S,5R)-4-AMINO-5-(METHYLTHIO)CYCLOPENTAN...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description The rate of hydrolysis catalyzed by drosophila golgi alpha-mannosidase II (dGMII)and in the presence of different concentration of inhibitor was meas... | Chembiochem 10: 268-77 (2009) Article DOI: 10.1002/cbic.200800538 BindingDB Entry DOI: 10.7270/Q2DZ06T7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327502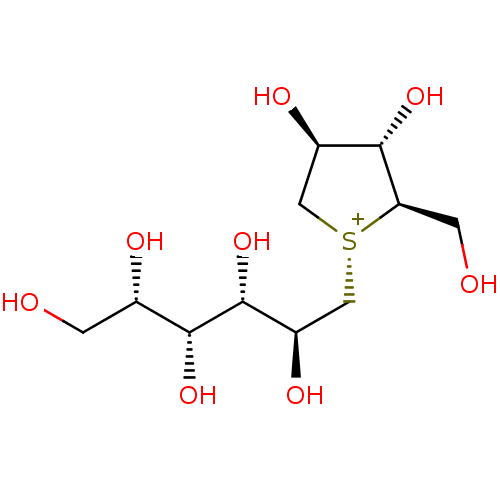 ((1R,2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-1-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human N-terminal maltase-glucoamylase | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327502 ((1R,2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-1-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem Lett 20: 5686-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.020 BindingDB Entry DOI: 10.7270/Q2WQ0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330960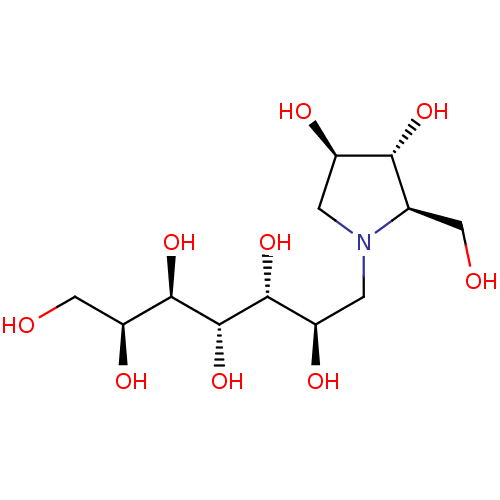 ((2R,3R,4R)-1-((2R,3R,4S,5R,6S)-2,3,4,5,6,7-hexahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM50088625 ((1R,2R,3R,4S,5R)-4-Amino-5-methoxy-cyclopentane-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description The rate of hydrolysis catalyzed by drosophila golgi alpha-mannosidase II (dGMII)and in the presence of different concentration of inhibitor was meas... | Chembiochem 10: 268-77 (2009) Article DOI: 10.1002/cbic.200800538 BindingDB Entry DOI: 10.7270/Q2DZ06T7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330959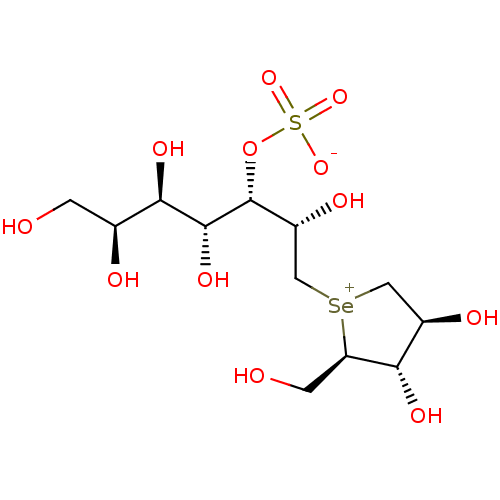 (CHEMBL1277153) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316180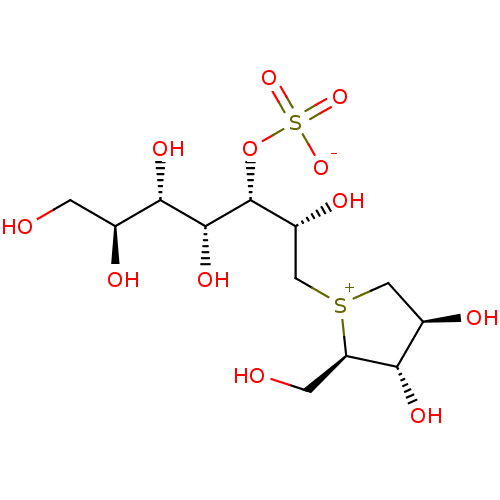 ((1S,2R,3S,4S)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domain | Bioorg Med Chem 18: 2829-35 (2010) Article DOI: 10.1016/j.bmc.2010.03.027 BindingDB Entry DOI: 10.7270/Q22B8Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50327502 ((1R,2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-1-((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316181 ((1S,2R,3S,4R)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domain | Bioorg Med Chem 18: 2829-35 (2010) Article DOI: 10.1016/j.bmc.2010.03.027 BindingDB Entry DOI: 10.7270/Q22B8Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM84614 (Mannostatin B, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description The rate of hydrolysis catalyzed by drosophila golgi alpha-mannosidase II (dGMII)and in the presence of different concentration of inhibitor was meas... | Chembiochem 10: 268-77 (2009) Article DOI: 10.1002/cbic.200800538 BindingDB Entry DOI: 10.7270/Q2DZ06T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327501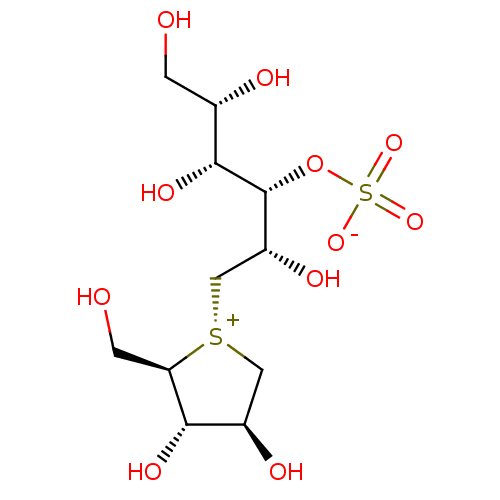 (CHEMBL1258528 | ponkoranol) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem Lett 20: 5686-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.020 BindingDB Entry DOI: 10.7270/Q2WQ0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316179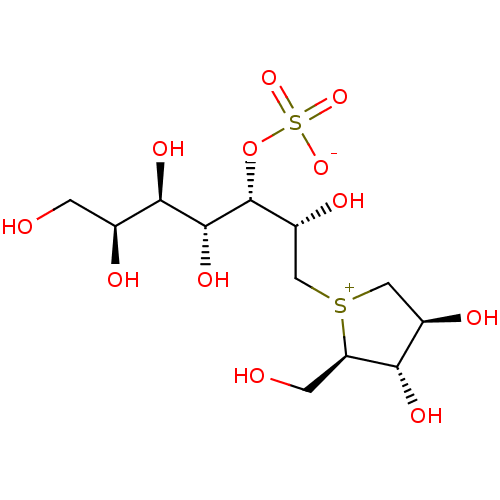 ((1S,2R,3R,4S)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316179 ((1S,2R,3R,4S)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domain | Bioorg Med Chem 18: 2829-35 (2010) Article DOI: 10.1016/j.bmc.2010.03.027 BindingDB Entry DOI: 10.7270/Q22B8Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316178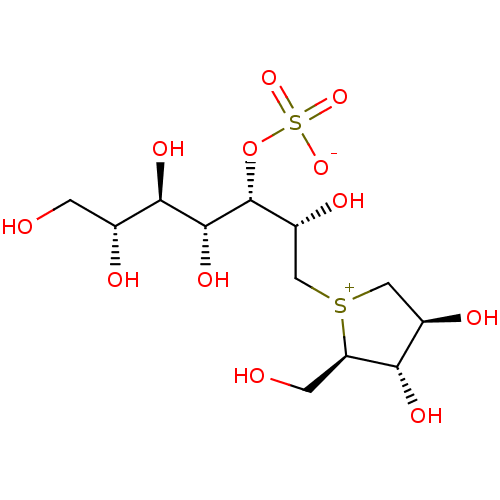 (1,4-Dideoxy-1,4-[[2S,3S,4R,5R,6R-2,4,5,6,7-pentahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domain | Bioorg Med Chem 18: 2829-35 (2010) Article DOI: 10.1016/j.bmc.2010.03.027 BindingDB Entry DOI: 10.7270/Q22B8Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM84615 (Mannostatin analogue, 4a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description The rate of hydrolysis catalyzed by drosophila golgi alpha-mannosidase II (dGMII)and in the presence of different concentration of inhibitor was meas... | Chembiochem 10: 268-77 (2009) Article DOI: 10.1002/cbic.200800538 BindingDB Entry DOI: 10.7270/Q2DZ06T7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50327502 ((1R,2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-1-((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal maltase-glucoamylase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem Lett 20: 5686-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.020 BindingDB Entry DOI: 10.7270/Q2WQ0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human N-terminal maltase-glucoamylase | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50263049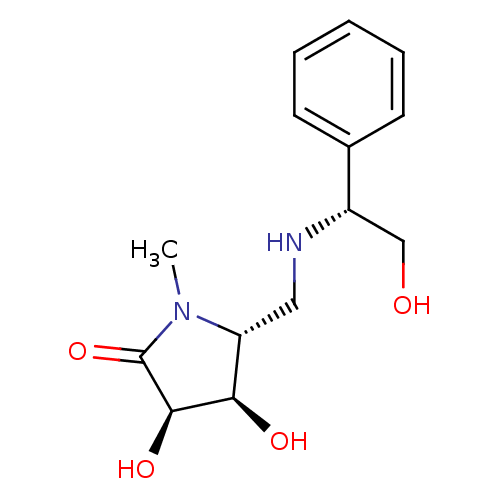 ((3R,4R,5R)-3,4-dihydroxy-5-(((R)-2-hydroxy-1-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human LNZ308 cells | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50263049 ((3R,4R,5R)-3,4-dihydroxy-5-(((R)-2-hydroxy-1-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human HCEC | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330955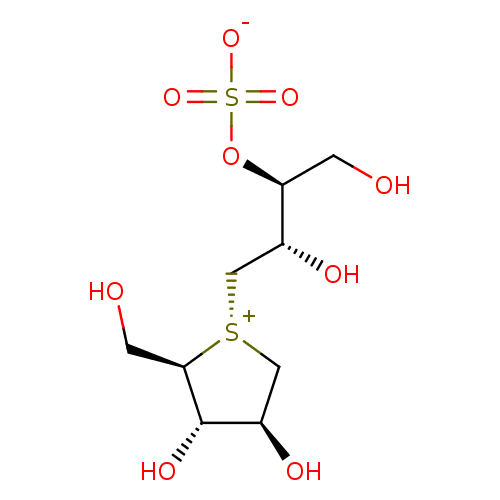 ((1S,2S)-3-[(2R,3S,4S)-3,4-dihydroxy-2-(hydroxymeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50242271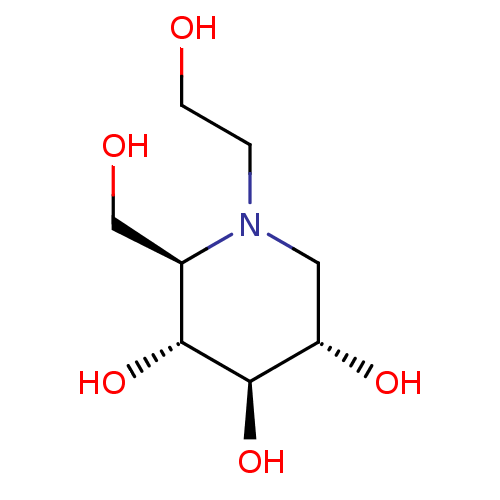 ((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330957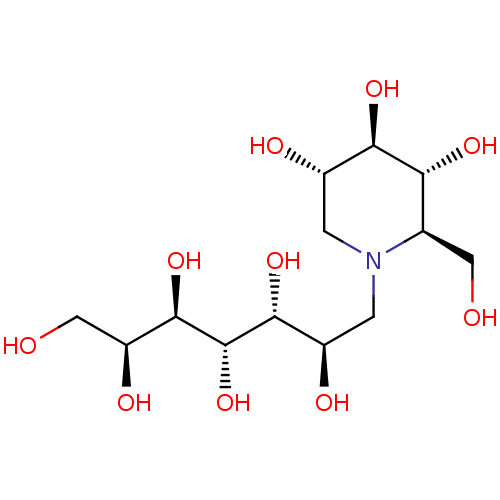 (7'-[(1,5-Dideoxy-1,5-imino-D-glucitol)-5-N-ammoniu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330956 (7'-[(1,5-Dideoxy-1,5-imino-D-glucitol)-5-N-ammoniu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50263049 ((3R,4R,5R)-3,4-dihydroxy-5-(((R)-2-hydroxy-1-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human LN18 cells | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50168988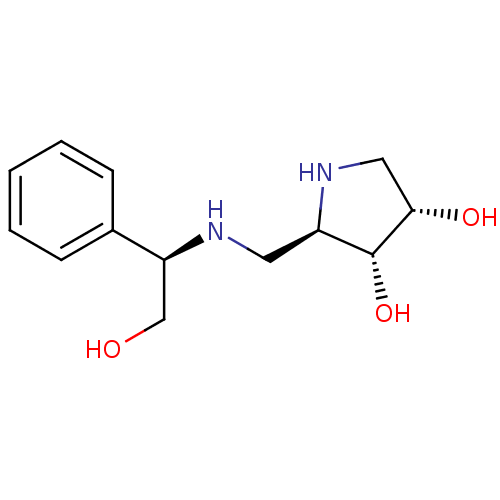 ((2R,3R,4S)-2-({[(1R)-2-HYDROXY-1-PHENYLETHYL]AMINO...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human HCEC | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50168988 ((2R,3R,4S)-2-({[(1R)-2-HYDROXY-1-PHENYLETHYL]AMINO...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human LN18 cells | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50168988 ((2R,3R,4S)-2-({[(1R)-2-HYDROXY-1-PHENYLETHYL]AMINO...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human LNZ308 cells | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330958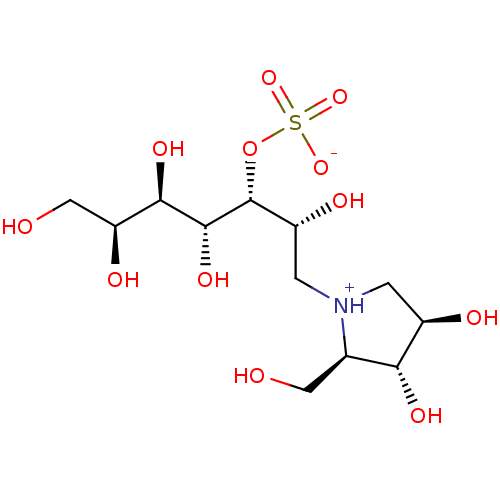 ((2R,3R,4R,5R,6S)-1-((2R,3R,4R)-3,4-dihydroxy-2-(hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM50088627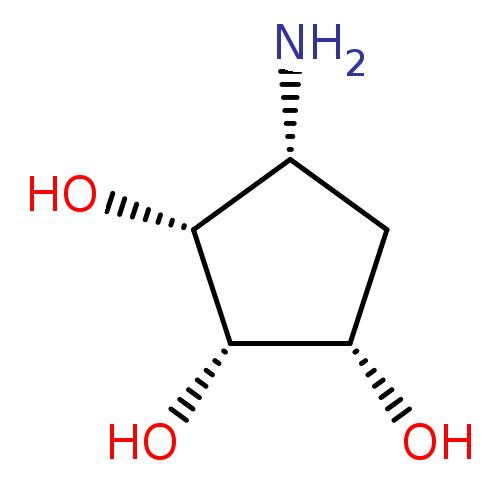 ((1S,2S,3R,4R)-4-Amino-cyclopentane-1,2,3-triol | (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description The rate of hydrolysis catalyzed by drosophila golgi alpha-mannosidase II (dGMII)and in the presence of different concentration of inhibitor was meas... | Chembiochem 10: 268-77 (2009) Article DOI: 10.1002/cbic.200800538 BindingDB Entry DOI: 10.7270/Q2DZ06T7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50168995 ((-)-swainsonine | (1S,2R,8R,8aR)-Octahydro-indoliz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human HCEC | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM84871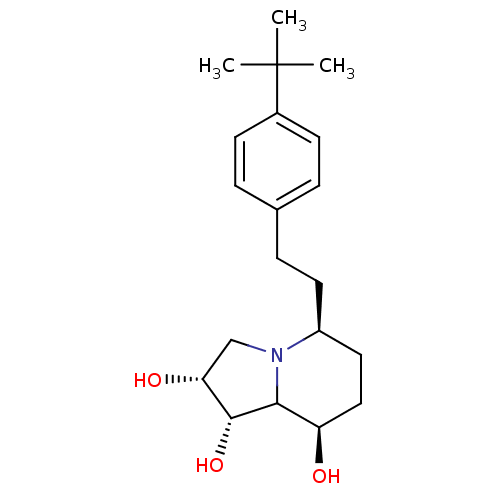 (Swainsonine derivative, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto | Assay Description Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... | Chembiochem 11: 673-80 (2010) Article DOI: 10.1002/cbic.200900750 BindingDB Entry DOI: 10.7270/Q2WS8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50168995 ((-)-swainsonine | (1S,2R,8R,8aR)-Octahydro-indoliz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human LN18 cells | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM84872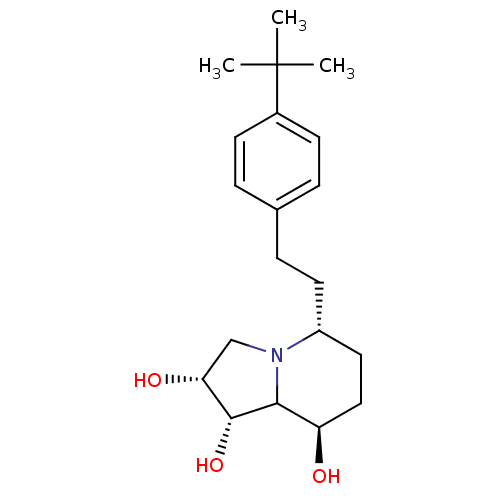 (Swainsonine derivative, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto | Assay Description Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... | Chembiochem 11: 673-80 (2010) Article DOI: 10.1002/cbic.200900750 BindingDB Entry DOI: 10.7270/Q2WS8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Drosophila melanogaster (Fruit fly)) | BDBM84870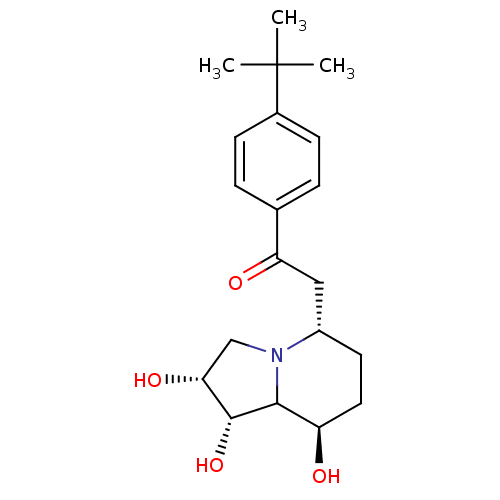 (Swainsonine derivative, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto | Assay Description Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... | Chembiochem 11: 673-80 (2010) Article DOI: 10.1002/cbic.200900750 BindingDB Entry DOI: 10.7270/Q2WS8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50263049 ((3R,4R,5R)-3,4-dihydroxy-5-(((R)-2-hydroxy-1-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human HCEC | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50263049 ((3R,4R,5R)-3,4-dihydroxy-5-(((R)-2-hydroxy-1-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human LNZ308 cells | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-mannosidase (Homo sapiens (Human)) | BDBM50263091 ((R)-2-(((2R,3R,4R)-3,4-dihydroxy-1-methyl-5-oxopyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique Fédérale de Lausanne Curated by ChEMBL | Assay Description Inhibition of alpha-mannosidase in human HCEC | Bioorg Med Chem 16: 7337-46 (2008) Article DOI: 10.1016/j.bmc.2008.06.021 BindingDB Entry DOI: 10.7270/Q2668D0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50067661 (CHEMBL137706 | Phosphoric acid mono-(4-{(S)-2-acet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Affinity for SH2 domain of src in Sf9 insect cells | J Med Chem 41: 4329-42 (1998) Article DOI: 10.1021/jm9802766 BindingDB Entry DOI: 10.7270/Q2ZW1K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50067665 (CHEMBL137715 | Phosphoric acid mono-(4-{(S)-2-acet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Affinity for SH2 domain of src in Sf9 insect cells | J Med Chem 41: 4329-42 (1998) Article DOI: 10.1021/jm9802766 BindingDB Entry DOI: 10.7270/Q2ZW1K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 87 total ) | Next | Last >> |