 Found 225 hits with Last Name = 'rupin' and Initial = 'a'
Found 225 hits with Last Name = 'rupin' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575780
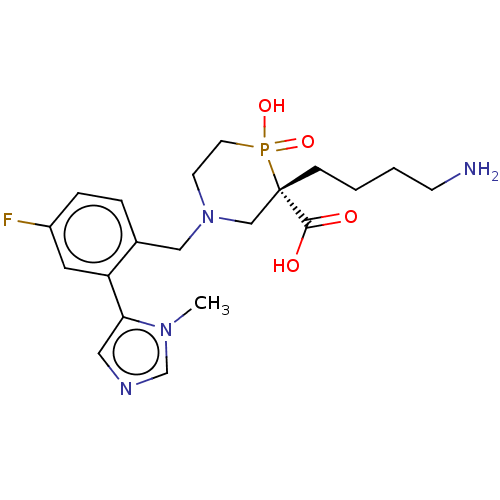
(CHEMBL4878039)Show SMILES Cn1cncc1-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50575780

(CHEMBL4878039)Show SMILES Cn1cncc1-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human pancreatic CPB incubated for 25 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50575780

(CHEMBL4878039)Show SMILES Cn1cncc1-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human plasma CPN incubated for 25 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575776
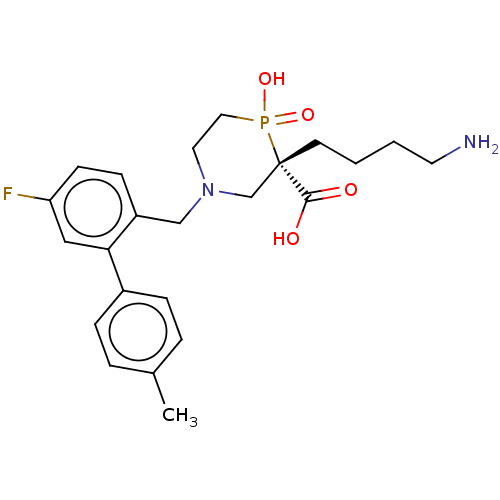
(CHEMBL4868605)Show SMILES Cc1ccc(cc1)-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575768
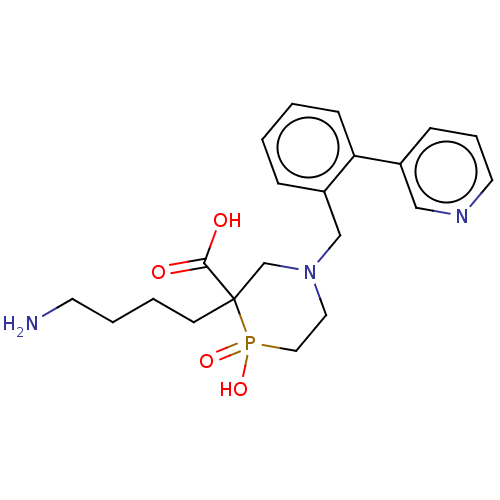
(CHEMBL4846664)Show SMILES NCCCCC1(CN(Cc2ccccc2-c2cccnc2)CCP1(O)=O)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373665

(CHEMBL442455)Show SMILES CC(C)CN(CP(O)(O)=O)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(O)=O Show InChI InChI=1S/C19H25N2O6P/c1-13(2)11-21(12-28(25,26)27)19(24)20-17(18(22)23)10-14-7-8-15-5-3-4-6-16(15)9-14/h3-9,13,17H,10-12H2,1-2H3,(H,20,24)(H,22,23)(H2,25,26,27)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373657
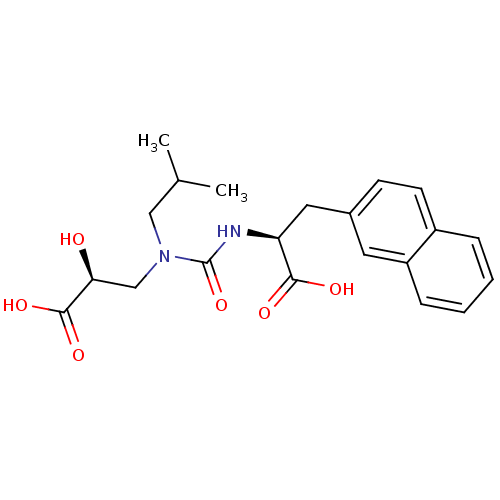
(CHEMBL450935)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(O)=O Show InChI InChI=1S/C21H26N2O6/c1-13(2)11-23(12-18(24)20(27)28)21(29)22-17(19(25)26)10-14-7-8-15-5-3-4-6-16(15)9-14/h3-9,13,17-18,24H,10-12H2,1-2H3,(H,22,29)(H,25,26)(H,27,28)/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575775
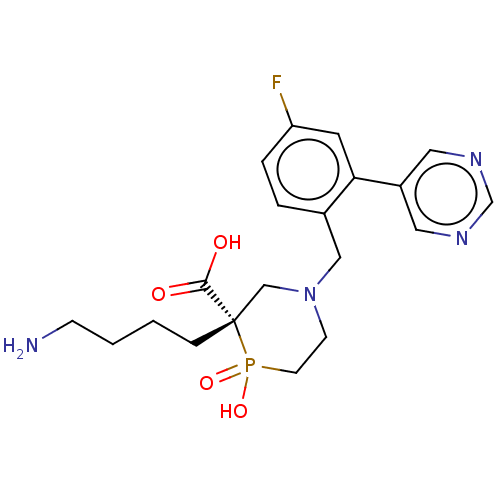
(CHEMBL4861532)Show SMILES NCCCC[C@]1(CN(Cc2ccc(F)cc2-c2cncnc2)CCP1(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575770
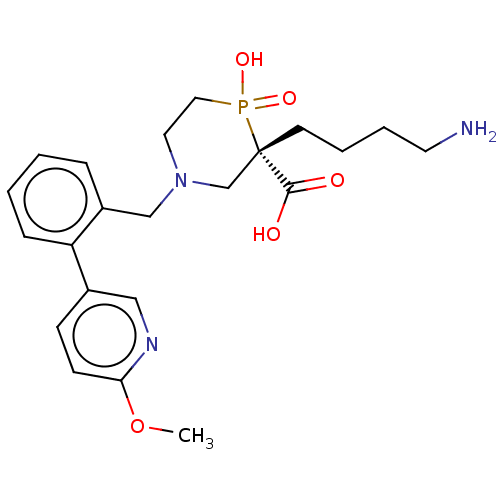
(CHEMBL4858095)Show SMILES COc1ccc(cn1)-c1ccccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373663
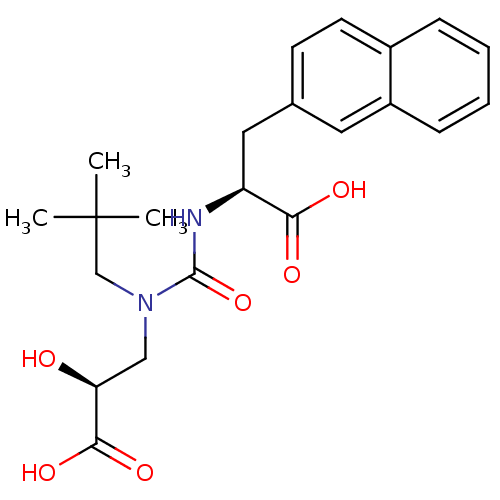
(CHEMBL273142)Show SMILES CC(C)(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(O)=O Show InChI InChI=1S/C22H28N2O6/c1-22(2,3)13-24(12-18(25)20(28)29)21(30)23-17(19(26)27)11-14-8-9-15-6-4-5-7-16(15)10-14/h4-10,17-18,25H,11-13H2,1-3H3,(H,23,30)(H,26,27)(H,28,29)/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575780

(CHEMBL4878039)Show SMILES Cn1cncc1-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373661
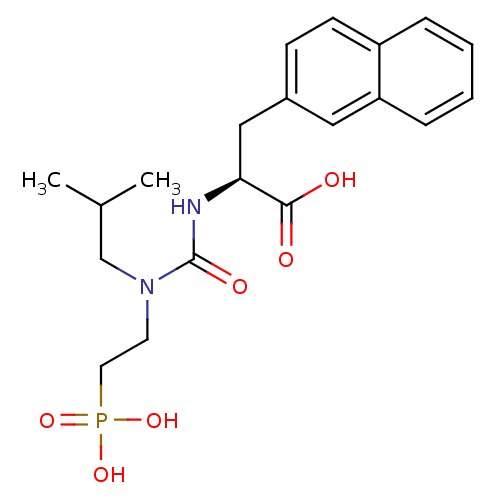
(CHEMBL257251)Show SMILES CC(C)CN(CCP(O)(O)=O)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(O)=O Show InChI InChI=1S/C20H27N2O6P/c1-14(2)13-22(9-10-29(26,27)28)20(25)21-18(19(23)24)12-15-7-8-16-5-3-4-6-17(16)11-15/h3-8,11,14,18H,9-10,12-13H2,1-2H3,(H,21,25)(H,23,24)(H2,26,27,28)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373668
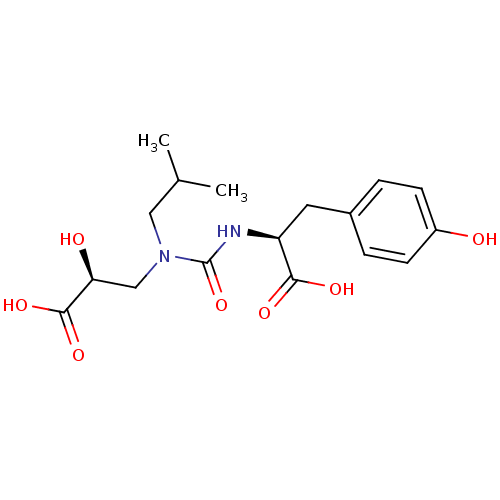
(CHEMBL270576)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C17H24N2O7/c1-10(2)8-19(9-14(21)16(24)25)17(26)18-13(15(22)23)7-11-3-5-12(20)6-4-11/h3-6,10,13-14,20-21H,7-9H2,1-2H3,(H,18,26)(H,22,23)(H,24,25)/t13-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575769
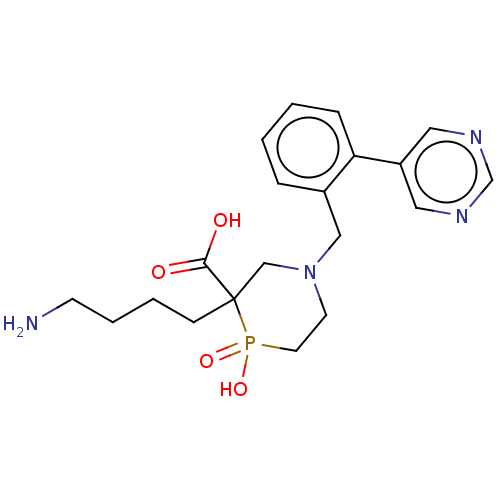
(CHEMBL4853133)Show SMILES NCCCCC1(CN(Cc2ccccc2-c2cncnc2)CCP1(O)=O)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575777
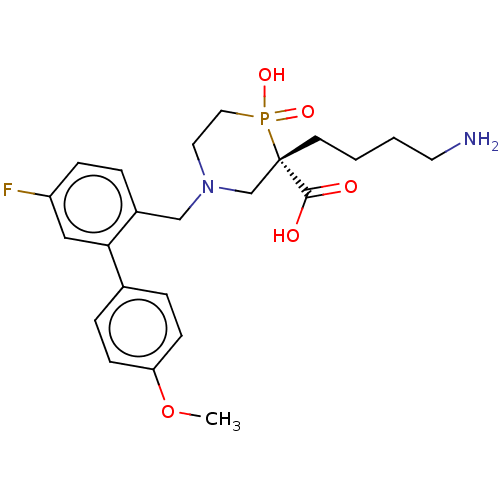
(CHEMBL4852722)Show SMILES COc1ccc(cc1)-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575779
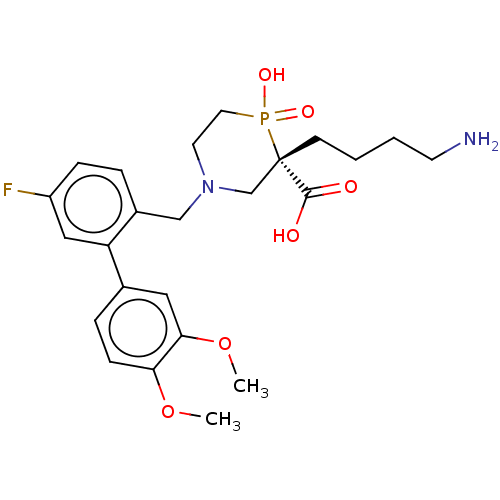
(CHEMBL4858909)Show SMILES COc1ccc(cc1OC)-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373660
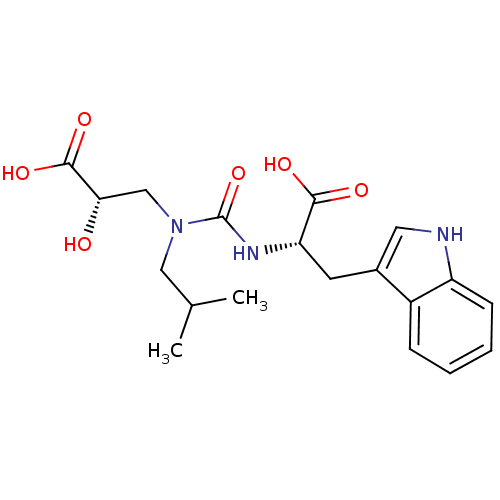
(CHEMBL255568)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C19H25N3O6/c1-11(2)9-22(10-16(23)18(26)27)19(28)21-15(17(24)25)7-12-8-20-14-6-4-3-5-13(12)14/h3-6,8,11,15-16,20,23H,7,9-10H2,1-2H3,(H,21,28)(H,24,25)(H,26,27)/t15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373664
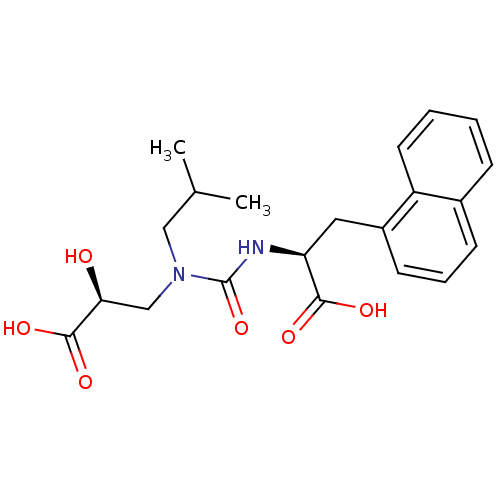
(CHEMBL409920)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(O)=O Show InChI InChI=1S/C21H26N2O6/c1-13(2)11-23(12-18(24)20(27)28)21(29)22-17(19(25)26)10-15-8-5-7-14-6-3-4-9-16(14)15/h3-9,13,17-18,24H,10-12H2,1-2H3,(H,22,29)(H,25,26)(H,27,28)/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575771

(CHEMBL4852607)Show SMILES Cn1nc(cc1-c1ccccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575772
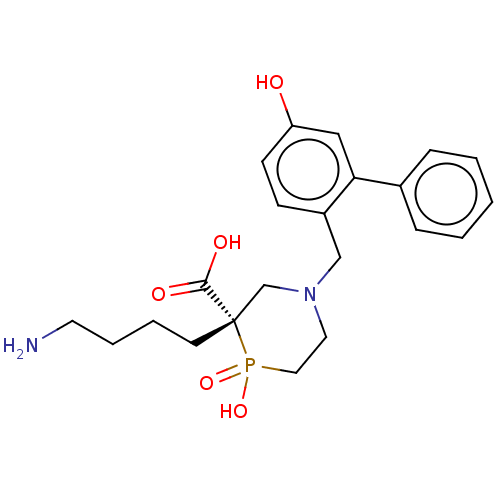
(CHEMBL4875723)Show SMILES NCCCC[C@]1(CN(Cc2ccc(O)cc2-c2ccccc2)CCP1(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575773
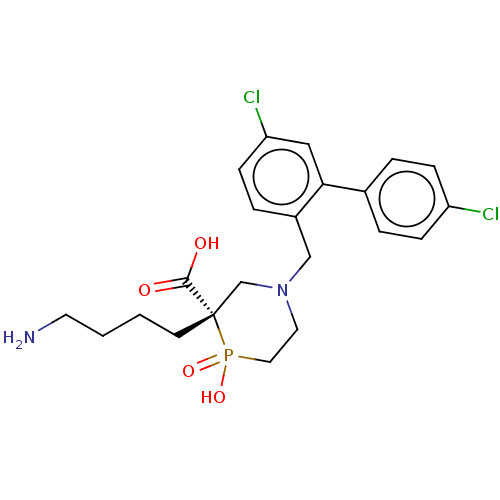
(CHEMBL4861840)Show SMILES NCCCC[C@]1(CN(Cc2ccc(Cl)cc2-c2ccc(Cl)cc2)CCP1(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373669
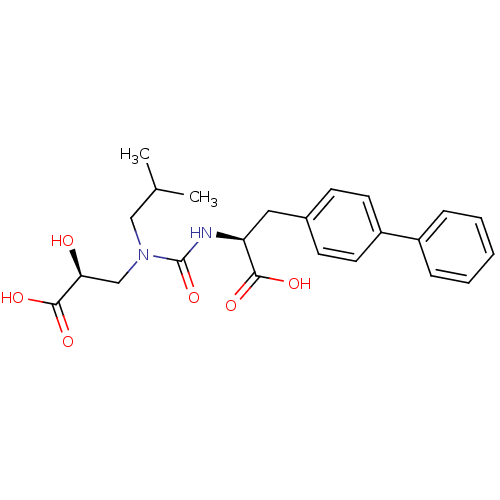
(CHEMBL409720)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C23H28N2O6/c1-15(2)13-25(14-20(26)22(29)30)23(31)24-19(21(27)28)12-16-8-10-18(11-9-16)17-6-4-3-5-7-17/h3-11,15,19-20,26H,12-14H2,1-2H3,(H,24,31)(H,27,28)(H,29,30)/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575774
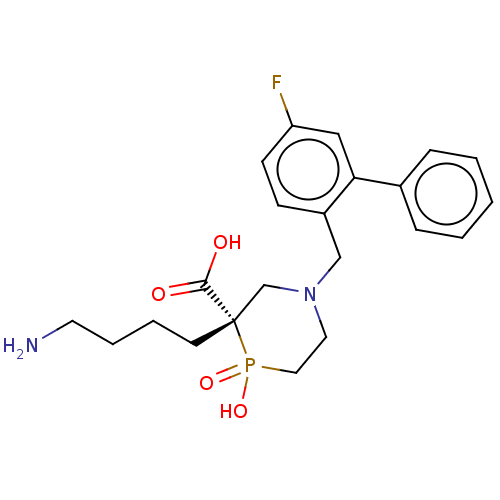
(CHEMBL4854445)Show SMILES NCCCC[C@]1(CN(Cc2ccc(F)cc2-c2ccccc2)CCP1(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575764

(CHEMBL4866725)Show SMILES NCCCCC1(CN(Cc2ccccc2-c2ccccc2)CCP1(O)=O)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373659
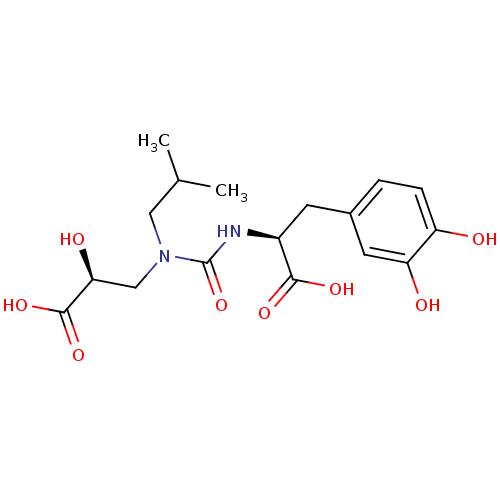
(CHEMBL258278)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1ccc(O)c(O)c1)C(O)=O Show InChI InChI=1S/C17H24N2O8/c1-9(2)7-19(8-14(22)16(25)26)17(27)18-11(15(23)24)5-10-3-4-12(20)13(21)6-10/h3-4,6,9,11,14,20-22H,5,7-8H2,1-2H3,(H,18,27)(H,23,24)(H,25,26)/t11-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50373659

(CHEMBL258278)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1ccc(O)c(O)c1)C(O)=O Show InChI InChI=1S/C17H24N2O8/c1-9(2)7-19(8-14(22)16(25)26)17(27)18-11(15(23)24)5-10-3-4-12(20)13(21)6-10/h3-4,6,9,11,14,20-22H,5,7-8H2,1-2H3,(H,18,27)(H,23,24)(H,25,26)/t11-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50373665

(CHEMBL442455)Show SMILES CC(C)CN(CP(O)(O)=O)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(O)=O Show InChI InChI=1S/C19H25N2O6P/c1-13(2)11-21(12-28(25,26)27)19(24)20-17(18(22)23)10-14-7-8-15-5-3-4-6-16(15)9-14/h3-9,13,17H,10-12H2,1-2H3,(H,20,24)(H,22,23)(H2,25,26,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575778

(CHEMBL4868496)Show SMILES COc1ccc(cc1)-c1cccc(F)c1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50373669

(CHEMBL409720)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C23H28N2O6/c1-15(2)13-25(14-20(26)22(29)30)23(31)24-19(21(27)28)12-16-8-10-18(11-9-16)17-6-4-3-5-7-17/h3-11,15,19-20,26H,12-14H2,1-2H3,(H,24,31)(H,27,28)(H,29,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373672
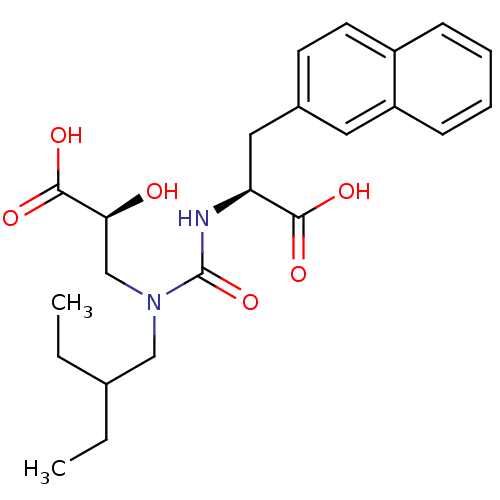
(CHEMBL257416)Show SMILES CCC(CC)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(O)=O Show InChI InChI=1S/C23H30N2O6/c1-3-15(4-2)13-25(14-20(26)22(29)30)23(31)24-19(21(27)28)12-16-9-10-17-7-5-6-8-18(17)11-16/h5-11,15,19-20,26H,3-4,12-14H2,1-2H3,(H,24,31)(H,27,28)(H,29,30)/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50373667
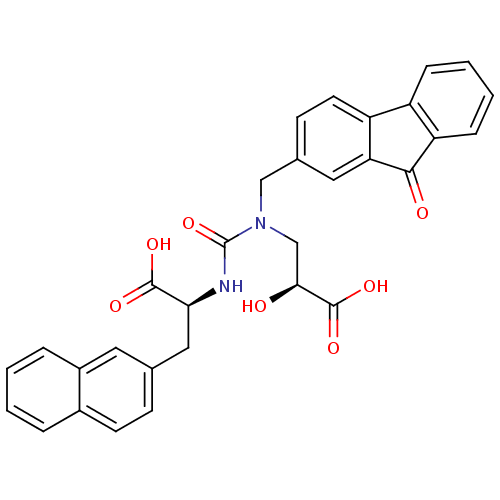
(CHEMBL258130)Show SMILES O[C@@H](CN(Cc1ccc2-c3ccccc3C(=O)c2c1)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(O)=O)C(O)=O Show InChI InChI=1S/C31H26N2O7/c34-27(30(38)39)17-33(16-19-10-12-23-22-7-3-4-8-24(22)28(35)25(23)14-19)31(40)32-26(29(36)37)15-18-9-11-20-5-1-2-6-21(20)13-18/h1-14,26-27,34H,15-17H2,(H,32,40)(H,36,37)(H,38,39)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50127745
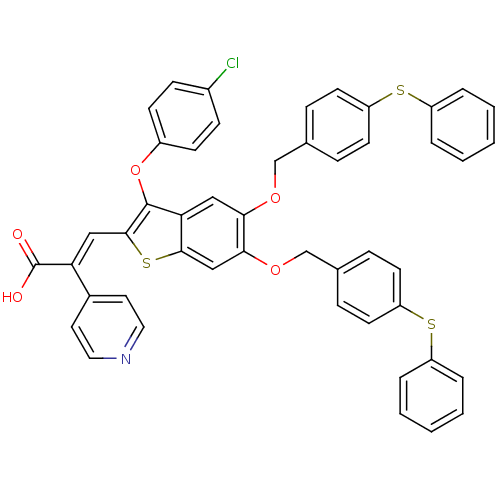
((E)-3-[3-(4-Chloro-phenoxy)-5,6-bis-(4-phenylsulfa...)Show SMILES OC(=O)C(=C\c1sc2cc(OCc3ccc(Sc4ccccc4)cc3)c(OCc3ccc(Sc4ccccc4)cc3)cc2c1Oc1ccc(Cl)cc1)\c1ccncc1 Show InChI InChI=1S/C48H34ClNO5S3/c49-35-15-17-36(18-16-35)55-47-42-27-43(53-30-32-11-19-39(20-12-32)56-37-7-3-1-4-8-37)44(54-31-33-13-21-40(22-14-33)57-38-9-5-2-6-10-38)29-45(42)58-46(47)28-41(48(51)52)34-23-25-50-26-24-34/h1-29H,30-31H2,(H,51,52)/b41-28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... |
Bioorg Med Chem Lett 13: 1705-8 (2003)
BindingDB Entry DOI: 10.7270/Q2J965SB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50373658

(CHEMBL273140)Show SMILES O[C@@H](CN(Cc1ccc2C(=O)c3ccccc3C(=O)c2c1)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(O)=O)C(O)=O Show InChI InChI=1S/C32H26N2O8/c35-27(31(40)41)17-34(16-19-10-12-24-25(14-19)29(37)23-8-4-3-7-22(23)28(24)36)32(42)33-26(30(38)39)15-18-9-11-20-5-1-2-6-21(20)13-18/h1-14,26-27,35H,15-17H2,(H,33,42)(H,38,39)(H,40,41)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50373657

(CHEMBL450935)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(O)=O Show InChI InChI=1S/C21H26N2O6/c1-13(2)11-23(12-18(24)20(27)28)21(29)22-17(19(25)26)10-14-7-8-15-5-3-4-6-16(15)9-14/h3-9,13,17-18,24H,10-12H2,1-2H3,(H,22,29)(H,25,26)(H,27,28)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50373668

(CHEMBL270576)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C17H24N2O7/c1-10(2)8-19(9-14(21)16(24)25)17(26)18-13(15(22)23)7-11-3-5-12(20)6-4-11/h3-6,10,13-14,20-21H,7-9H2,1-2H3,(H,18,26)(H,22,23)(H,24,25)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575763
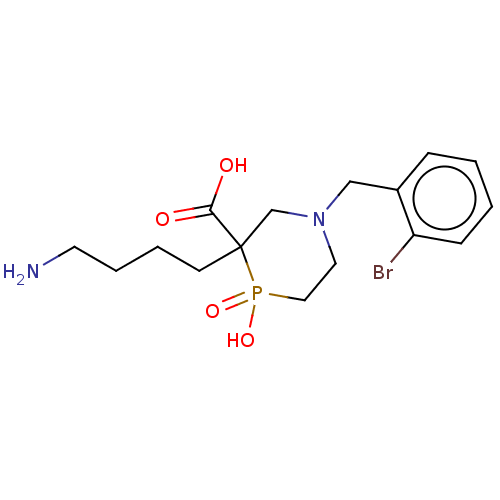
(CHEMBL4858525) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50373671
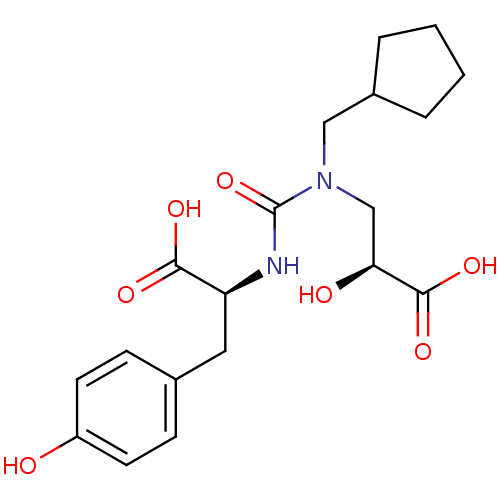
(CHEMBL272575)Show SMILES O[C@@H](CN(CC1CCCC1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)C(O)=O Show InChI InChI=1S/C19H26N2O7/c22-14-7-5-12(6-8-14)9-15(17(24)25)20-19(28)21(11-16(23)18(26)27)10-13-3-1-2-4-13/h5-8,13,15-16,22-23H,1-4,9-11H2,(H,20,28)(H,24,25)(H,26,27)/t15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50193234
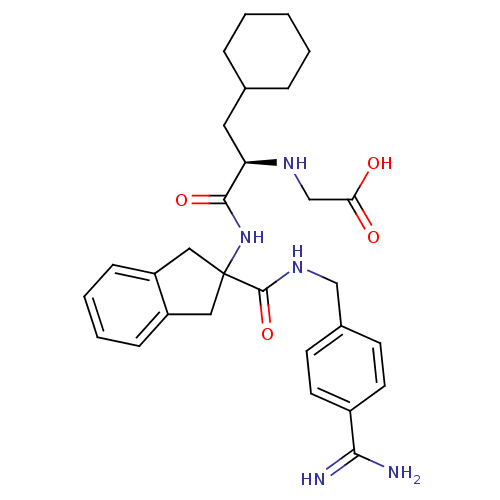
(CHEMBL211973 | {(R)-1-[2-(4-carbamimidoyl-benzylca...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 Show InChI InChI=1S/C29H37N5O4/c30-26(31)21-12-10-20(11-13-21)17-33-28(38)29(15-22-8-4-5-9-23(22)16-29)34-27(37)24(32-18-25(35)36)14-19-6-2-1-3-7-19/h4-5,8-13,19,24,32H,1-3,6-7,14-18H2,(H3,30,31)(H,33,38)(H,34,37)(H,35,36)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of human aPC |
J Med Chem 49: 5047-50 (2006)
Article DOI: 10.1021/jm0606950
BindingDB Entry DOI: 10.7270/Q2H994VN |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575741

(CHEMBL4856598)Show SMILES NCCCC[C@]1(CN(Cc2ccccc2)CCP1(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50127735
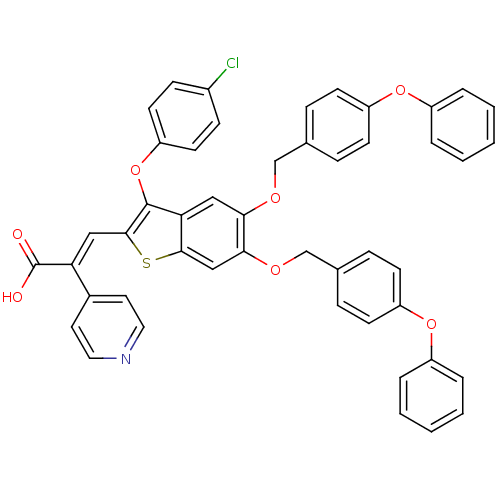
((E)-3-[3-(4-Chloro-phenoxy)-5,6-bis-(4-phenoxy-ben...)Show SMILES OC(=O)C(=C\c1sc2cc(OCc3ccc(Oc4ccccc4)cc3)c(OCc3ccc(Oc4ccccc4)cc3)cc2c1Oc1ccc(Cl)cc1)\c1ccncc1 Show InChI InChI=1S/C48H34ClNO7S/c49-35-15-21-40(22-16-35)57-47-42-27-43(53-30-32-11-17-38(18-12-32)55-36-7-3-1-4-8-36)44(54-31-33-13-19-39(20-14-33)56-37-9-5-2-6-10-37)29-45(42)58-46(47)28-41(48(51)52)34-23-25-50-26-24-34/h1-29H,30-31H2,(H,51,52)/b41-28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... |
Bioorg Med Chem Lett 13: 1705-8 (2003)
BindingDB Entry DOI: 10.7270/Q2J965SB |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50127733
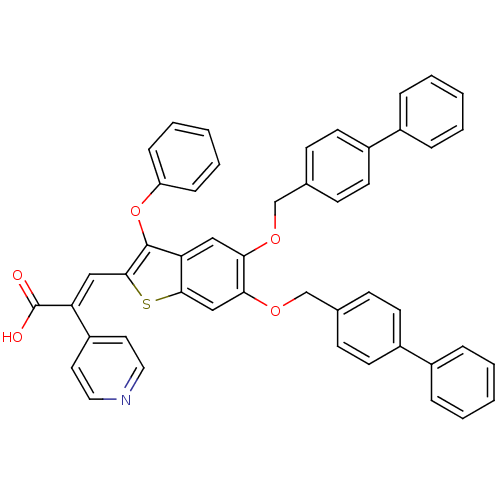
((E)-3-[5,6-Bis-(biphenyl-4-ylmethoxy)-3-phenoxy-be...)Show SMILES OC(=O)C(=C\c1sc2cc(OCc3ccc(cc3)-c3ccccc3)c(OCc3ccc(cc3)-c3ccccc3)cc2c1Oc1ccccc1)\c1ccncc1 Show InChI InChI=1S/C48H35NO5S/c50-48(51)41(39-24-26-49-27-25-39)29-46-47(54-40-14-8-3-9-15-40)42-28-43(52-31-33-16-20-37(21-17-33)35-10-4-1-5-11-35)44(30-45(42)55-46)53-32-34-18-22-38(23-19-34)36-12-6-2-7-13-36/h1-30H,31-32H2,(H,50,51)/b41-29+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... |
Bioorg Med Chem Lett 13: 1705-8 (2003)
BindingDB Entry DOI: 10.7270/Q2J965SB |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575757

(CHEMBL4861694) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50373660

(CHEMBL255568)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C19H25N3O6/c1-11(2)9-22(10-16(23)18(26)27)19(28)21-15(17(24)25)7-12-8-20-14-6-4-3-5-13(12)14/h3-6,8,11,15-16,20,23H,7,9-10H2,1-2H3,(H,21,28)(H,24,25)(H,26,27)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50373664

(CHEMBL409920)Show SMILES CC(C)CN(C[C@H](O)C(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(O)=O Show InChI InChI=1S/C21H26N2O6/c1-13(2)11-23(12-18(24)20(27)28)21(29)22-17(19(25)26)10-15-8-5-7-14-6-3-4-9-16(14)15/h3-9,13,17-18,24H,10-12H2,1-2H3,(H,22,29)(H,25,26)(H,27,28)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575761

(CHEMBL4846016) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50127743
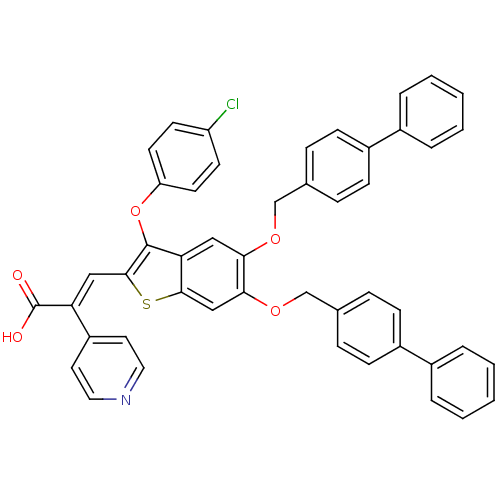
((E)-3-[5,6-Bis-(biphenyl-4-ylmethoxy)-3-(4-chloro-...)Show SMILES OC(=O)C(=C\c1sc2cc(OCc3ccc(cc3)-c3ccccc3)c(OCc3ccc(cc3)-c3ccccc3)cc2c1Oc1ccc(Cl)cc1)\c1ccncc1 Show InChI InChI=1S/C48H34ClNO5S/c49-39-19-21-40(22-20-39)55-47-42-27-43(53-30-32-11-15-36(16-12-32)34-7-3-1-4-8-34)44(54-31-33-13-17-37(18-14-33)35-9-5-2-6-10-35)29-45(42)56-46(47)28-41(48(51)52)38-23-25-50-26-24-38/h1-29H,30-31H2,(H,51,52)/b41-28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... |
Bioorg Med Chem Lett 13: 1705-8 (2003)
BindingDB Entry DOI: 10.7270/Q2J965SB |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50127732
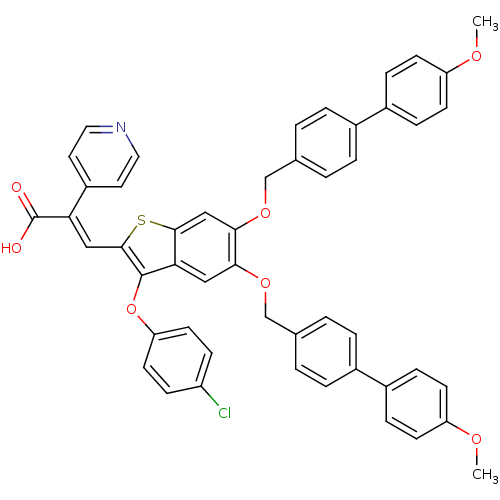
((E)-3-[3-(4-Chloro-phenoxy)-5,6-bis-(4'-methoxy-bi...)Show SMILES COc1ccc(cc1)-c1ccc(COc2cc3sc(\C=C(\C(O)=O)c4ccncc4)c(Oc4ccc(Cl)cc4)c3cc2OCc2ccc(cc2)-c2ccc(OC)cc2)cc1 Show InChI InChI=1S/C50H38ClNO7S/c1-55-40-17-11-36(12-18-40)34-7-3-32(4-8-34)30-57-45-27-44-47(29-46(45)58-31-33-5-9-35(10-6-33)37-13-19-41(56-2)20-14-37)60-48(49(44)59-42-21-15-39(51)16-22-42)28-43(50(53)54)38-23-25-52-26-24-38/h3-29H,30-31H2,1-2H3,(H,53,54)/b43-28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... |
Bioorg Med Chem Lett 13: 1705-8 (2003)
BindingDB Entry DOI: 10.7270/Q2J965SB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50373662
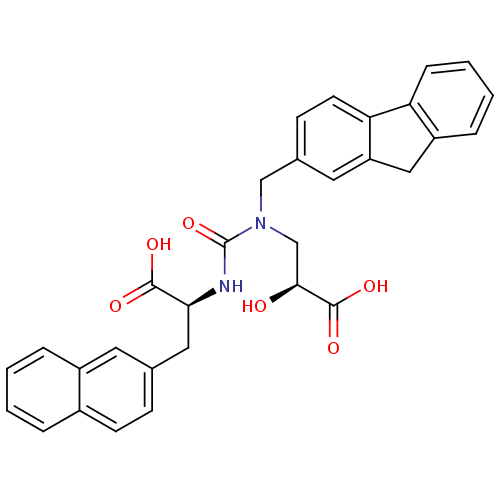
(CHEMBL256895)Show SMILES O[C@@H](CN(Cc1ccc-2c(Cc3ccccc-23)c1)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(O)=O)C(O)=O Show InChI InChI=1S/C31H28N2O6/c34-28(30(37)38)18-33(17-20-10-12-26-24(14-20)16-23-7-3-4-8-25(23)26)31(39)32-27(29(35)36)15-19-9-11-21-5-1-2-6-22(21)13-19/h1-14,27-28,34H,15-18H2,(H,32,39)(H,35,36)(H,37,38)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem Lett 18: 1058-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.013
BindingDB Entry DOI: 10.7270/Q23N2483 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575760

(CHEMBL4855391) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575758

(CHEMBL4847492) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data