 Found 693 hits with Last Name = 'schultz' and Initial = 'm'
Found 693 hits with Last Name = 'schultz' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50288820
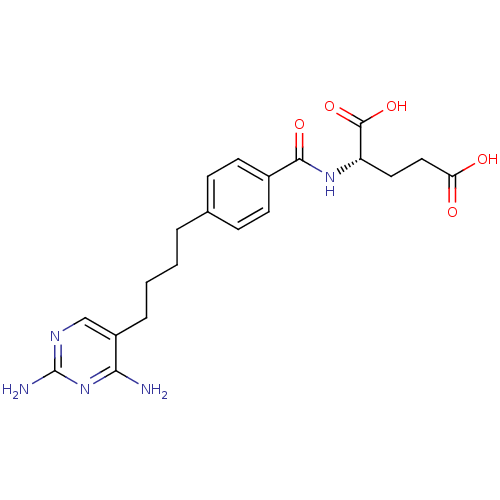
((S)-2-{(S)-4-[4-(2,4-Diamino-pyrimidin-5-yl)-butyl...)Show SMILES Nc1ncc(CCCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C20H25N5O5/c21-17-14(11-23-20(22)25-17)4-2-1-3-12-5-7-13(8-6-12)18(28)24-15(19(29)30)9-10-16(26)27/h5-8,11,15H,1-4,9-10H2,(H,24,28)(H,26,27)(H,29,30)(H4,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50288821

((S)-2-{(S)-4-[3-(2,4-Diamino-pyrimidin-5-yl)-propy...)Show SMILES Nc1ncc(CCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C19H23N5O5/c20-16-13(10-22-19(21)24-16)3-1-2-11-4-6-12(7-5-11)17(27)23-14(18(28)29)8-9-15(25)26/h4-7,10,14H,1-3,8-9H2,(H,23,27)(H,25,26)(H,28,29)(H4,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50073754
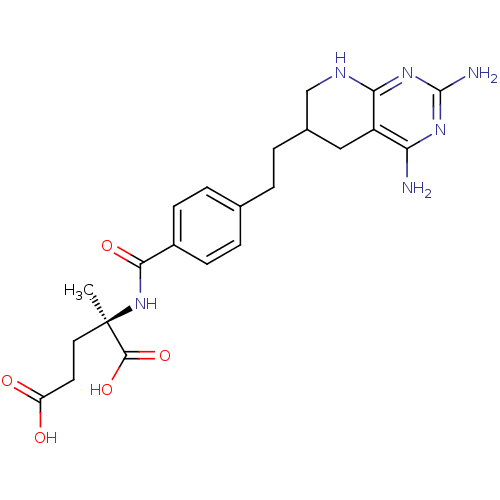
((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)cc1)C(O)=O Show InChI InChI=1S/C22H28N6O5/c1-22(20(32)33,9-8-16(29)30)28-19(31)14-6-4-12(5-7-14)2-3-13-10-15-17(23)26-21(24)27-18(15)25-11-13/h4-7,13H,2-3,8-11H2,1H3,(H,28,31)(H,29,30)(H,32,33)(H5,23,24,25,26,27)/t13?,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50073752
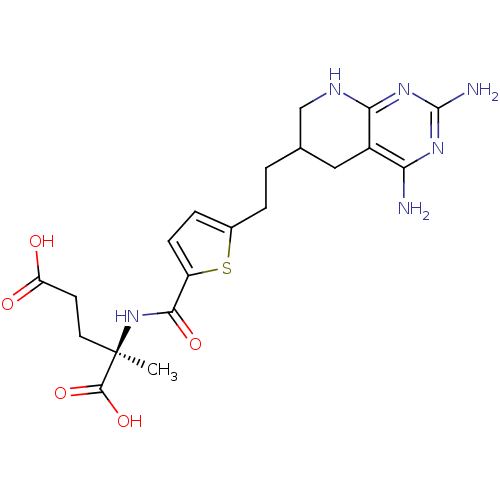
((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)s1)C(O)=O Show InChI InChI=1S/C20H26N6O5S/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50052337
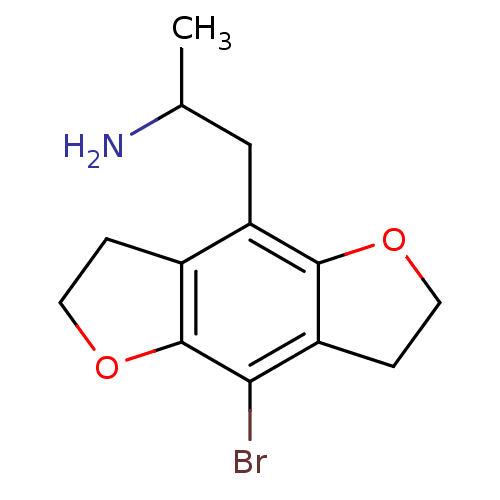
(2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...)Show InChI InChI=1S/C13H16BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h7H,2-6,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-La Crosse
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem 16: 6242-51 (2008)
Article DOI: 10.1016/j.bmc.2008.04.030
BindingDB Entry DOI: 10.7270/Q2QZ29R4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50271489
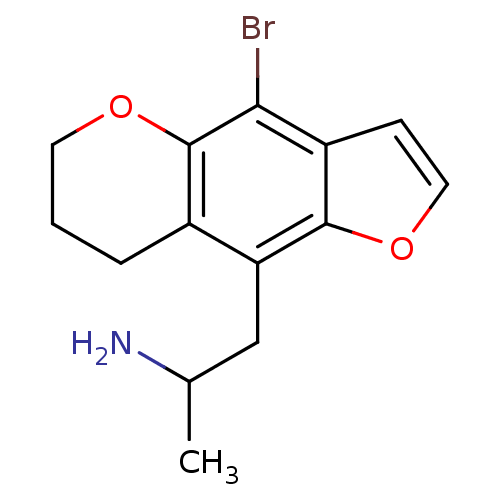
(1-(4-Bromo-7,8-dihydro-6H-furo[2,3-g]chromen-9-yl)...)Show InChI InChI=1S/C14H16BrNO2/c1-8(16)7-11-9-3-2-5-17-14(9)12(15)10-4-6-18-13(10)11/h4,6,8H,2-3,5,7,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-La Crosse
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem 16: 6242-51 (2008)
Article DOI: 10.1016/j.bmc.2008.04.030
BindingDB Entry DOI: 10.7270/Q2QZ29R4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50271484
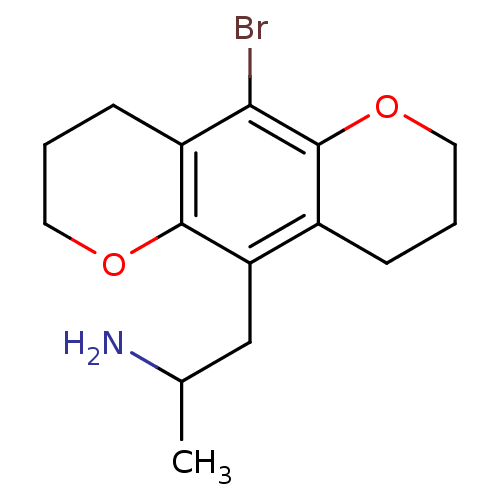
(2-(10-Bromo-2,3,4,7,8,9-hexahydro-pyrano[2,3-g]chr...)Show InChI InChI=1S/C15H20BrNO2/c1-9(17)8-12-10-4-2-7-19-15(10)13(16)11-5-3-6-18-14(11)12/h9H,2-8,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-La Crosse
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem 16: 6242-51 (2008)
Article DOI: 10.1016/j.bmc.2008.04.030
BindingDB Entry DOI: 10.7270/Q2QZ29R4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50271486
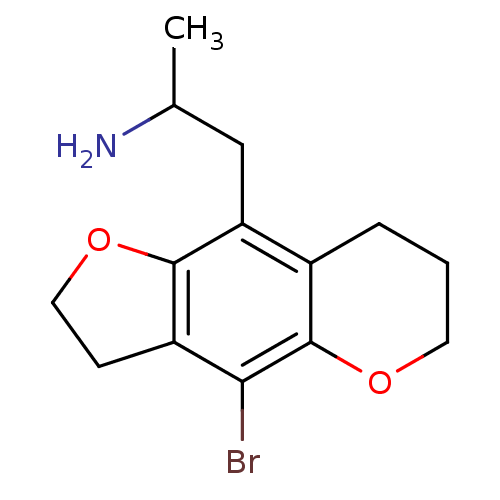
(1-(4-Bromo-3,6,7,8-tetrahydro-2H-furo[2,3-g]chrome...)Show InChI InChI=1S/C14H18BrNO2/c1-8(16)7-11-9-3-2-5-17-14(9)12(15)10-4-6-18-13(10)11/h8H,2-7,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-La Crosse
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem 16: 6242-51 (2008)
Article DOI: 10.1016/j.bmc.2008.04.030
BindingDB Entry DOI: 10.7270/Q2QZ29R4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50271483
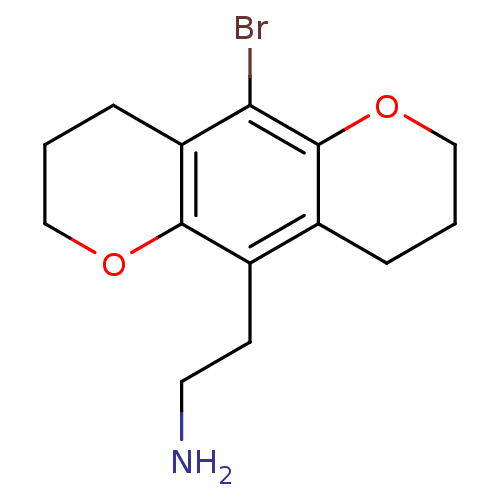
(2-(10-Bromo-2,3,4,7,8,9-hexahydro-pyrano[2,3-g]chr...)Show InChI InChI=1S/C14H18BrNO2/c15-12-11-4-2-7-17-13(11)10(5-6-16)9-3-1-8-18-14(9)12/h1-8,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-La Crosse
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem 16: 6242-51 (2008)
Article DOI: 10.1016/j.bmc.2008.04.030
BindingDB Entry DOI: 10.7270/Q2QZ29R4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50271488
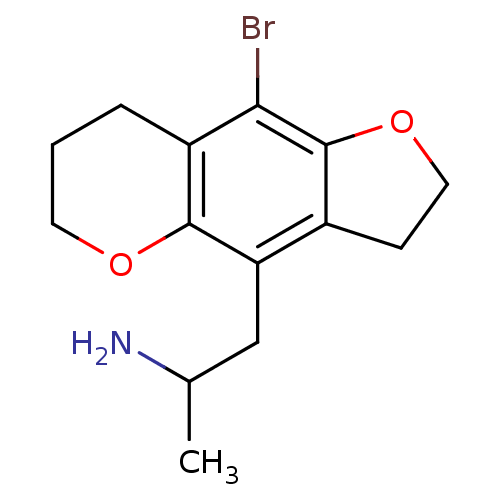
(1-(9-Bromo-3,6,7,8-tetrahydro-2H-furo[2,3-g]chrome...)Show InChI InChI=1S/C14H18BrNO2/c1-8(16)7-11-9-4-6-18-14(9)12(15)10-3-2-5-17-13(10)11/h8H,2-7,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-La Crosse
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem 16: 6242-51 (2008)
Article DOI: 10.1016/j.bmc.2008.04.030
BindingDB Entry DOI: 10.7270/Q2QZ29R4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50271487
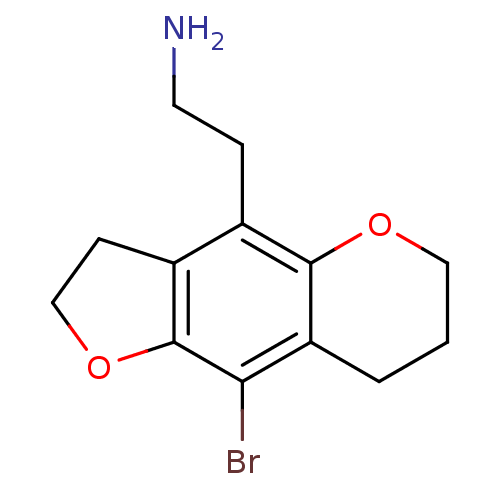
(2-(9-Bromo-3,6,7,8-tetrahydro-2H-furo[2,3-g]chrome...)Show InChI InChI=1S/C13H16BrNO2/c14-11-10-2-1-6-16-12(10)8(3-5-15)9-4-7-17-13(9)11/h1-7,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-La Crosse
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem 16: 6242-51 (2008)
Article DOI: 10.1016/j.bmc.2008.04.030
BindingDB Entry DOI: 10.7270/Q2QZ29R4 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50073753

((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)o1)C(O)=O Show InChI InChI=1S/C20H26N6O6/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50271485
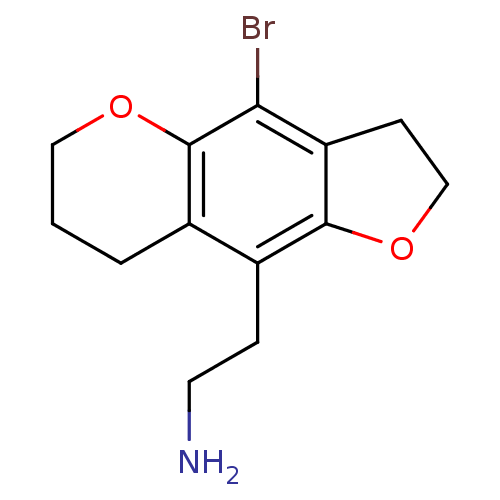
(2-(4-Bromo-3,6,7,8-tetrahydro-2H-furo[2,3-g]chrome...)Show InChI InChI=1S/C13H16BrNO2/c14-11-10-4-7-17-12(10)9(3-5-15)8-2-1-6-16-13(8)11/h1-7,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-La Crosse
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem 16: 6242-51 (2008)
Article DOI: 10.1016/j.bmc.2008.04.030
BindingDB Entry DOI: 10.7270/Q2QZ29R4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50005257
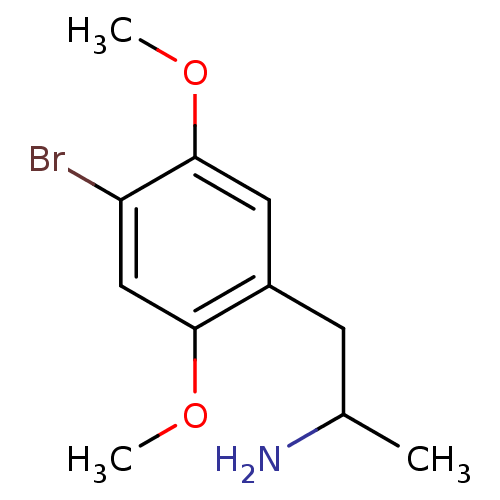
((+)-2-(4-Bromo-2,5-dimethoxy-phenyl)-1-methyl-ethy...)Show InChI InChI=1S/C11H16BrNO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-La Crosse
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem 16: 6242-51 (2008)
Article DOI: 10.1016/j.bmc.2008.04.030
BindingDB Entry DOI: 10.7270/Q2QZ29R4 |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50073753

((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)o1)C(O)=O Show InChI InChI=1S/C20H26N6O6/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine glycinamide ribonucleotide formyltransferase (GARFT) enzyme |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50073754

((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)cc1)C(O)=O Show InChI InChI=1S/C22H28N6O5/c1-22(20(32)33,9-8-16(29)30)28-19(31)14-6-4-12(5-7-14)2-3-13-10-15-17(23)26-21(24)27-18(15)25-11-13/h4-7,13H,2-3,8-11H2,1H3,(H,28,31)(H,29,30)(H,32,33)(H5,23,24,25,26,27)/t13?,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Thymidylate synthase |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50073753

((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)o1)C(O)=O Show InChI InChI=1S/C20H26N6O6/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Thymidylate synthase enzyme |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50073752

((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)s1)C(O)=O Show InChI InChI=1S/C20H26N6O5S/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Thymidylate synthase enzyme |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50288820

((S)-2-{(S)-4-[4-(2,4-Diamino-pyrimidin-5-yl)-butyl...)Show SMILES Nc1ncc(CCCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C20H25N5O5/c21-17-14(11-23-20(22)25-17)4-2-1-3-12-5-7-13(8-6-12)18(28)24-15(19(29)30)9-10-16(26)27/h5-8,11,15H,1-4,9-10H2,(H,24,28)(H,26,27)(H,29,30)(H4,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human thymidylate synthase (TS). |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50288821

((S)-2-{(S)-4-[3-(2,4-Diamino-pyrimidin-5-yl)-propy...)Show SMILES Nc1ncc(CCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C19H23N5O5/c20-16-13(10-22-19(21)24-16)3-1-2-11-4-6-12(7-5-11)17(27)23-14(18(28)29)8-9-15(25)26/h4-7,10,14H,1-3,8-9H2,(H,23,27)(H,25,26)(H,28,29)(H4,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human thymidylate synthase (TS) |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50073752

((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)s1)C(O)=O Show InChI InChI=1S/C20H26N6O5S/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine glycinamide ribonucleotide formyltransferase (GARFT) enzyme |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50073754

((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)cc1)C(O)=O Show InChI InChI=1S/C22H28N6O5/c1-22(20(32)33,9-8-16(29)30)28-19(31)14-6-4-12(5-7-14)2-3-13-10-15-17(23)26-21(24)27-18(15)25-11-13/h4-7,13H,2-3,8-11H2,1H3,(H,28,31)(H,29,30)(H,32,33)(H5,23,24,25,26,27)/t13?,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine glycinamide ribonucleotide formyltransferase (GARFT) enzyme |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50354719
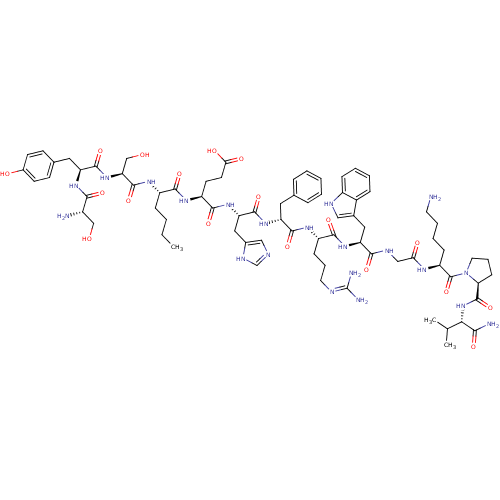
(CHEMBL1834391)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:26.27,8.10,4.4,74.76,92.96,108.113,wD:14.23,33.33,42.42,52.53,63.65,104.110,(10.67,7.94,;10.68,6.4,;9.35,5.63,;9.35,4.09,;8.01,3.31,;6.68,4.09,;5.35,3.31,;5.35,1.78,;4.01,4.09,;4.01,5.63,;5.35,6.4,;2.68,3.31,;1.35,4.09,;1.35,5.63,;.01,3.31,;.01,1.78,;1.35,1,;2.67,1.77,;4,1.01,;4,-.54,;5.33,-1.31,;2.67,-1.31,;1.35,-.53,;-1.32,4.09,;-2.66,3.31,;-2.66,1.78,;-3.99,4.09,;-5.32,3.31,;-3.99,5.63,;-5.32,6.4,;8.01,1.78,;6.68,1,;9.35,1,;9.35,-.53,;8.01,-1.31,;8.01,-2.84,;6.68,-3.62,;5.35,-2.84,;6.68,-5.15,;10.68,-1.31,;12.02,-.53,;10.68,-2.84,;12.02,-3.62,;12.02,-5.15,;10.68,-5.93,;10.52,-7.46,;9.02,-7.78,;8.25,-6.45,;9.28,-5.3,;13.35,-2.84,;13.35,-1.31,;14.68,-3.62,;16.02,-2.84,;16.02,-1.31,;17.36,-.53,;18.68,-1.3,;20.02,-.53,;20.02,1,;18.68,1.78,;17.36,1,;17.36,-3.62,;17.36,-5.15,;18.69,-2.84,;20.02,-3.62,;20.02,-5.15,;21.35,-5.93,;21.35,-7.46,;22.69,-8.24,;22.69,-9.77,;21.35,-10.55,;24.02,-10.55,;21.35,-2.84,;21.35,-1.31,;22.69,-3.62,;24.02,-2.84,;24.02,-1.31,;25.36,-.53,;26.76,-1.16,;27.79,-.02,;27.03,1.32,;27.5,2.78,;26.48,3.93,;24.97,3.61,;24.49,2.15,;25.52,1,;25.36,-3.62,;25.36,-5.15,;26.7,-2.84,;28.03,-3.62,;29.36,-2.84,;29.36,-1.31,;30.69,-3.62,;32.03,-2.84,;32.03,-1.31,;30.69,-.53,;30.69,1,;32.03,1.78,;32.03,3.31,;33.37,-3.62,;34.7,-2.84,;33.37,-5.15,;32.11,-6.07,;32.59,-7.53,;34.13,-7.53,;34.61,-6.06,;35.91,-5.24,;35.92,-3.7,;37.24,-6.01,;38.58,-5.25,;38.58,-3.72,;39.92,-2.96,;37.26,-2.94,;39.9,-6.04,;39.89,-7.58,;41.24,-5.28,)| Show InChI InChI=1S/C76H109N21O18/c1-4-5-18-51(89-73(113)59(40-99)95-71(111)55(91-65(105)49(78)39-98)33-44-23-25-47(100)26-24-44)67(107)90-53(27-28-62(102)103)69(109)94-58(35-46-37-82-41-86-46)72(112)92-56(32-43-15-7-6-8-16-43)70(110)88-52(21-13-30-83-76(80)81)68(108)93-57(34-45-36-84-50-19-10-9-17-48(45)50)66(106)85-38-61(101)87-54(20-11-12-29-77)75(115)97-31-14-22-60(97)74(114)96-63(42(2)3)64(79)104/h6-10,15-17,19,23-26,36-37,41-42,49,51-60,63,84,98-100H,4-5,11-14,18,20-22,27-35,38-40,77-78H2,1-3H3,(H2,79,104)(H,82,86)(H,85,106)(H,87,101)(H,88,110)(H,89,113)(H,90,107)(H,91,105)(H,92,112)(H,93,108)(H,94,109)(H,95,111)(H,96,114)(H,102,103)(H4,80,81,83)/t49-,51-,52-,53-,54-,55-,56+,57-,58-,59-,60-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle4-D-Phe7]-alpha-MSH from MC1R in mouse B16-F10 cells after 1.5 hrs by gamma counting |
Bioorg Med Chem Lett 21: 5757-61 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.017
BindingDB Entry DOI: 10.7270/Q2H132DZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50354718
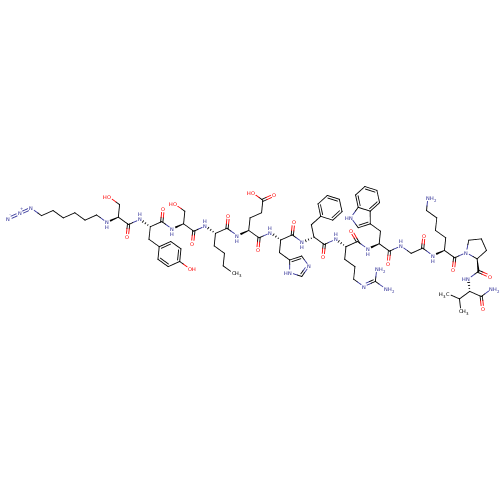
(CHEMBL1834392)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NCCCCCCN=[N+]=[N-])C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:26.29,8.10,4.4,83.85,101.105,117.122,wD:14.23,42.42,51.51,61.62,72.74,113.119,(18.25,-8.83,;18.25,-10.37,;16.92,-11.14,;16.92,-12.68,;15.59,-13.46,;14.26,-12.68,;12.92,-13.46,;12.92,-14.99,;11.58,-12.68,;11.58,-11.14,;12.92,-10.37,;10.25,-13.46,;8.92,-12.68,;8.92,-11.14,;7.58,-13.46,;7.58,-14.99,;8.92,-15.77,;10.25,-15,;11.57,-15.76,;11.57,-17.31,;12.9,-18.08,;10.24,-18.08,;8.92,-17.3,;6.25,-12.68,;4.91,-13.46,;4.91,-14.99,;3.58,-12.68,;3.58,-11.14,;2.25,-10.37,;2.25,-13.46,;.91,-12.69,;-.41,-13.46,;-1.75,-12.7,;-3.08,-13.47,;-4.4,-12.71,;-5.73,-13.48,;-7.06,-12.72,;-8.6,-12.71,;-10.14,-12.7,;15.59,-14.99,;14.26,-15.77,;16.92,-15.77,;16.92,-17.3,;15.59,-18.08,;15.59,-19.61,;14.26,-20.39,;12.92,-19.61,;14.26,-21.93,;18.25,-18.08,;19.59,-17.3,;18.25,-19.61,;19.59,-20.39,;19.59,-21.93,;18.25,-22.7,;18.1,-24.23,;16.59,-24.55,;15.82,-23.22,;16.86,-22.07,;20.93,-19.61,;20.93,-18.08,;22.26,-20.39,;23.59,-19.61,;23.59,-18.08,;24.93,-17.3,;26.26,-18.08,;27.59,-17.3,;27.59,-15.77,;26.26,-14.99,;24.93,-15.77,;24.93,-20.39,;24.93,-21.93,;26.27,-19.61,;27.6,-20.39,;27.6,-21.93,;28.93,-22.7,;28.93,-24.24,;30.27,-25.01,;30.27,-26.55,;28.93,-27.33,;31.6,-27.33,;28.93,-19.61,;28.93,-18.08,;30.27,-20.39,;31.6,-19.61,;31.6,-18.08,;32.94,-17.3,;34.34,-17.93,;35.38,-16.79,;34.61,-15.45,;35.09,-13.99,;34.06,-12.84,;32.55,-13.16,;32.07,-14.62,;33.1,-15.77,;32.94,-20.39,;32.94,-21.93,;34.28,-19.61,;35.61,-20.39,;36.94,-19.61,;36.94,-18.08,;38.28,-20.39,;39.61,-19.61,;39.61,-18.08,;38.28,-17.3,;38.28,-15.77,;39.61,-14.99,;39.61,-13.46,;40.95,-20.39,;42.28,-19.61,;40.95,-21.93,;39.7,-22.84,;40.17,-24.3,;41.71,-24.3,;42.2,-22.84,;43.49,-22.01,;43.5,-20.47,;44.82,-22.79,;46.16,-22.03,;46.17,-20.49,;47.51,-19.73,;44.84,-19.71,;47.49,-22.81,;47.47,-24.35,;48.83,-22.05,)| Show InChI InChI=1S/C82H120N24O18/c1-4-5-22-56(97-79(122)65(46-108)103-76(119)61(39-50-27-29-53(109)30-28-50)100-78(121)64(45-107)89-34-15-6-7-16-36-94-105-87)72(115)98-58(31-32-68(111)112)74(117)102-63(41-52-43-88-47-93-52)77(120)99-60(38-49-19-9-8-10-20-49)75(118)96-57(25-17-35-90-82(85)86)73(116)101-62(40-51-42-91-55-23-12-11-21-54(51)55)71(114)92-44-67(110)95-59(24-13-14-33-83)81(124)106-37-18-26-66(106)80(123)104-69(48(2)3)70(84)113/h8-12,19-21,23,27-30,42-43,47-48,56-66,69,89,91,107-109H,4-7,13-18,22,24-26,31-41,44-46,83H2,1-3H3,(H2,84,113)(H,88,93)(H,92,114)(H,95,110)(H,96,118)(H,97,122)(H,98,115)(H,99,120)(H,100,121)(H,101,116)(H,102,117)(H,103,119)(H,104,123)(H,111,112)(H4,85,86,90)/t56-,57-,58-,59-,60+,61-,62-,63-,64-,65-,66-,69-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle4-D-Phe7]-alpha-MSH from MC1R in mouse B16-F10 cells after 1.5 hrs by gamma counting |
Bioorg Med Chem Lett 21: 5757-61 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.017
BindingDB Entry DOI: 10.7270/Q2H132DZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50354717

(CHEMBL1834393)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:26.29,8.10,4.4,101.104,119.124,135.141,wD:14.23,60.61,69.70,79.81,90.93,131.138,(18.92,-28.29,;18.93,-29.83,;17.6,-30.6,;17.6,-32.14,;16.26,-32.92,;14.93,-32.14,;13.59,-32.92,;13.59,-34.45,;12.25,-32.14,;12.25,-30.6,;13.59,-29.83,;10.92,-32.92,;9.58,-32.14,;9.58,-30.6,;8.25,-32.92,;8.25,-34.45,;9.58,-35.23,;10.91,-34.46,;12.24,-35.22,;12.24,-36.77,;13.58,-37.54,;10.91,-37.54,;9.58,-36.76,;6.92,-32.14,;5.57,-32.92,;5.57,-34.45,;4.24,-32.14,;4.24,-30.6,;2.91,-29.83,;2.91,-32.92,;1.57,-32.15,;1.57,-30.61,;.24,-32.92,;-1.09,-32.16,;.38,-31.8,;.38,-30.27,;-1.12,-29.93,;-.04,-28.83,;-.45,-27.35,;-1.93,-26.96,;.64,-26.26,;-1.48,-28.45,;-3.03,-28.45,;-3.37,-29.93,;-4.47,-28.83,;-5.96,-29.23,;-7.04,-28.15,;-6.35,-30.72,;-4.84,-30.29,;-4.83,-31.81,;-3.32,-32.14,;-4.42,-33.23,;-4.03,-34.72,;-2.55,-35.12,;-5.12,-35.8,;-2.97,-33.65,;-1.43,-33.65,;16.26,-34.45,;14.93,-35.23,;17.6,-35.23,;17.6,-36.77,;16.26,-37.54,;16.26,-39.08,;14.93,-39.86,;13.59,-39.08,;14.93,-41.39,;18.93,-37.54,;20.27,-36.77,;18.93,-39.08,;20.27,-39.86,;20.27,-41.39,;18.93,-42.17,;18.77,-43.7,;17.27,-44.02,;16.5,-42.69,;17.53,-41.54,;21.6,-39.08,;21.6,-37.54,;22.94,-39.86,;24.27,-39.08,;24.27,-37.54,;25.61,-36.77,;26.94,-37.54,;28.28,-36.76,;28.28,-35.23,;26.94,-34.45,;25.61,-35.23,;25.61,-39.86,;25.61,-41.39,;26.95,-39.08,;28.28,-39.86,;28.28,-41.39,;29.62,-42.17,;29.62,-43.71,;30.96,-44.48,;30.96,-46.02,;29.62,-46.8,;32.29,-46.8,;29.62,-39.08,;29.62,-37.54,;30.96,-39.86,;32.29,-39.08,;32.29,-37.54,;33.63,-36.77,;35.03,-37.39,;36.07,-36.25,;35.3,-34.91,;35.78,-33.45,;34.75,-32.3,;33.24,-32.62,;32.76,-34.09,;33.79,-35.23,;33.63,-39.86,;33.63,-41.39,;34.97,-39.08,;36.3,-39.86,;37.63,-39.08,;37.63,-37.54,;38.97,-39.86,;40.3,-39.08,;40.3,-37.54,;38.97,-36.77,;38.97,-35.23,;40.3,-34.45,;40.3,-32.92,;41.64,-39.86,;42.98,-39.08,;41.64,-41.39,;40.39,-42.31,;40.87,-43.77,;42.41,-43.77,;42.89,-42.31,;44.19,-41.48,;44.2,-39.94,;45.52,-42.26,;46.86,-41.49,;46.87,-39.95,;48.21,-39.2,;45.54,-39.17,;48.19,-42.28,;48.17,-43.82,;49.53,-41.52,)| Show InChI InChI=1S/C92H135N25O25/c1-4-5-18-62(105-89(140)71(52-119)111-86(137)67(41-56-23-25-59(120)26-24-56)108-88(139)70(51-118)103-74(122)47-113-32-34-114(48-76(125)126)36-38-116(50-78(129)130)39-37-115(35-33-113)49-77(127)128)82(133)106-64(27-28-75(123)124)84(135)110-69(43-58-45-97-53-101-58)87(138)107-66(40-55-15-7-6-8-16-55)85(136)104-63(21-13-30-98-92(95)96)83(134)109-68(42-57-44-99-61-19-10-9-17-60(57)61)81(132)100-46-73(121)102-65(20-11-12-29-93)91(142)117-31-14-22-72(117)90(141)112-79(54(2)3)80(94)131/h6-10,15-17,19,23-26,44-45,53-54,62-72,79,99,118-120H,4-5,11-14,18,20-22,27-43,46-52,93H2,1-3H3,(H2,94,131)(H,97,101)(H,100,132)(H,102,121)(H,103,122)(H,104,136)(H,105,140)(H,106,133)(H,107,138)(H,108,139)(H,109,134)(H,110,135)(H,111,137)(H,112,141)(H,123,124)(H,125,126)(H,127,128)(H,129,130)(H4,95,96,98)/t62-,63-,64-,65-,66+,67-,68-,69-,70-,71-,72-,79-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle4-D-Phe7]-alpha-MSH from MC1R in mouse B16-F10 cells after 1.5 hrs by gamma counting |
Bioorg Med Chem Lett 21: 5757-61 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.017
BindingDB Entry DOI: 10.7270/Q2H132DZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50354716

(CHEMBL1834394)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NCCCCCCn1nnc2c1CCCCCC2(F)C(=O)NCCCCNC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:26.29,8.10,4.4,127.132,145.152,161.169,wD:14.23,86.89,95.98,105.109,116.121,157.166,(27.78,-6.77,;27.78,-8.31,;26.45,-9.09,;26.45,-10.63,;25.11,-11.4,;23.78,-10.63,;22.45,-11.4,;22.45,-12.94,;21.1,-10.63,;21.1,-9.09,;22.45,-8.31,;19.77,-11.4,;18.44,-10.63,;18.44,-9.09,;17.1,-11.4,;17.1,-12.94,;18.44,-13.72,;19.77,-12.95,;21.09,-13.71,;21.09,-15.26,;22.43,-16.03,;19.76,-16.03,;18.44,-15.25,;15.77,-10.63,;14.43,-11.4,;14.43,-12.94,;13.09,-10.63,;13.09,-9.09,;11.76,-8.31,;11.76,-11.4,;10.42,-10.64,;9.1,-11.41,;7.76,-10.65,;6.43,-11.42,;5.1,-10.66,;3.77,-11.43,;2.44,-10.66,;2.27,-9.13,;.77,-8.81,;.14,-10.04,;1.04,-11.29,;.79,-12.81,;-.44,-13.71,;-1.97,-13.47,;-2.87,-12.23,;-2.63,-10.7,;-1.38,-9.8,;-.62,-8.46,;-2.72,-9.02,;-4.05,-9.78,;-2.71,-7.48,;-4.03,-6.7,;-4.02,-5.16,;-5.35,-4.39,;-5.34,-2.85,;-6.67,-2.07,;-6.66,-.53,;-7.98,.24,;-5.32,.23,;-5.31,1.76,;-4.02,.95,;-2.93,2.02,;-3.73,3.34,;-2.18,3.32,;-1.4,4.64,;-2.16,5.98,;.14,4.63,;-2.91,4.63,;-3.98,5.73,;-5.29,4.95,;-5.27,6.5,;-6.59,7.29,;-6.56,8.84,;-7.93,6.54,;-6.57,5.76,;-7.66,4.69,;-6.84,3.37,;-8.39,3.41,;-9.18,2.09,;-10.72,2.13,;-8.44,.75,;-7.68,2.07,;-6.62,.97,;25.11,-12.94,;23.78,-13.72,;26.45,-13.72,;26.45,-15.25,;25.11,-16.03,;25.11,-17.57,;23.78,-18.34,;22.45,-17.57,;23.78,-19.88,;27.78,-16.03,;29.12,-15.25,;27.78,-17.57,;29.12,-18.34,;29.12,-19.88,;27.78,-20.66,;27.63,-22.19,;26.12,-22.51,;25.35,-21.18,;26.38,-20.03,;30.46,-17.57,;30.46,-16.03,;31.79,-18.34,;33.13,-17.57,;33.13,-16.03,;34.47,-15.25,;35.8,-16.03,;37.13,-15.25,;37.13,-13.72,;35.8,-12.94,;34.47,-13.72,;34.47,-18.34,;34.47,-19.88,;35.8,-17.57,;37.14,-18.34,;37.14,-19.88,;38.47,-20.66,;38.47,-22.2,;39.81,-22.97,;39.81,-24.51,;38.47,-25.29,;41.15,-25.29,;38.47,-17.57,;38.47,-16.03,;39.81,-18.34,;41.15,-17.57,;41.15,-16.03,;42.48,-15.25,;43.89,-15.88,;44.92,-14.74,;44.15,-13.4,;44.63,-11.94,;43.6,-10.79,;42.1,-11.11,;41.61,-12.57,;42.65,-13.72,;42.48,-18.34,;42.48,-19.88,;43.82,-17.57,;45.16,-18.34,;46.49,-17.57,;46.49,-16.03,;47.82,-18.34,;49.16,-17.57,;49.16,-16.03,;47.82,-15.25,;47.82,-13.72,;49.16,-12.94,;49.16,-11.4,;50.5,-18.34,;51.83,-17.57,;50.5,-19.88,;49.25,-20.8,;49.72,-22.26,;51.26,-22.26,;51.75,-20.79,;53.04,-19.97,;53.06,-18.42,;54.38,-20.74,;55.72,-19.98,;55.72,-18.44,;57.07,-17.69,;54.4,-17.66,;57.04,-20.77,;57.03,-22.31,;58.39,-20.01,)| Show InChI InChI=1S/C111H167FN30O26/c1-4-5-27-77(127-106(165)86(67-144)133-103(162)82(56-71-33-35-74(145)36-34-71)130-105(164)85(66-143)118-41-18-6-7-21-46-142-87-31-12-9-16-39-111(112,96(87)135-136-142)109(168)120-43-20-19-42-119-90(147)62-137-47-49-138(63-92(150)151)51-53-140(65-94(154)155)54-52-139(50-48-137)64-93(152)153)99(158)128-79(37-38-91(148)149)101(160)132-84(58-73-60-117-68-124-73)104(163)129-81(55-70-24-10-8-11-25-70)102(161)126-78(30-22-44-121-110(115)116)100(159)131-83(57-72-59-122-76-28-14-13-26-75(72)76)98(157)123-61-89(146)125-80(29-15-17-40-113)108(167)141-45-23-32-88(141)107(166)134-95(69(2)3)97(114)156/h8,10-11,13-14,24-26,28,33-36,59-60,68-69,77-86,88,95,118,122,143-145H,4-7,9,12,15-23,27,29-32,37-58,61-67,113H2,1-3H3,(H2,114,156)(H,117,124)(H,119,147)(H,120,168)(H,123,157)(H,125,146)(H,126,161)(H,127,165)(H,128,158)(H,129,163)(H,130,164)(H,131,159)(H,132,160)(H,133,162)(H,134,166)(H,148,149)(H,150,151)(H,152,153)(H,154,155)(H4,115,116,121)/t77-,78-,79-,80-,81+,82-,83-,84-,85-,86-,88-,95-,111?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle4-D-Phe7]-alpha-MSH from MC1R in mouse B16-F10 cells after 1.5 hrs by gamma counting |
Bioorg Med Chem Lett 21: 5757-61 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.017
BindingDB Entry DOI: 10.7270/Q2H132DZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50354715

(CHEMBL1834395)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NCCCCCCn1nnc2c1CCCCCC2(F)C(=O)NCCCCNC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:26.29,8.10,4.4,120.125,138.145,154.162,wD:14.23,79.82,88.91,98.102,109.114,150.159,(30.34,-37.71,;30.34,-39.25,;29.01,-40.02,;29.01,-41.56,;27.68,-42.34,;26.34,-41.56,;25.01,-42.34,;25.01,-43.87,;23.67,-41.56,;23.67,-40.02,;25.01,-39.25,;22.33,-42.34,;21,-41.56,;21,-40.02,;19.66,-42.34,;19.66,-43.87,;21,-44.65,;22.33,-43.88,;23.66,-44.65,;23.66,-46.19,;24.99,-46.96,;22.32,-46.96,;21,-46.18,;18.33,-41.56,;16.99,-42.34,;16.99,-43.87,;15.66,-41.56,;15.66,-40.02,;14.32,-39.25,;14.32,-42.34,;12.99,-41.57,;11.66,-42.35,;10.32,-41.58,;9,-42.35,;7.67,-41.59,;6.34,-42.36,;5,-41.6,;4.83,-40.06,;3.33,-39.75,;2.7,-40.98,;3.6,-42.22,;3.36,-43.75,;2.12,-44.64,;.6,-44.4,;-.31,-43.16,;-.07,-41.64,;1.18,-40.73,;1.94,-39.4,;-.16,-39.95,;-1.49,-40.71,;-.15,-38.41,;-1.47,-37.63,;-1.46,-36.1,;-2.79,-35.32,;-2.78,-33.78,;-4.1,-33,;-4.09,-31.47,;-5.42,-30.69,;-2.76,-30.71,;-2.74,-29.17,;-1.3,-28.62,;-.56,-27.29,;-.86,-25.78,;.46,-24.99,;1.81,-25.73,;3.12,-24.93,;1.84,-27.27,;-2.04,-24.82,;-3.58,-24.85,;-4.74,-25.85,;-6.09,-25.1,;-7.41,-25.89,;-8.75,-25.14,;-7.38,-27.43,;-4.98,-27.36,;-4.19,-28.68,;27.68,-43.87,;26.34,-44.65,;29.01,-44.65,;29.01,-46.19,;27.68,-46.96,;27.68,-48.5,;26.34,-49.28,;25.01,-48.5,;26.34,-50.81,;30.34,-46.96,;31.69,-46.19,;30.34,-48.5,;31.69,-49.28,;31.69,-50.81,;30.34,-51.59,;30.19,-53.12,;28.68,-53.45,;27.91,-52.11,;28.94,-50.96,;33.02,-48.5,;33.02,-46.96,;34.35,-49.28,;35.69,-48.5,;35.69,-46.96,;37.03,-46.19,;38.36,-46.96,;39.69,-46.19,;39.69,-44.65,;38.36,-43.87,;37.03,-44.65,;37.03,-49.28,;37.03,-50.81,;38.36,-48.5,;39.7,-49.28,;39.7,-50.81,;41.03,-51.59,;41.03,-53.13,;42.37,-53.91,;42.37,-55.44,;41.03,-56.22,;43.71,-56.22,;41.03,-48.5,;41.03,-46.96,;42.37,-49.28,;43.71,-48.5,;43.71,-46.96,;45.04,-46.19,;46.45,-46.81,;47.48,-45.67,;46.71,-44.33,;47.19,-42.87,;46.17,-41.72,;44.66,-42.04,;44.17,-43.51,;45.21,-44.65,;45.04,-49.28,;45.04,-50.81,;46.38,-48.5,;47.72,-49.28,;49.05,-48.5,;49.05,-46.96,;50.39,-49.28,;51.72,-48.5,;51.72,-46.96,;50.39,-46.19,;50.39,-44.65,;51.72,-43.87,;51.72,-42.34,;53.06,-49.28,;54.4,-48.5,;53.06,-50.81,;51.81,-51.73,;52.28,-53.19,;53.83,-53.19,;54.31,-51.73,;55.6,-50.9,;55.62,-49.36,;56.94,-51.68,;58.28,-50.92,;58.29,-49.38,;59.63,-48.62,;56.96,-48.59,;59.61,-51.7,;59.59,-53.25,;60.95,-50.94,)| Show InChI InChI=1S/C107H160FN29O24/c1-4-5-27-74(123-102(158)83(64-139)129-99(155)79(54-68-33-35-71(140)36-34-68)126-101(157)82(63-138)114-41-18-6-7-21-46-137-84-31-12-9-16-39-107(108,92(84)131-132-137)105(161)116-43-20-19-42-115-87(142)60-133-47-49-134(61-89(145)146)51-52-135(50-48-133)62-90(147)148)95(151)124-76(37-38-88(143)144)97(153)128-81(56-70-58-113-65-120-70)100(156)125-78(53-67-24-10-8-11-25-67)98(154)122-75(30-22-44-117-106(111)112)96(152)127-80(55-69-57-118-73-28-14-13-26-72(69)73)94(150)119-59-86(141)121-77(29-15-17-40-109)104(160)136-45-23-32-85(136)103(159)130-91(66(2)3)93(110)149/h8,10-11,13-14,24-26,28,33-36,57-58,65-66,74-83,85,91,114,118,138-140H,4-7,9,12,15-23,27,29-32,37-56,59-64,109H2,1-3H3,(H2,110,149)(H,113,120)(H,115,142)(H,116,161)(H,119,150)(H,121,141)(H,122,154)(H,123,158)(H,124,151)(H,125,156)(H,126,157)(H,127,152)(H,128,153)(H,129,155)(H,130,159)(H,143,144)(H,145,146)(H,147,148)(H4,111,112,117)/t74-,75-,76-,77-,78+,79-,80-,81-,82-,83-,85-,91-,107?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle4-D-Phe7]-alpha-MSH from MC1R in mouse B16-F10 cells after 1.5 hrs by gamma counting |
Bioorg Med Chem Lett 21: 5757-61 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.017
BindingDB Entry DOI: 10.7270/Q2H132DZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6811
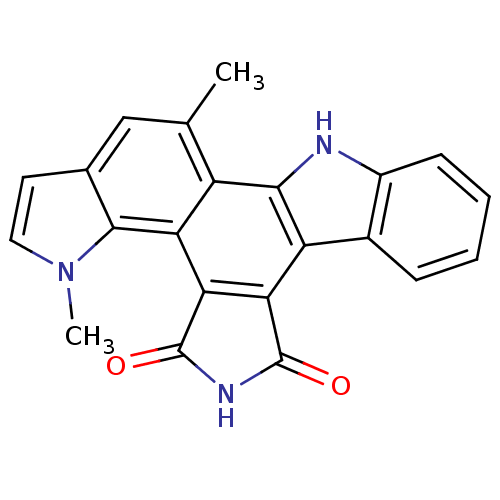
(18,23-dimethyl-3,13,18-triazahexacyclo[14.7.0.0^{2...)Show SMILES Cc1cc2ccn(C)c2c2c3C(=O)NC(=O)c3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C22H15N3O2/c1-10-9-11-7-8-25(2)20(11)16-14(10)19-15(12-5-3-4-6-13(12)23-19)17-18(16)22(27)24-21(17)26/h3-9,23H,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Lilly Research Laboratories
| Assay Description
In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... |
Bioorg Med Chem Lett 13: 2261-7 (2003)
Article DOI: 10.1016/s0960-894x(03)00461-x
BindingDB Entry DOI: 10.7270/Q29W0CPW |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6745

(1,7-Annulated indolocarbazole deriv. 13Ab | 7-fluo...)Show SMILES OCC1CCc2cccc3c4c5C(=O)NC(=O)c5c5c6ccc(F)cc6[nH]c5c4n1c23 Show InChI InChI=1S/C24H16FN3O3/c25-11-5-7-13-15(8-11)26-20-16(13)18-19(24(31)27-23(18)30)17-14-3-1-2-10-4-6-12(9-29)28(21(10)14)22(17)20/h1-3,5,7-8,12,26,29H,4,6,9H2,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Lilly Research Laboratories
| Assay Description
In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... |
Bioorg Med Chem Lett 14: 3057-61 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.033
BindingDB Entry DOI: 10.7270/Q2KD1W3H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50164242
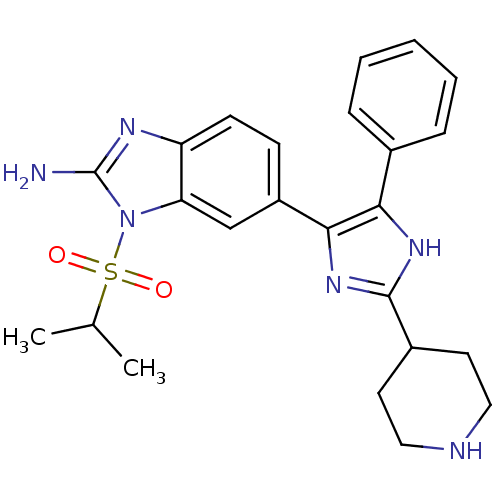
(6-(5-Phenyl-2-piperidin-4-yl-3H-imidazol-4-yl)-1-(...)Show SMILES CC(C)S(=O)(=O)n1c(N)nc2ccc(cc12)-c1nc([nH]c1-c1ccccc1)C1CCNCC1 Show InChI InChI=1S/C24H28N6O2S/c1-15(2)33(31,32)30-20-14-18(8-9-19(20)27-24(30)25)22-21(16-6-4-3-5-7-16)28-23(29-22)17-10-12-26-13-11-17/h3-9,14-15,17,26H,10-13H2,1-2H3,(H2,25,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide |
J Med Chem 48: 2270-3 (2005)
Article DOI: 10.1021/jm048978k
BindingDB Entry DOI: 10.7270/Q2M90869 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6832
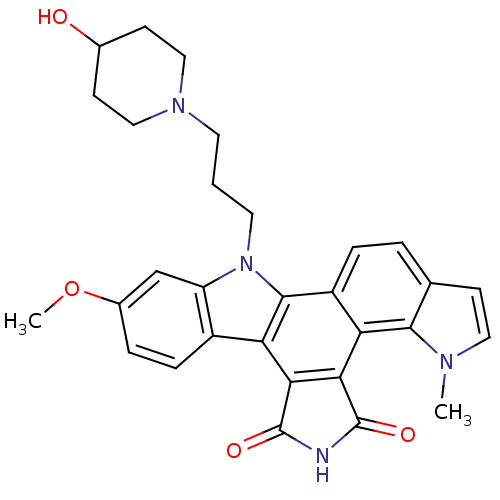
(3-[3-(4-hydroxypiperidin-1-yl)propyl]-6-methoxy-18...)Show SMILES COc1ccc2c(c1)n(CCCN1CCC(O)CC1)c1c2c2C(=O)NC(=O)c2c2c3n(C)ccc3ccc12 Show InChI InChI=1S/C30H30N4O4/c1-32-13-8-17-4-6-21-24(27(17)32)26-25(29(36)31-30(26)37)23-20-7-5-19(38-2)16-22(20)34(28(21)23)12-3-11-33-14-9-18(35)10-15-33/h4-8,13,16,18,35H,3,9-12,14-15H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
Lilly Research Laboratories
| Assay Description
In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... |
Bioorg Med Chem Lett 13: 2261-7 (2003)
Article DOI: 10.1016/s0960-894x(03)00461-x
BindingDB Entry DOI: 10.7270/Q29W0CPW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50390701
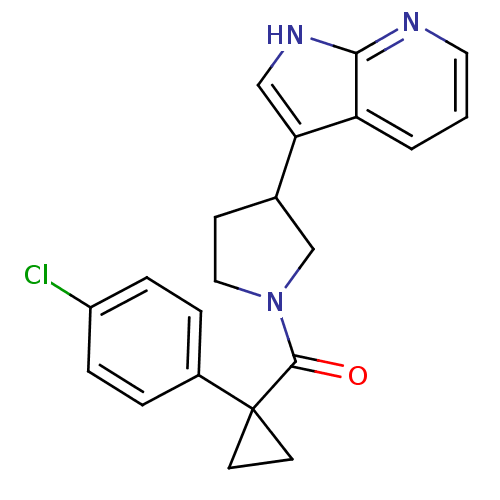
(CHEMBL2070229)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CCC(C1)c1c[nH]c2ncccc12 Show InChI InChI=1S/C21H20ClN3O/c22-16-5-3-15(4-6-16)21(8-9-21)20(26)25-11-7-14(13-25)18-12-24-19-17(18)2-1-10-23-19/h1-6,10,12,14H,7-9,11,13H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono S.A.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as conversion of cortisone to cortisol level after 150 mins by HTR... |
Bioorg Med Chem Lett 22: 5909-14 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.070
BindingDB Entry DOI: 10.7270/Q2Z89DH5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50164228
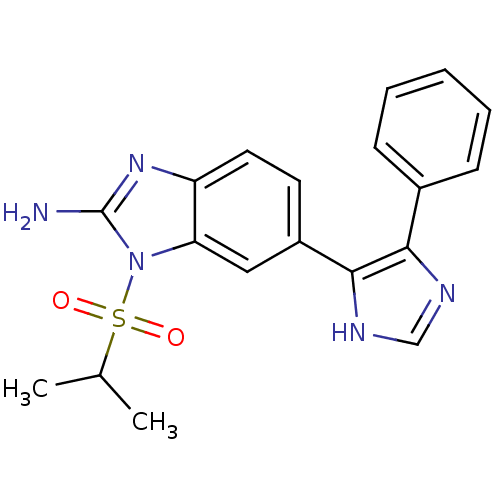
(1-(isopropylsulfonyl)-6-(4-phenyl-1H-imidazol-5-yl...)Show SMILES CC(C)S(=O)(=O)n1c(N)nc2ccc(cc12)-c1[nH]cnc1-c1ccccc1 Show InChI InChI=1S/C19H19N5O2S/c1-12(2)27(25,26)24-16-10-14(8-9-15(16)23-19(24)20)18-17(21-11-22-18)13-6-4-3-5-7-13/h3-12H,1-2H3,(H2,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide |
J Med Chem 48: 2270-3 (2005)
Article DOI: 10.1021/jm048978k
BindingDB Entry DOI: 10.7270/Q2M90869 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50164240

(6-[5-(4-Fluoro-phenyl)-3H-imidazol-4-yl]-1-(propan...)Show SMILES CC(C)S(=O)(=O)n1c(N)nc2ccc(cc12)-c1nc[nH]c1-c1ccc(F)cc1 Show InChI InChI=1S/C19H18FN5O2S/c1-11(2)28(26,27)25-16-9-13(5-8-15(16)24-19(25)21)18-17(22-10-23-18)12-3-6-14(20)7-4-12/h3-11H,1-2H3,(H2,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide |
J Med Chem 48: 2270-3 (2005)
Article DOI: 10.1021/jm048978k
BindingDB Entry DOI: 10.7270/Q2M90869 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50164236

(6-[2-tert-Butyl-5-(2,4-difluoro-phenyl)-3H-imidazo...)Show SMILES CC(C)S(=O)(=O)n1c(N)nc2ccc(cc12)-c1[nH]c(nc1-c1ccc(F)cc1F)C(C)(C)C Show InChI InChI=1S/C23H25F2N5O2S/c1-12(2)33(31,32)30-18-10-13(6-9-17(18)27-22(30)26)19-20(29-21(28-19)23(3,4)5)15-8-7-14(24)11-16(15)25/h6-12H,1-5H3,(H2,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide |
J Med Chem 48: 2270-3 (2005)
Article DOI: 10.1021/jm048978k
BindingDB Entry DOI: 10.7270/Q2M90869 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50164230
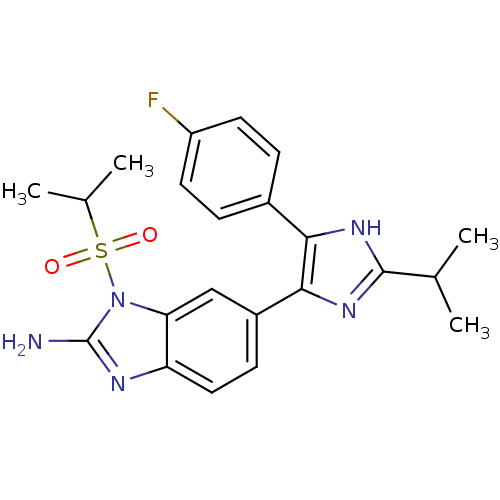
(6-[5-(4-Fluoro-phenyl)-2-isopropyl-3H-imidazol-4-y...)Show SMILES CC(C)c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccc2nc(N)n(c2c1)S(=O)(=O)C(C)C Show InChI InChI=1S/C22H24FN5O2S/c1-12(2)21-26-19(14-5-8-16(23)9-6-14)20(27-21)15-7-10-17-18(11-15)28(22(24)25-17)31(29,30)13(3)4/h5-13H,1-4H3,(H2,24,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide |
J Med Chem 48: 2270-3 (2005)
Article DOI: 10.1021/jm048978k
BindingDB Entry DOI: 10.7270/Q2M90869 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50164239
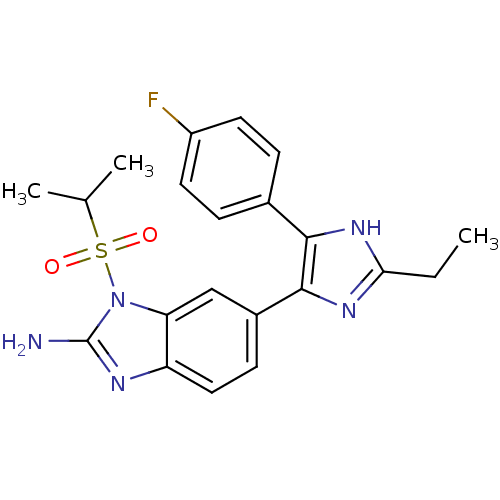
(6-[2-Ethyl-5-(4-fluoro-phenyl)-3H-imidazol-4-yl]-1...)Show SMILES CCc1nc(c([nH]1)-c1ccc(F)cc1)-c1ccc2nc(N)n(c2c1)S(=O)(=O)C(C)C Show InChI InChI=1S/C21H22FN5O2S/c1-4-18-25-19(13-5-8-15(22)9-6-13)20(26-18)14-7-10-16-17(11-14)27(21(23)24-16)30(28,29)12(2)3/h5-12H,4H2,1-3H3,(H2,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide |
J Med Chem 48: 2270-3 (2005)
Article DOI: 10.1021/jm048978k
BindingDB Entry DOI: 10.7270/Q2M90869 |
More data for this
Ligand-Target Pair | |
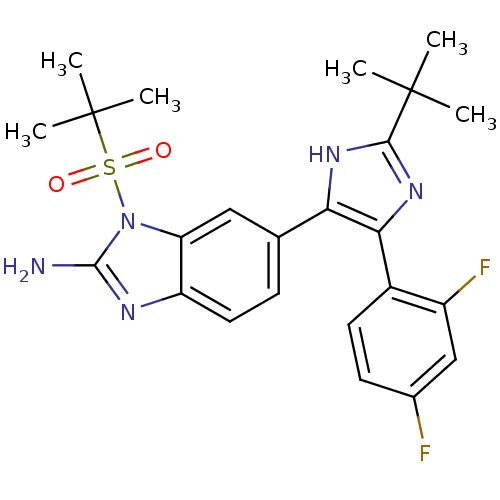
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50164229
(6-[2-tert-Butyl-5-(2,4-difluoro-phenyl)-3H-imidazo...)Show SMILES CC(C)(C)c1nc(c([nH]1)-c1ccc2nc(N)n(c2c1)S(=O)(=O)C(C)(C)C)-c1ccc(F)cc1F Show InChI InChI=1S/C24H27F2N5O2S/c1-23(2,3)21-29-19(20(30-21)15-9-8-14(25)12-16(15)26)13-7-10-17-18(11-13)31(22(27)28-17)34(32,33)24(4,5)6/h7-12H,1-6H3,(H2,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide |
J Med Chem 48: 2270-3 (2005)
Article DOI: 10.1021/jm048978k
BindingDB Entry DOI: 10.7270/Q2M90869 |
More data for this
Ligand-Target Pair | |
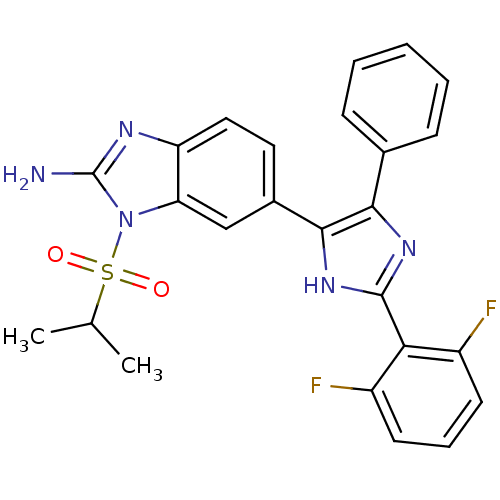
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50164232
(6-(2-(2,6-difluorophenyl)-4-phenyl-1H-imidazol-5-y...)Show SMILES CC(C)S(=O)(=O)n1c(N)nc2ccc(cc12)-c1[nH]c(nc1-c1ccccc1)-c1c(F)cccc1F Show InChI InChI=1S/C25H21F2N5O2S/c1-14(2)35(33,34)32-20-13-16(11-12-19(20)29-25(32)28)23-22(15-7-4-3-5-8-15)30-24(31-23)21-17(26)9-6-10-18(21)27/h3-14H,1-2H3,(H2,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide |
J Med Chem 48: 2270-3 (2005)
Article DOI: 10.1021/jm048978k
BindingDB Entry DOI: 10.7270/Q2M90869 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6838
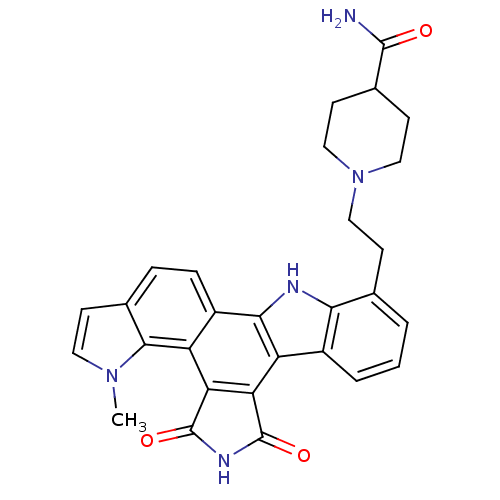
(1-(2-{18-methyl-12,14-dioxo-3,13,18-triazahexacycl...)Show SMILES Cn1ccc2ccc3c4[nH]c5c(CCN6CCC(CC6)C(N)=O)cccc5c4c4C(=O)NC(=O)c4c3c12 Show InChI InChI=1S/C29H27N5O3/c1-33-11-7-16-5-6-19-21(26(16)33)23-22(28(36)32-29(23)37)20-18-4-2-3-15(24(18)31-25(19)20)8-12-34-13-9-17(10-14-34)27(30)35/h2-7,11,17,31H,8-10,12-14H2,1H3,(H2,30,35)(H,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 25 |
Lilly Research Laboratories
| Assay Description
In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... |
Bioorg Med Chem Lett 13: 2261-7 (2003)
Article DOI: 10.1016/s0960-894x(03)00461-x
BindingDB Entry DOI: 10.7270/Q29W0CPW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50390710

(CHEMBL2070319)Show SMILES Cc1[nH]c2ncccc2c1C1CCN(C1)C(=O)C1(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C22H22FN3O/c1-14-19(18-3-2-11-24-20(18)25-14)15-8-12-26(13-15)21(27)22(9-10-22)16-4-6-17(23)7-5-16/h2-7,11,15H,8-10,12-13H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono S.A.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as conversion of cortisone to cortisol level after 150 mins by HTR... |
Bioorg Med Chem Lett 22: 5909-14 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.070
BindingDB Entry DOI: 10.7270/Q2Z89DH5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6846
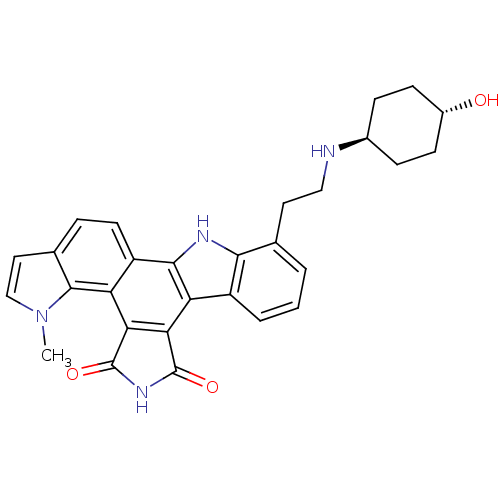
(5-{2-[(4-hydroxycyclohexyl)amino]ethyl}-18-methyl-...)Show SMILES Cn1ccc2ccc3c4[nH]c5c(CCN[C@H]6CC[C@H](O)CC6)cccc5c4c4C(=O)NC(=O)c4c3c12 |r,wU:18.18,wD:15.14,(3.5,4.11,;3.82,2.6,;5.23,1.97,;5.06,.44,;3.56,.12,;2.79,-1.21,;1.25,-1.21,;.48,.12,;-1.06,.12,;-2.09,-1.02,;-3.5,-.4,;-4.91,-1.02,;-5.07,-2.55,;-6.44,-3.25,;-6.52,-4.79,;-7.89,-5.49,;-9.23,-4.72,;-10.56,-5.49,;-10.56,-7.03,;-11.89,-7.8,;-9.23,-7.8,;-7.89,-7.03,;-6.15,-.12,;-5.99,1.42,;-4.58,2.04,;-3.34,1.14,;-1.83,1.46,;-1.06,2.79,;-1.54,4.25,;-3,4.73,;-.29,5.16,;.95,4.25,;2.42,4.73,;.48,2.79,;1.25,1.46,;2.79,1.46,)| Show InChI InChI=1S/C29H28N4O3/c1-33-14-12-16-5-10-20-22(27(16)33)24-23(28(35)32-29(24)36)21-19-4-2-3-15(25(19)31-26(20)21)11-13-30-17-6-8-18(34)9-7-17/h2-5,10,12,14,17-18,30-31,34H,6-9,11,13H2,1H3,(H,32,35,36)/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 25 |
Lilly Research Laboratories
| Assay Description
In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... |
Bioorg Med Chem Lett 13: 2261-7 (2003)
Article DOI: 10.1016/s0960-894x(03)00461-x
BindingDB Entry DOI: 10.7270/Q29W0CPW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50164231
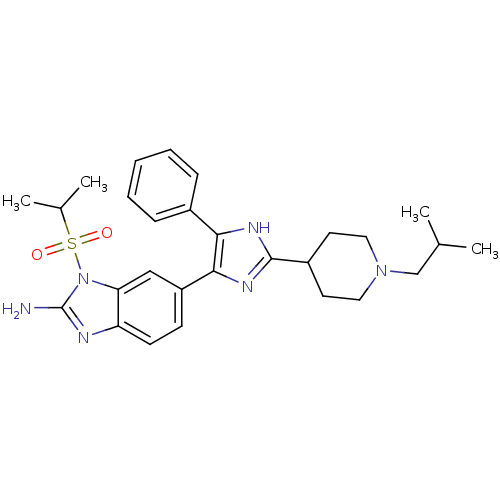
(6-[2-(1-Isobutyl-piperidin-4-yl)-5-phenyl-3H-imida...)Show SMILES CC(C)CN1CCC(CC1)c1nc(c([nH]1)-c1ccccc1)-c1ccc2nc(N)n(c2c1)S(=O)(=O)C(C)C Show InChI InChI=1S/C28H36N6O2S/c1-18(2)17-33-14-12-21(13-15-33)27-31-25(20-8-6-5-7-9-20)26(32-27)22-10-11-23-24(16-22)34(28(29)30-23)37(35,36)19(3)4/h5-11,16,18-19,21H,12-15,17H2,1-4H3,(H2,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide |
J Med Chem 48: 2270-3 (2005)
Article DOI: 10.1021/jm048978k
BindingDB Entry DOI: 10.7270/Q2M90869 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6803
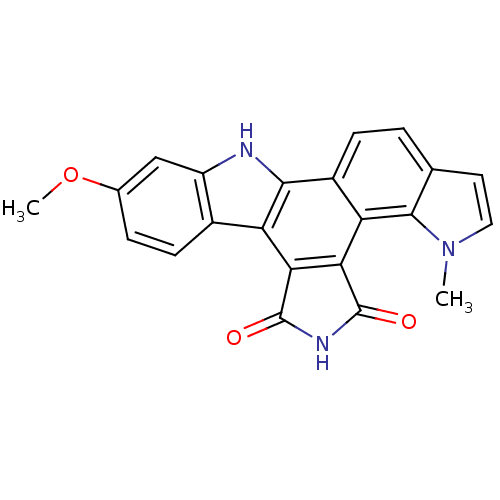
(6-methoxy-18-methyl-3,13,18-triazahexacyclo[14.7.0...)Show SMILES COc1ccc2c(c1)[nH]c1c2c2C(=O)NC(=O)c2c2c3n(C)ccc3ccc12 Show InChI InChI=1S/C22H15N3O3/c1-25-8-7-10-3-5-13-16(20(10)25)18-17(21(26)24-22(18)27)15-12-6-4-11(28-2)9-14(12)23-19(13)15/h3-9,23H,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Lilly Research Laboratories
| Assay Description
In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... |
Bioorg Med Chem Lett 13: 2261-7 (2003)
Article DOI: 10.1016/s0960-894x(03)00461-x
BindingDB Entry DOI: 10.7270/Q29W0CPW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM6800
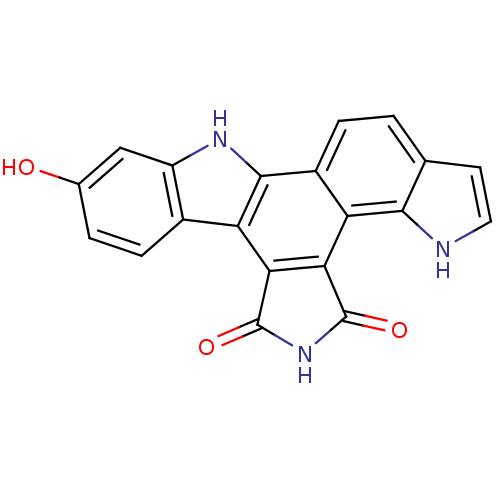
(6-hydroxy-3,13,18-triazahexacyclo[14.7.0.0^{2,10}....)Show SMILES Oc1ccc2c(c1)[nH]c1c2c2C(=O)NC(=O)c2c2c3[nH]ccc3ccc12 Show InChI InChI=1S/C20H11N3O3/c24-9-2-4-10-12(7-9)22-18-11-3-1-8-5-6-21-17(8)14(11)16-15(13(10)18)19(25)23-20(16)26/h1-7,21-22,24H,(H,23,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Lilly Research Laboratories
| Assay Description
In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... |
Bioorg Med Chem Lett 13: 2261-7 (2003)
Article DOI: 10.1016/s0960-894x(03)00461-x
BindingDB Entry DOI: 10.7270/Q29W0CPW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6815
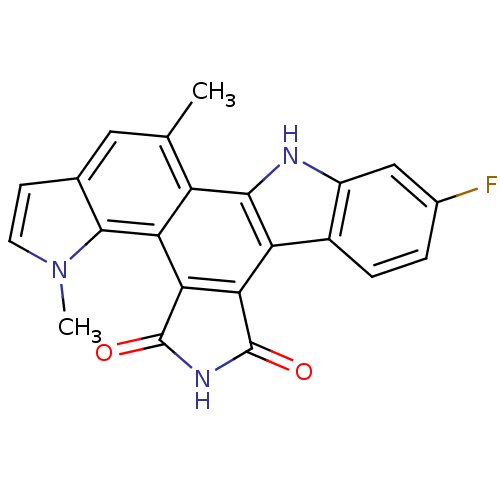
(6-fluoro-18,23-dimethyl-3,13,18-triazahexacyclo[14...)Show SMILES Cc1cc2ccn(C)c2c2c3C(=O)NC(=O)c3c3c4ccc(F)cc4[nH]c3c12 Show InChI InChI=1S/C22H14FN3O2/c1-9-7-10-5-6-26(2)20(10)16-14(9)19-15(17-18(16)22(28)25-21(17)27)12-4-3-11(23)8-13(12)24-19/h3-8,24H,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Lilly Research Laboratories
| Assay Description
In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... |
Bioorg Med Chem Lett 13: 2261-7 (2003)
Article DOI: 10.1016/s0960-894x(03)00461-x
BindingDB Entry DOI: 10.7270/Q29W0CPW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50164232

(6-(2-(2,6-difluorophenyl)-4-phenyl-1H-imidazol-5-y...)Show SMILES CC(C)S(=O)(=O)n1c(N)nc2ccc(cc12)-c1[nH]c(nc1-c1ccccc1)-c1c(F)cccc1F Show InChI InChI=1S/C25H21F2N5O2S/c1-14(2)35(33,34)32-20-13-16(11-12-19(20)29-25(32)28)23-22(15-7-4-3-5-8-15)30-24(31-23)21-17(26)9-6-10-18(21)27/h3-14H,1-2H3,(H2,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Cytochrome P450 3A4 |
J Med Chem 48: 2270-3 (2005)
Article DOI: 10.1021/jm048978k
BindingDB Entry DOI: 10.7270/Q2M90869 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6816
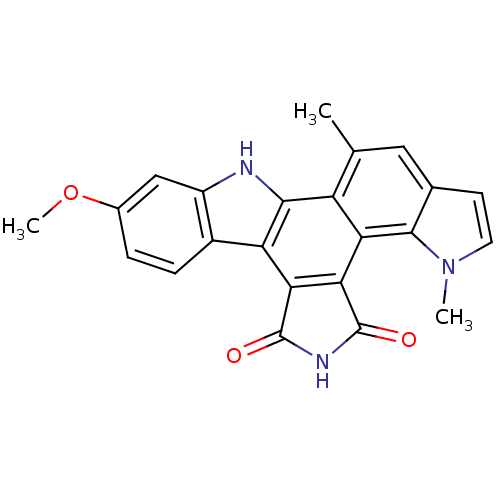
(6-methoxy-18,23-dimethyl-3,13,18-triazahexacyclo[1...)Show SMILES COc1ccc2c(c1)[nH]c1c2c2C(=O)NC(=O)c2c2c3n(C)ccc3cc(C)c12 Show InChI InChI=1S/C23H17N3O3/c1-10-8-11-6-7-26(2)21(11)17-15(10)20-16(18-19(17)23(28)25-22(18)27)13-5-4-12(29-3)9-14(13)24-20/h4-9,24H,1-3H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | 25 |
Lilly Research Laboratories
| Assay Description
In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... |
Bioorg Med Chem Lett 13: 2261-7 (2003)
Article DOI: 10.1016/s0960-894x(03)00461-x
BindingDB Entry DOI: 10.7270/Q29W0CPW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50390703
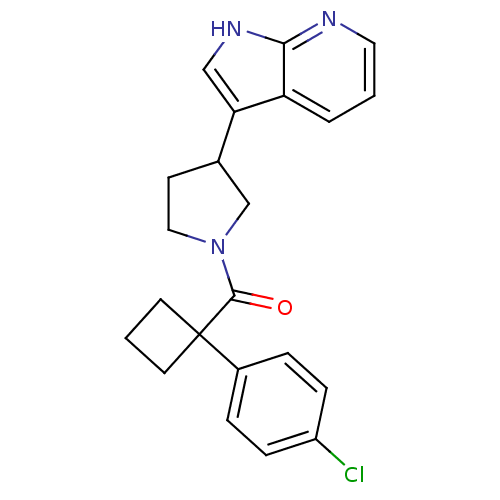
(CHEMBL2070231)Show SMILES Clc1ccc(cc1)C1(CCC1)C(=O)N1CCC(C1)c1c[nH]c2ncccc12 Show InChI InChI=1S/C22H22ClN3O/c23-17-6-4-16(5-7-17)22(9-2-10-22)21(27)26-12-8-15(14-26)19-13-25-20-18(19)3-1-11-24-20/h1,3-7,11,13,15H,2,8-10,12,14H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono S.A.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as conversion of cortisone to cortisol level after 150 mins by HTR... |
Bioorg Med Chem Lett 22: 5909-14 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.070
BindingDB Entry DOI: 10.7270/Q2Z89DH5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data