 Found 161 hits with Last Name = 'searle' and Initial = 'll'
Found 161 hits with Last Name = 'searle' and Initial = 'll' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377274
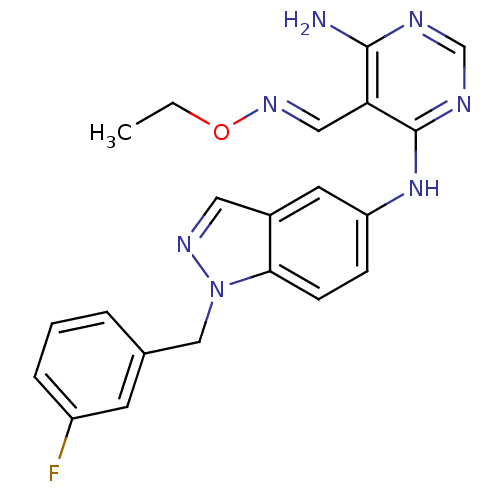
(CHEMBL402294)Show SMILES CCO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C21H20FN7O/c1-2-30-27-11-18-20(23)24-13-25-21(18)28-17-6-7-19-15(9-17)10-26-29(19)12-14-4-3-5-16(22)8-14/h3-11,13H,2,12H2,1H3,(H3,23,24,25,28)/b27-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377285
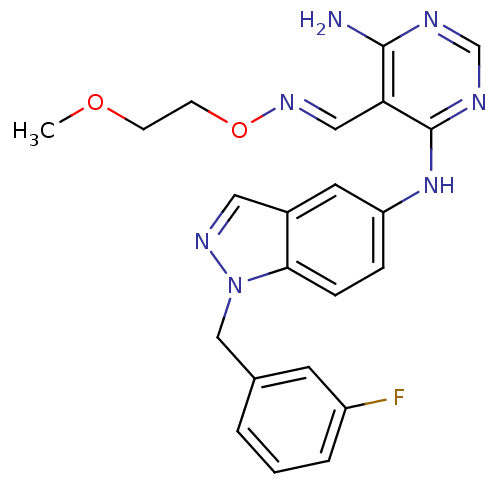
(CHEMBL255237)Show SMILES COCCO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C22H22FN7O2/c1-31-7-8-32-28-12-19-21(24)25-14-26-22(19)29-18-5-6-20-16(10-18)11-27-30(20)13-15-3-2-4-17(23)9-15/h2-6,9-12,14H,7-8,13H2,1H3,(H3,24,25,26,29)/b28-12+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377274

(CHEMBL402294)Show SMILES CCO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C21H20FN7O/c1-2-30-27-11-18-20(23)24-13-25-21(18)28-17-6-7-19-15(9-17)10-26-29(19)12-14-4-3-5-16(22)8-14/h3-11,13H,2,12H2,1H3,(H3,23,24,25,28)/b27-11+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50240214
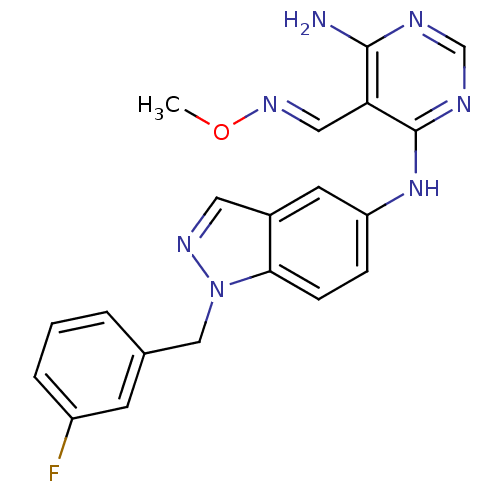
(4-(1-(3-fluorobenzyl)-1H-indazol-5-ylamino)-6-amin...)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C20H18FN7O/c1-29-26-10-17-19(22)23-12-24-20(17)27-16-5-6-18-14(8-16)9-25-28(18)11-13-3-2-4-15(21)7-13/h2-10,12H,11H2,1H3,(H3,22,23,24,27)/b26-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377276
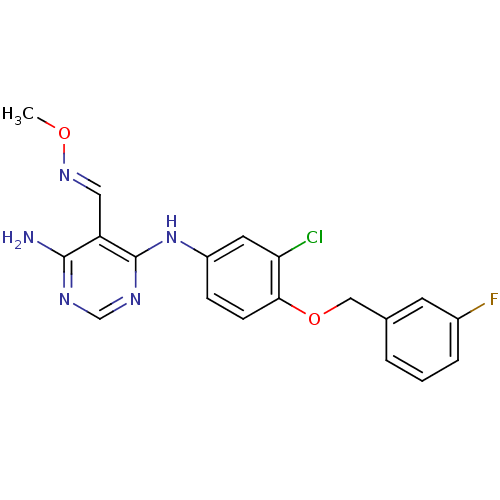
(CHEMBL256297)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc(OCc2cccc(F)c2)c(Cl)c1 Show InChI InChI=1S/C19H17ClFN5O2/c1-27-25-9-15-18(22)23-11-24-19(15)26-14-5-6-17(16(20)8-14)28-10-12-3-2-4-13(21)7-12/h2-9,11H,10H2,1H3,(H3,22,23,24,26)/b25-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377275
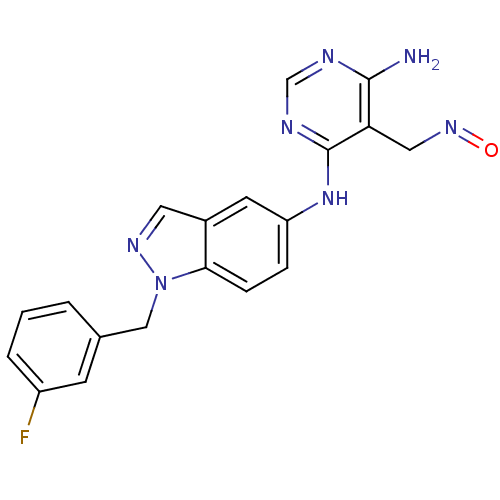
(CHEMBL256295)Show SMILES Nc1ncnc(Nc2ccc3n(Cc4cccc(F)c4)ncc3c2)c1CN=O Show InChI InChI=1S/C19H16FN7O/c20-14-3-1-2-12(6-14)10-27-17-5-4-15(7-13(17)8-24-27)26-19-16(9-25-28)18(21)22-11-23-19/h1-8,11H,9-10H2,(H3,21,22,23,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50240214

(4-(1-(3-fluorobenzyl)-1H-indazol-5-ylamino)-6-amin...)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C20H18FN7O/c1-29-26-10-17-19(22)23-12-24-20(17)27-16-5-6-18-14(8-16)9-25-28(18)11-13-3-2-4-15(21)7-13/h2-10,12H,11H2,1H3,(H3,22,23,24,27)/b26-10+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336739
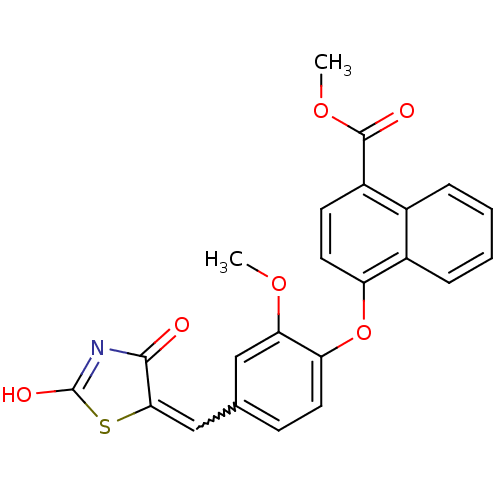
(4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...)Show SMILES COC(=O)c1ccc(Oc2ccc(C=C3SC(O)=NC3=O)cc2OC)c2ccccc12 |w:13.12,c:17| Show InChI InChI=1S/C23H17NO6S/c1-28-19-11-13(12-20-21(25)24-23(27)31-20)7-9-18(19)30-17-10-8-16(22(26)29-2)14-5-3-4-6-15(14)17/h3-12H,1-2H3,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377279
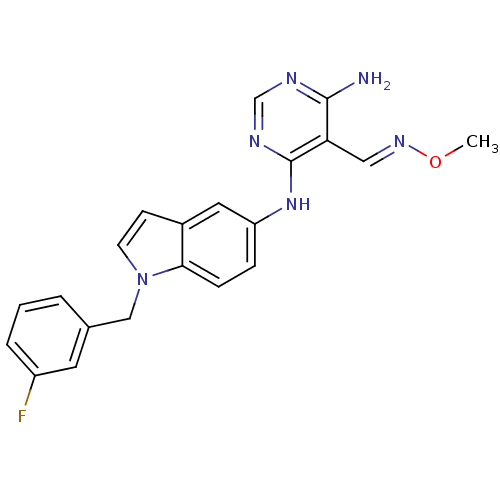
(CHEMBL257816)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ccc2c1 Show InChI InChI=1S/C21H19FN6O/c1-29-26-11-18-20(23)24-13-25-21(18)27-17-5-6-19-15(10-17)7-8-28(19)12-14-3-2-4-16(22)9-14/h2-11,13H,12H2,1H3,(H3,23,24,25,27)/b26-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377286
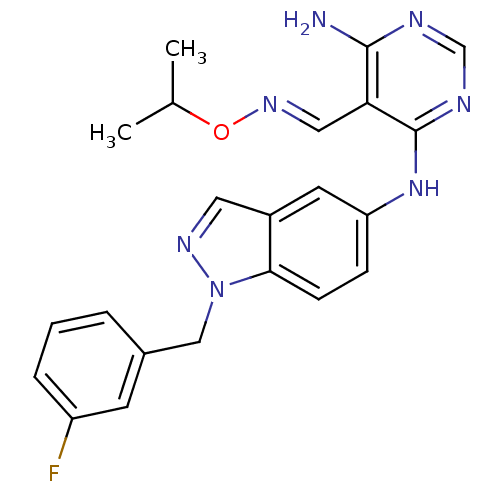
(CHEMBL402293)Show SMILES CC(C)O\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C22H22FN7O/c1-14(2)31-28-11-19-21(24)25-13-26-22(19)29-18-6-7-20-16(9-18)10-27-30(20)12-15-4-3-5-17(23)8-15/h3-11,13-14H,12H2,1-2H3,(H3,24,25,26,29)/b28-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377285

(CHEMBL255237)Show SMILES COCCO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C22H22FN7O2/c1-31-7-8-32-28-12-19-21(24)25-14-26-22(19)29-18-5-6-20-16(10-18)11-27-30(20)13-15-3-2-4-17(23)9-15/h2-6,9-12,14H,7-8,13H2,1H3,(H3,24,25,26,29)/b28-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336759
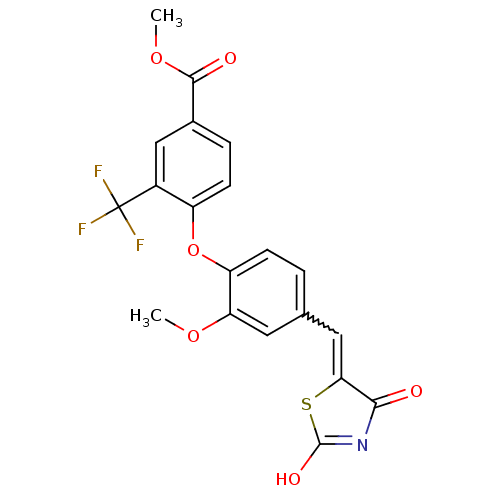
(4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...)Show SMILES COC(=O)c1ccc(Oc2ccc(C=C3SC(O)=NC3=O)cc2OC)c(c1)C(F)(F)F |w:13.12,c:17| Show InChI InChI=1S/C20H14F3NO6S/c1-28-15-7-10(8-16-17(25)24-19(27)31-16)3-5-14(15)30-13-6-4-11(18(26)29-2)9-12(13)20(21,22)23/h3-9H,1-2H3,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377277

(CHEMBL257815)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc(OCc2ccccc2)c(Cl)c1 Show InChI InChI=1S/C19H18ClN5O2/c1-26-24-10-15-18(21)22-12-23-19(15)25-14-7-8-17(16(20)9-14)27-11-13-5-3-2-4-6-13/h2-10,12H,11H2,1H3,(H3,21,22,23,25)/b24-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377276

(CHEMBL256297)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc(OCc2cccc(F)c2)c(Cl)c1 Show InChI InChI=1S/C19H17ClFN5O2/c1-27-25-9-15-18(22)23-11-24-19(15)26-14-5-6-17(16(20)8-14)28-10-12-3-2-4-13(21)7-12/h2-9,11H,10H2,1H3,(H3,22,23,24,26)/b25-9+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377282
(CHEMBL255865)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(c3)C#N)ncc2c1 Show InChI InChI=1S/C21H18N8O/c1-30-27-11-18-20(23)24-13-25-21(18)28-17-5-6-19-16(8-17)10-26-29(19)12-15-4-2-3-14(7-15)9-22/h2-8,10-11,13H,12H2,1H3,(H3,23,24,25,28)/b27-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377288
(CHEMBL402113)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3ccccc3)ncc2c1 Show InChI InChI=1S/C20H19N7O/c1-28-25-11-17-19(21)22-13-23-20(17)26-16-7-8-18-15(9-16)10-24-27(18)12-14-5-3-2-4-6-14/h2-11,13H,12H2,1H3,(H3,21,22,23,26)/b25-11+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377272

(CHEMBL255438)Show SMILES Nc1ncnc(Nc2ccc3n(Cc4cccc(F)c4)ncc3c2)c1\C=N\OCc1ccccc1 Show InChI InChI=1S/C26H22FN7O/c27-21-8-4-7-19(11-21)15-34-24-10-9-22(12-20(24)13-31-34)33-26-23(25(28)29-17-30-26)14-32-35-16-18-5-2-1-3-6-18/h1-14,17H,15-16H2,(H3,28,29,30,33)/b32-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377283

(CHEMBL255656)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(Cl)c3)ncc2c1 Show InChI InChI=1S/C20H18ClN7O/c1-29-26-10-17-19(22)23-12-24-20(17)27-16-5-6-18-14(8-16)9-25-28(18)11-13-3-2-4-15(21)7-13/h2-10,12H,11H2,1H3,(H3,22,23,24,27)/b26-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377269

(CHEMBL402149)Show InChI InChI=1S/C12H12BrN5O/c1-19-17-6-10-11(14)15-7-16-12(10)18-9-4-2-3-8(13)5-9/h2-7H,1H3,(H3,14,15,16,18)/b17-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377286

(CHEMBL402293)Show SMILES CC(C)O\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C22H22FN7O/c1-14(2)31-28-11-19-21(24)25-13-26-22(19)29-18-6-7-20-16(9-18)10-27-30(20)12-15-4-3-5-17(23)8-15/h3-11,13-14H,12H2,1-2H3,(H3,24,25,26,29)/b28-11+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377279

(CHEMBL257816)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ccc2c1 Show InChI InChI=1S/C21H19FN6O/c1-29-26-11-18-20(23)24-13-25-21(18)27-17-5-6-19-15(10-17)7-8-28(19)12-14-3-2-4-16(22)9-14/h2-11,13H,12H2,1H3,(H3,23,24,25,27)/b26-11+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377283

(CHEMBL255656)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(Cl)c3)ncc2c1 Show InChI InChI=1S/C20H18ClN7O/c1-29-26-10-17-19(22)23-12-24-20(17)27-16-5-6-18-14(8-16)9-25-28(18)11-13-3-2-4-15(21)7-13/h2-10,12H,11H2,1H3,(H3,22,23,24,27)/b26-10+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377273

(CHEMBL430031)Show SMILES CC(C)CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C23H24FN7O/c1-15(2)13-32-29-11-20-22(25)26-14-27-23(20)30-19-6-7-21-17(9-19)10-28-31(21)12-16-4-3-5-18(24)8-16/h3-11,14-15H,12-13H2,1-2H3,(H3,25,26,27,30)/b29-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377281

(CHEMBL256529)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc(OCc2cc(F)cc(F)c2)c(Cl)c1 Show InChI InChI=1S/C19H16ClF2N5O2/c1-28-26-8-15-18(23)24-10-25-19(15)27-14-2-3-17(16(20)7-14)29-9-11-4-12(21)6-13(22)5-11/h2-8,10H,9H2,1H3,(H3,23,24,25,27)/b26-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336760
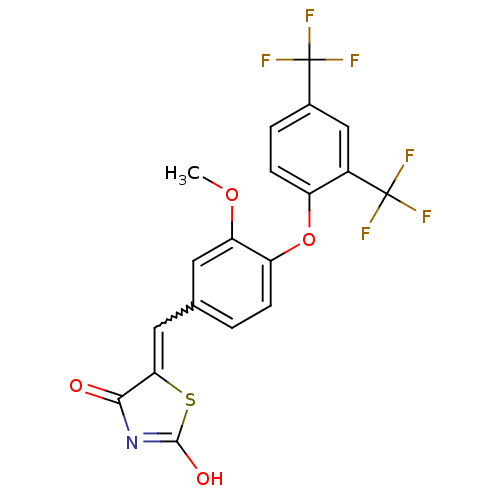
(5-[4-(2,4-Bis-trifluoromethylphenoxy)-3-methoxyben...)Show SMILES COc1cc(C=C2SC(O)=NC2=O)ccc1Oc1ccc(cc1C(F)(F)F)C(F)(F)F |w:5.4,c:9| Show InChI InChI=1S/C19H11F6NO4S/c1-29-14-6-9(7-15-16(27)26-17(28)31-15)2-4-13(14)30-12-5-3-10(18(20,21)22)8-11(12)19(23,24)25/h2-8H,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336730

(4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...)Show SMILES COc1cc(C=C2SC(O)=NC2=O)ccc1Oc1ccc(cc1C(F)(F)F)C#N |w:5.4,c:9| Show InChI InChI=1S/C19H11F3N2O4S/c1-27-15-7-10(8-16-17(25)24-18(26)29-16)2-5-14(15)28-13-4-3-11(9-23)6-12(13)19(20,21)22/h2-8H,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377275

(CHEMBL256295)Show SMILES Nc1ncnc(Nc2ccc3n(Cc4cccc(F)c4)ncc3c2)c1CN=O Show InChI InChI=1S/C19H16FN7O/c20-14-3-1-2-12(6-14)10-27-17-5-4-15(7-13(17)8-24-27)26-19-16(9-25-28)18(21)22-11-23-19/h1-8,11H,9-10H2,(H3,21,22,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377288

(CHEMBL402113)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3ccccc3)ncc2c1 Show InChI InChI=1S/C20H19N7O/c1-28-25-11-17-19(21)22-13-23-20(17)26-16-7-8-18-15(9-16)10-24-27(18)12-14-5-3-2-4-6-14/h2-11,13H,12H2,1H3,(H3,21,22,23,26)/b25-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377282

(CHEMBL255865)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(c3)C#N)ncc2c1 Show InChI InChI=1S/C21H18N8O/c1-30-27-11-18-20(23)24-13-25-21(18)28-17-5-6-19-16(8-17)10-26-29(19)12-15-4-2-3-14(7-15)9-22/h2-8,10-11,13H,12H2,1H3,(H3,23,24,25,28)/b27-11+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377271

(CHEMBL255236)Show SMILES COc1ccccc1CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C27H24FN7O2/c1-36-25-8-3-2-6-19(25)16-37-33-14-23-26(29)30-17-31-27(23)34-22-9-10-24-20(12-22)13-32-35(24)15-18-5-4-7-21(28)11-18/h2-14,17H,15-16H2,1H3,(H3,29,30,31,34)/b33-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336753
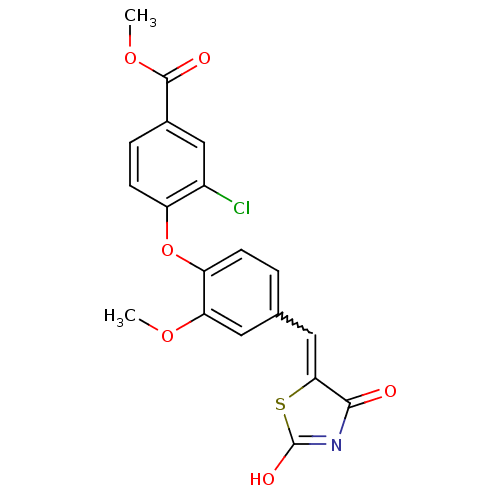
(4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...)Show SMILES COC(=O)c1ccc(Oc2ccc(C=C3SC(O)=NC3=O)cc2OC)c(Cl)c1 |w:13.12,c:17| Show InChI InChI=1S/C19H14ClNO6S/c1-25-15-7-10(8-16-17(22)21-19(24)28-16)3-5-14(15)27-13-6-4-11(9-12(13)20)18(23)26-2/h3-9H,1-2H3,(H,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377287
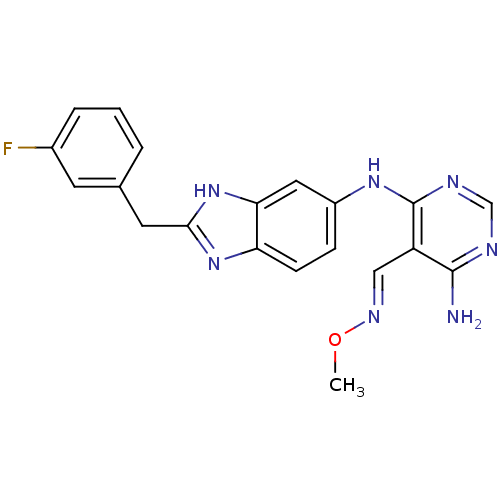
(CHEMBL402316)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2nc(Cc3cccc(F)c3)[nH]c2c1 Show InChI InChI=1S/C20H18FN7O/c1-29-25-10-15-19(22)23-11-24-20(15)26-14-5-6-16-17(9-14)28-18(27-16)8-12-3-2-4-13(21)7-12/h2-7,9-11H,8H2,1H3,(H,27,28)(H3,22,23,24,26)/b25-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377278

(CHEMBL257814)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2N(Cc3cccc(F)c3)CCc2c1 Show InChI InChI=1S/C21H21FN6O/c1-29-26-11-18-20(23)24-13-25-21(18)27-17-5-6-19-15(10-17)7-8-28(19)12-14-3-2-4-16(22)9-14/h2-6,9-11,13H,7-8,12H2,1H3,(H3,23,24,25,27)/b26-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336731
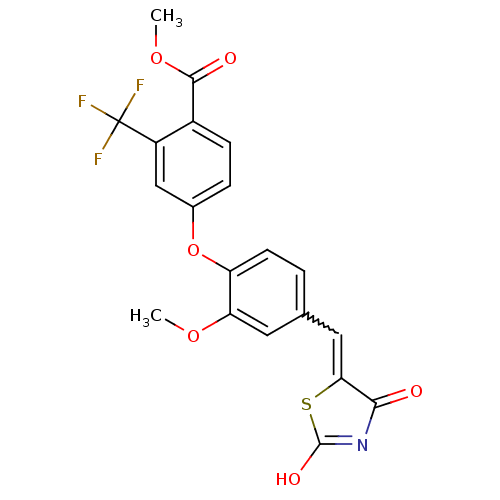
(4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...)Show SMILES COC(=O)c1ccc(Oc2ccc(C=C3SC(O)=NC3=O)cc2OC)cc1C(F)(F)F |w:13.12,c:17| Show InChI InChI=1S/C20H14F3NO6S/c1-28-15-7-10(8-16-17(25)24-19(27)31-16)3-6-14(15)30-11-4-5-12(18(26)29-2)13(9-11)20(21,22)23/h3-9H,1-2H3,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336738
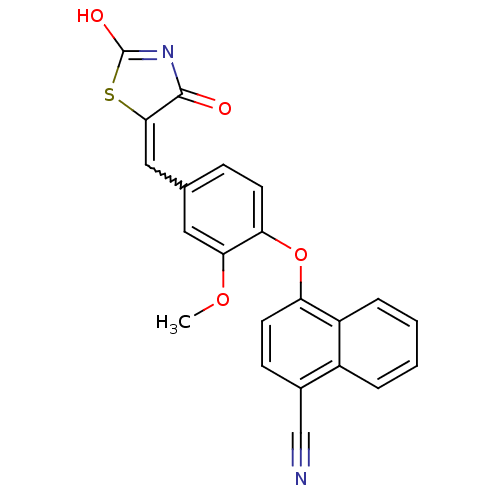
(4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...)Show SMILES COc1cc(C=C2SC(O)=NC2=O)ccc1Oc1ccc(C#N)c2ccccc12 |w:5.4,c:9| Show InChI InChI=1S/C22H14N2O4S/c1-27-19-10-13(11-20-21(25)24-22(26)29-20)6-8-18(19)28-17-9-7-14(12-23)15-4-2-3-5-16(15)17/h2-11H,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336761
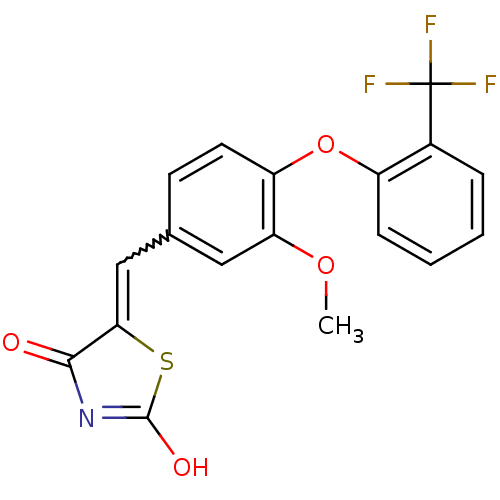
(5-[4-(2-Trifluoromethylphenoxy)-3-methoxybenzylide...)Show SMILES COc1cc(C=C2SC(O)=NC2=O)ccc1Oc1ccccc1C(F)(F)F |w:5.4,c:9| Show InChI InChI=1S/C18H12F3NO4S/c1-25-14-8-10(9-15-16(23)22-17(24)27-15)6-7-13(14)26-12-5-3-2-4-11(12)18(19,20)21/h2-9H,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377270
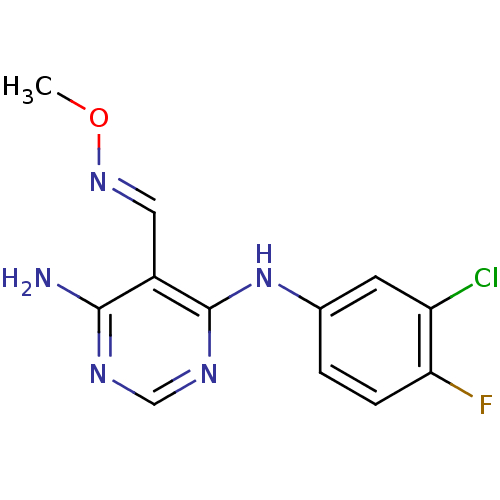
(CHEMBL255490)Show InChI InChI=1S/C12H11ClFN5O/c1-20-18-5-8-11(15)16-6-17-12(8)19-7-2-3-10(14)9(13)4-7/h2-6H,1H3,(H3,15,16,17,19)/b18-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336762
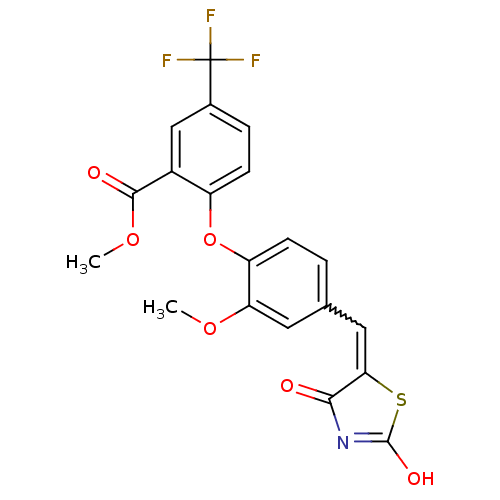
(2-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...)Show SMILES COC(=O)c1cc(ccc1Oc1ccc(C=C2SC(O)=NC2=O)cc1OC)C(F)(F)F |w:15.15,c:20| Show InChI InChI=1S/C20H14F3NO6S/c1-28-15-7-10(8-16-17(25)24-19(27)31-16)3-5-14(15)30-13-6-4-11(20(21,22)23)9-12(13)18(26)29-2/h3-9H,1-2H3,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377287

(CHEMBL402316)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2nc(Cc3cccc(F)c3)[nH]c2c1 Show InChI InChI=1S/C20H18FN7O/c1-29-25-10-15-19(22)23-11-24-20(15)26-14-5-6-16-17(9-14)28-18(27-16)8-12-3-2-4-13(21)7-12/h2-7,9-11H,8H2,1H3,(H,27,28)(H3,22,23,24,26)/b25-10+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336756
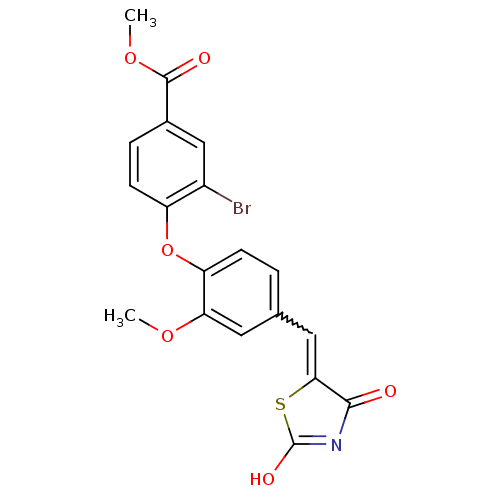
(4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...)Show SMILES COC(=O)c1ccc(Oc2ccc(C=C3SC(O)=NC3=O)cc2OC)c(Br)c1 |w:13.12,c:17| Show InChI InChI=1S/C19H14BrNO6S/c1-25-15-7-10(8-16-17(22)21-19(24)28-16)3-5-14(15)27-13-6-4-11(9-12(13)20)18(23)26-2/h3-9H,1-2H3,(H,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336739

(4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...)Show SMILES COC(=O)c1ccc(Oc2ccc(C=C3SC(O)=NC3=O)cc2OC)c2ccccc12 |w:13.12,c:17| Show InChI InChI=1S/C23H17NO6S/c1-28-19-11-13(12-20-21(25)24-23(27)31-20)7-9-18(19)30-17-10-8-16(22(26)29-2)14-5-3-4-6-15(14)17/h3-12H,1-2H3,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377272

(CHEMBL255438)Show SMILES Nc1ncnc(Nc2ccc3n(Cc4cccc(F)c4)ncc3c2)c1\C=N\OCc1ccccc1 Show InChI InChI=1S/C26H22FN7O/c27-21-8-4-7-19(11-21)15-34-24-10-9-22(12-20(24)13-31-34)33-26-23(25(28)29-17-30-26)14-32-35-16-18-5-2-1-3-6-18/h1-14,17H,15-16H2,(H3,28,29,30,33)/b32-14+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377280

(CHEMBL256527)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3ccc(F)cc3)ncc2c1 Show InChI InChI=1S/C20H18FN7O/c1-29-26-10-17-19(22)23-12-24-20(17)27-16-6-7-18-14(8-16)9-25-28(18)11-13-2-4-15(21)5-3-13/h2-10,12H,11H2,1H3,(H3,22,23,24,27)/b26-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336757

(5-[4-(2-Bromo-4-trifluoromethylphenoxy)-3-methoxyb...)Show SMILES COc1cc(C=C2SC(O)=NC2=O)ccc1Oc1ccc(cc1Br)C(F)(F)F |w:5.4,c:9| Show InChI InChI=1S/C18H11BrF3NO4S/c1-26-14-6-9(7-15-16(24)23-17(25)28-15)2-4-13(14)27-12-5-3-10(8-11(12)19)18(20,21)22/h2-8H,1H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377277

(CHEMBL257815)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc(OCc2ccccc2)c(Cl)c1 Show InChI InChI=1S/C19H18ClN5O2/c1-26-24-10-15-18(21)22-12-23-19(15)25-14-7-8-17(16(20)9-14)27-11-13-5-3-2-4-6-13/h2-10,12H,11H2,1H3,(H3,21,22,23,25)/b24-10+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM50336754
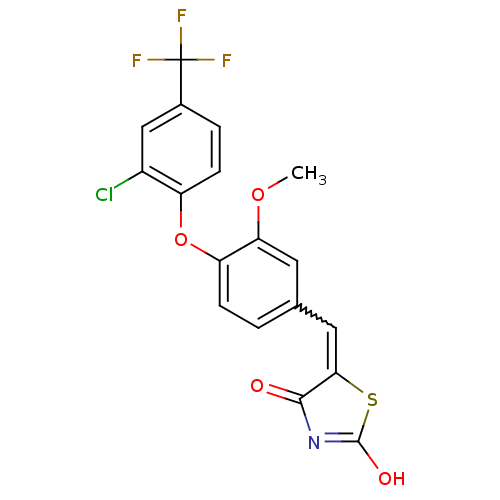
(5-[4-(2-Chloro-4-trifluoromethylphenoxy)-3-methoxy...)Show SMILES COc1cc(C=C2SC(O)=NC2=O)ccc1Oc1ccc(cc1Cl)C(F)(F)F |w:5.4,c:9| Show InChI InChI=1S/C18H11ClF3NO4S/c1-26-14-6-9(7-15-16(24)23-17(25)28-15)2-4-13(14)27-12-5-3-10(8-11(12)19)18(20,21)22/h2-8H,1H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377273

(CHEMBL430031)Show SMILES CC(C)CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C23H24FN7O/c1-15(2)13-32-29-11-20-22(25)26-14-27-23(20)30-19-6-7-21-17(9-19)10-28-31(21)12-16-4-3-5-18(24)8-16/h3-11,14-15H,12-13H2,1-2H3,(H3,25,26,27,30)/b29-11+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377280

(CHEMBL256527)Show SMILES CO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3ccc(F)cc3)ncc2c1 Show InChI InChI=1S/C20H18FN7O/c1-29-26-10-17-19(22)23-12-24-20(17)27-16-6-7-18-14(8-16)9-25-28(18)11-13-2-4-15(21)5-3-13/h2-10,12H,11H2,1H3,(H3,22,23,24,27)/b26-10+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Steroid hormone receptor ERR1
(Homo sapiens (Human)) | BDBM22420
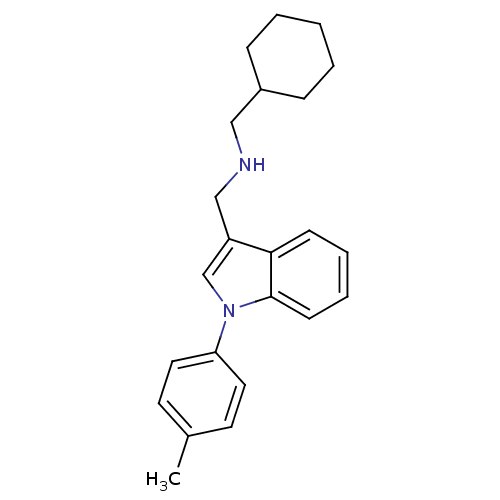
((cyclohexylmethyl)({[1-(4-methylphenyl)-1H-indol-3...)Show InChI InChI=1S/C23H28N2/c1-18-11-13-21(14-12-18)25-17-20(22-9-5-6-10-23(22)25)16-24-15-19-7-3-2-4-8-19/h5-6,9-14,17,19,24H,2-4,7-8,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377270

(CHEMBL255490)Show InChI InChI=1S/C12H11ClFN5O/c1-20-18-5-8-11(15)16-6-17-12(8)19-7-2-3-10(14)9(13)4-7/h2-6H,1H3,(H3,15,16,17,19)/b18-5+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data