 Found 220 hits with Last Name = 'seki' and Initial = 'j'
Found 220 hits with Last Name = 'seki' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50168287
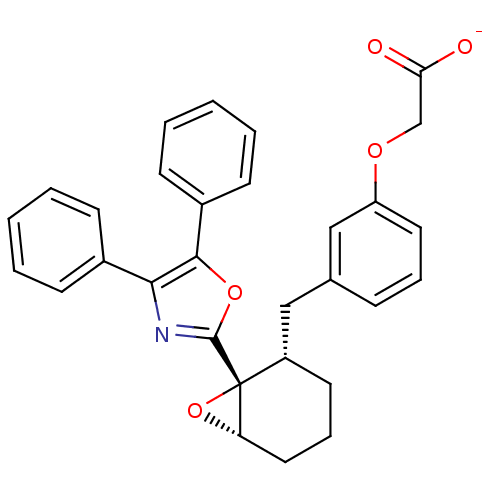
(CHEMBL363800 | Sodium; {3-[(1R,2S,6S)-1-(4,5-diphe...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC[C@@H]3O[C@]23c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C30H27NO5/c32-26(33)19-34-24-15-7-9-20(18-24)17-23-14-8-16-25-30(23,36-25)29-31-27(21-10-3-1-4-11-21)28(35-29)22-12-5-2-6-13-22/h1-7,9-13,15,18,23,25H,8,14,16-17,19H2,(H,32,33)/p-1/t23-,25-,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptor |
Bioorg Med Chem Lett 15: 3279-83 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.042
BindingDB Entry DOI: 10.7270/Q2J103X8 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167890
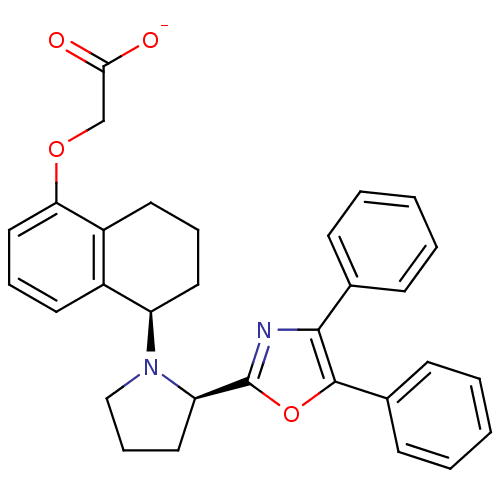
(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptor |
Bioorg Med Chem Lett 15: 3279-83 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.042
BindingDB Entry DOI: 10.7270/Q2J103X8 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50168291
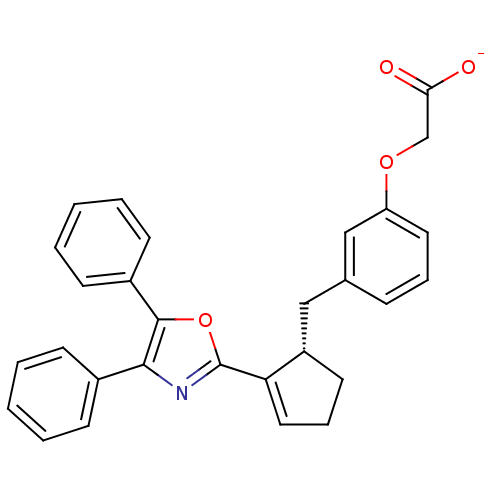
(CHEMBL363350 | Sodium; {3-[(S)-2-(4,5-diphenyl-oxa...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:14| Show InChI InChI=1S/C29H25NO4/c31-26(32)19-33-24-15-7-9-20(18-24)17-23-14-8-16-25(23)29-30-27(21-10-3-1-4-11-21)28(34-29)22-12-5-2-6-13-22/h1-7,9-13,15-16,18,23H,8,14,17,19H2,(H,31,32)/p-1/t23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50370452
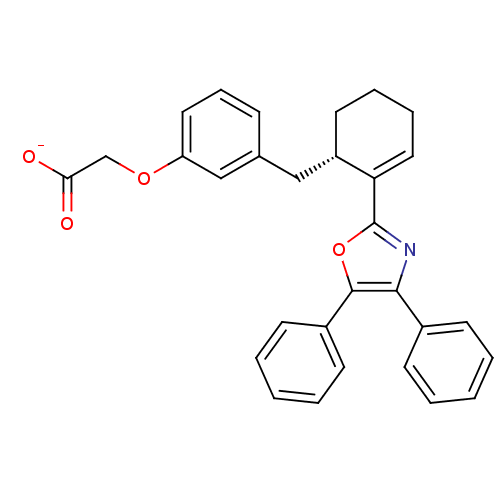
(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptor |
Bioorg Med Chem Lett 15: 3279-83 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.042
BindingDB Entry DOI: 10.7270/Q2J103X8 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50136234
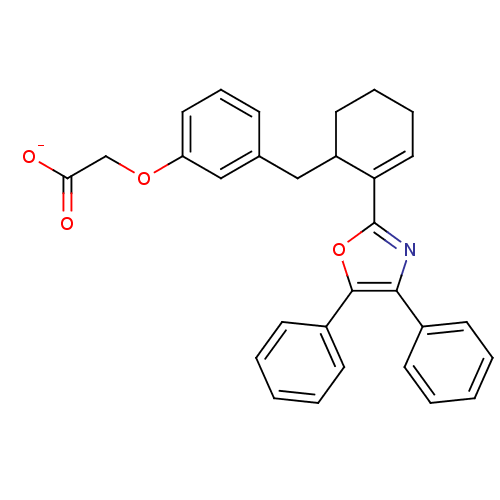
(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from cloned human PGI2 receptor |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50191133

(CHEMBL439357 | sodium (R)-2-(3-((2-(4,5-diphenylox...)Show SMILES [O-]C(=O)COc1cccc(CN2CCC[C@@H]2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C28H26N2O4/c31-25(32)19-33-23-14-7-9-20(17-23)18-30-16-8-15-24(30)28-29-26(21-10-3-1-4-11-21)27(34-28)22-12-5-2-6-13-22/h1-7,9-14,17,24H,8,15-16,18-19H2,(H,31,32)/p-1/t24-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from cloned human PGI2 receptor |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50095202
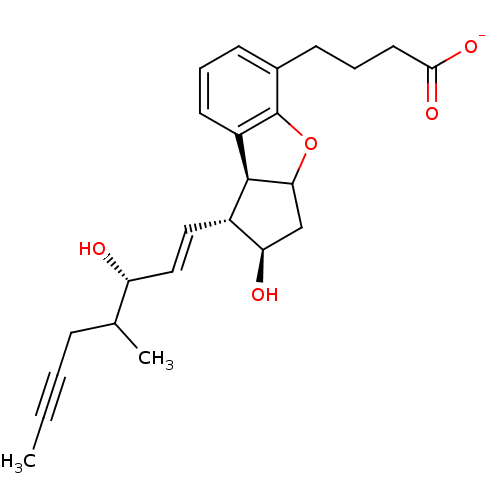
(4-[2-Hydroxy-3-((E)-3-hydroxy-4-methyl-oct-1-en-6-...)Show SMILES CC#CCC(C)[C@H](O)\C=C\[C@H]1[C@H](O)CC2Oc3c(cccc3CCCC([O-])=O)[C@H]12 Show InChI InChI=1S/C24H30O5/c1-3-4-7-15(2)19(25)13-12-17-20(26)14-21-23(17)18-10-5-8-16(24(18)29-21)9-6-11-22(27)28/h5,8,10,12-13,15,17,19-21,23,25-26H,6-7,9,11,14H2,1-2H3,(H,27,28)/p-1/b13-12+/t15?,17-,19+,20+,21?,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Tested for inhibition of 3[H]-iloprost binding to human IP receptor |
Bioorg Med Chem Lett 10: 2787-90 (2000)
BindingDB Entry DOI: 10.7270/Q26972TT |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50333455
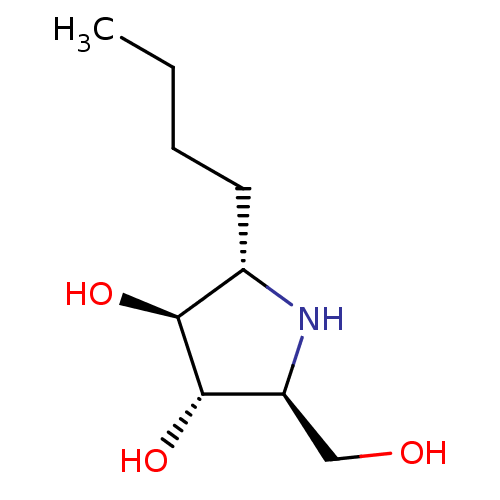
((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-6-8(12)9(13)7(5-11)10-6/h6-13H,2-5H2,1H3/t6-,7-,8-,9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50095203

(CHEMBL94751 | [(S)-6-(3-Benzhydryl-6-oxo-6H-pyrida...)Show SMILES OC(=O)COc1cccc2C[C@@H](Cn3nc(ccc3=O)C(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C30H28N2O4/c33-28-17-16-26(30(22-8-3-1-4-9-22)23-10-5-2-6-11-23)31-32(28)19-21-14-15-25-24(18-21)12-7-13-27(25)36-20-29(34)35/h1-13,16-17,21,30H,14-15,18-20H2,(H,34,35)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Tested for inhibition of 3[H]-iloprost binding to human IP receptor |
Bioorg Med Chem Lett 10: 2787-90 (2000)
BindingDB Entry DOI: 10.7270/Q26972TT |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167891
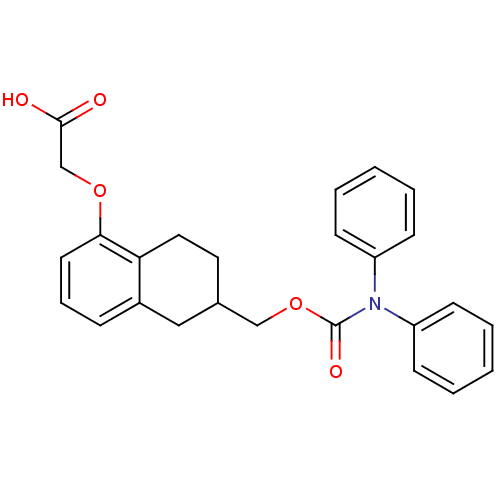
(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50242271
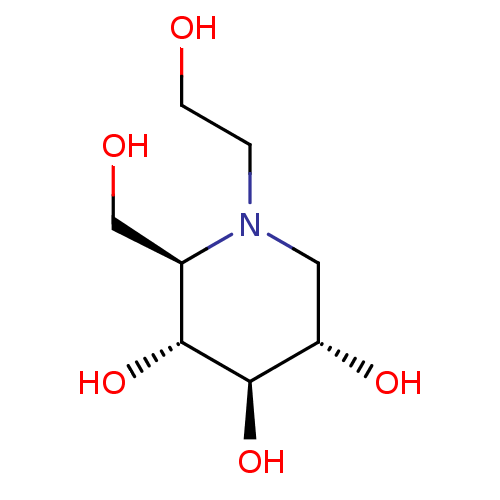
((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...)Show SMILES OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C8H17NO5/c10-2-1-9-3-6(12)8(14)7(13)5(9)4-11/h5-8,10-14H,1-4H2/t5-,6+,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50168287

(CHEMBL363800 | Sodium; {3-[(1R,2S,6S)-1-(4,5-diphe...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC[C@@H]3O[C@]23c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C30H27NO5/c32-26(33)19-34-24-15-7-9-20(18-24)17-23-14-8-16-25-30(23,36-25)29-31-27(21-10-3-1-4-11-21)28(35-29)22-12-5-2-6-13-22/h1-7,9-13,15,18,23,25H,8,14,16-17,19H2,(H,32,33)/p-1/t23-,25-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-SQ-29,548 binding to human Prostanoid TP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-SQ-29,548 binding to human Prostanoid TP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ-29,548 from human Prostanoid TP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF-2 from human Prostanoid FP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF-2 from human Prostanoid FP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD-2 from human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ-29,548 from human Prostanoid TP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD-2 from human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGD-2 binding to human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGF-2 binding to human Prostanoid FP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50168287

(CHEMBL363800 | Sodium; {3-[(1R,2S,6S)-1-(4,5-diphe...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC[C@@H]3O[C@]23c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C30H27NO5/c32-26(33)19-34-24-15-7-9-20(18-24)17-23-14-8-16-25-30(23,36-25)29-31-27(21-10-3-1-4-11-21)28(35-29)22-12-5-2-6-13-22/h1-7,9-13,15,18,23,25H,8,14,16-17,19H2,(H,32,33)/p-1/t23-,25-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD-2 from human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50168287

(CHEMBL363800 | Sodium; {3-[(1R,2S,6S)-1-(4,5-diphe...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC[C@@H]3O[C@]23c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C30H27NO5/c32-26(33)19-34-24-15-7-9-20(18-24)17-23-14-8-16-25-30(23,36-25)29-31-27(21-10-3-1-4-11-21)28(35-29)22-12-5-2-6-13-22/h1-7,9-13,15,18,23,25H,8,14,16-17,19H2,(H,32,33)/p-1/t23-,25-,30+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGD-2 binding to human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50168287

(CHEMBL363800 | Sodium; {3-[(1R,2S,6S)-1-(4,5-diphe...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC[C@@H]3O[C@]23c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C30H27NO5/c32-26(33)19-34-24-15-7-9-20(18-24)17-23-14-8-16-25-30(23,36-25)29-31-27(21-10-3-1-4-11-21)28(35-29)22-12-5-2-6-13-22/h1-7,9-13,15,18,23,25H,8,14,16-17,19H2,(H,32,33)/p-1/t23-,25-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50168287

(CHEMBL363800 | Sodium; {3-[(1R,2S,6S)-1-(4,5-diphe...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC[C@@H]3O[C@]23c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C30H27NO5/c32-26(33)19-34-24-15-7-9-20(18-24)17-23-14-8-16-25-30(23,36-25)29-31-27(21-10-3-1-4-11-21)28(35-29)22-12-5-2-6-13-22/h1-7,9-13,15,18,23,25H,8,14,16-17,19H2,(H,32,33)/p-1/t23-,25-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data