

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50133069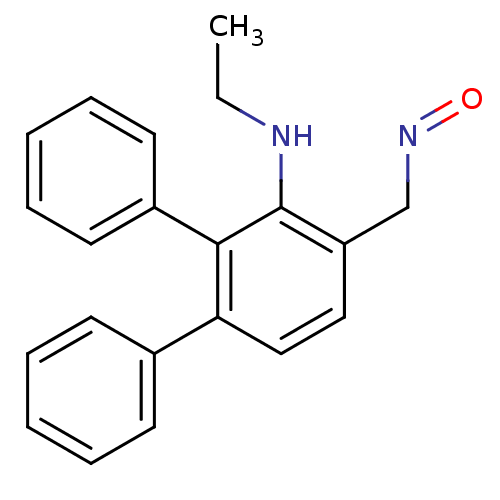 (3'-Ethylamino-[1,1';2',1'']terphenyl-4'-carbaldehy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50133070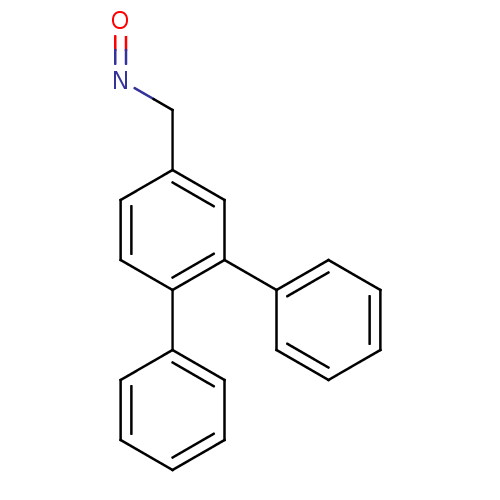 (CHEMBL335465 | [1,1';2',1'']Terphenyl-4'-carbaldeh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50133070 (CHEMBL335465 | [1,1';2',1'']Terphenyl-4'-carbaldeh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50133069 (3'-Ethylamino-[1,1';2',1'']terphenyl-4'-carbaldehy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50133071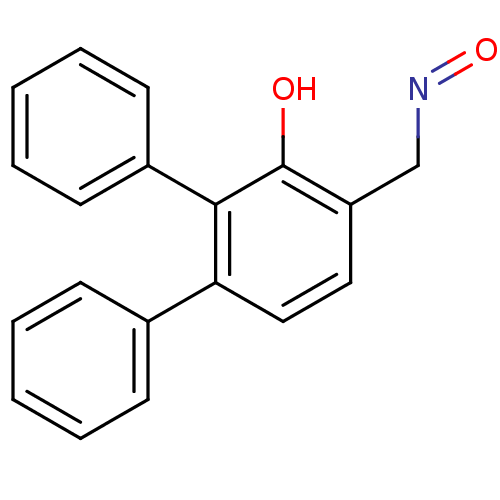 (3'-Hydroxy-[1,1';2',1'']terphenyl-4'-carbaldehyde ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50133071 (3'-Hydroxy-[1,1';2',1'']terphenyl-4'-carbaldehyde ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50133072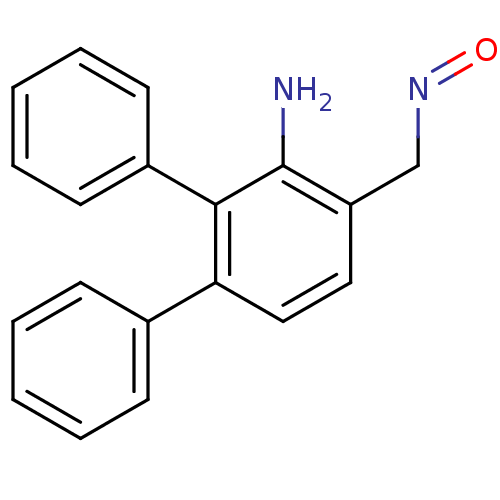 (3'-Amino-[1,1';2',1'']terphenyl-4'-carbaldehyde ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50133072 (3'-Amino-[1,1';2',1'']terphenyl-4'-carbaldehyde ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50133068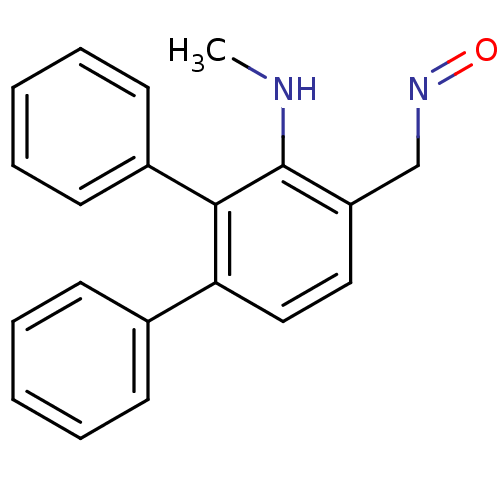 (3'-Methylamino-[1,1';2',1'']terphenyl-4'-carbaldeh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50133068 (3'-Methylamino-[1,1';2',1'']terphenyl-4'-carbaldeh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19455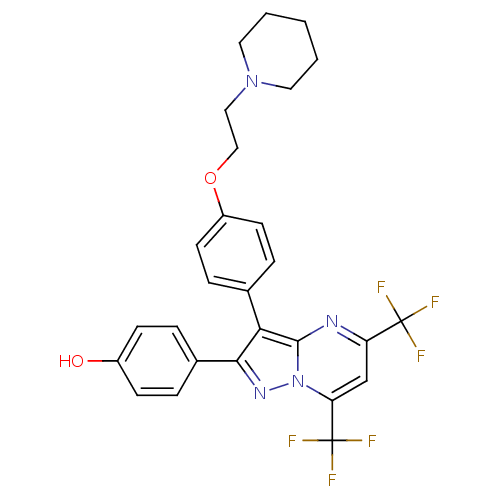 (4-(3-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-5,7-bis(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -42.6 | n/a | n/a | 90 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19454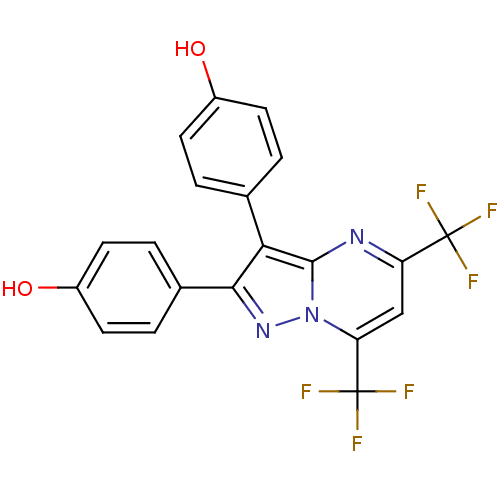 (4-[2-(4-hydroxyphenyl)-5,7-bis(trifluoromethyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -39.7 | n/a | n/a | 6.00E+3 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19456 (4-(3-{4-[2-(dimethylamino)ethoxy]phenyl}-5,7-bis(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 47 | -38.3 | n/a | n/a | 1.00E+3 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19454 (4-[2-(4-hydroxyphenyl)-5,7-bis(trifluoromethyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 48 | -38.3 | n/a | n/a | 600 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19453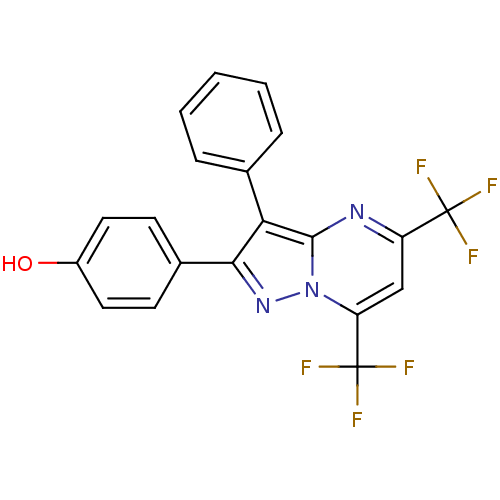 (4-[3-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 51 | -38.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19455 (4-(3-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-5,7-bis(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 71 | -37.4 | n/a | n/a | 40 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Binding affinity for human estrogen receptor alpha by displacement of [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19453 (4-[3-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 122 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19452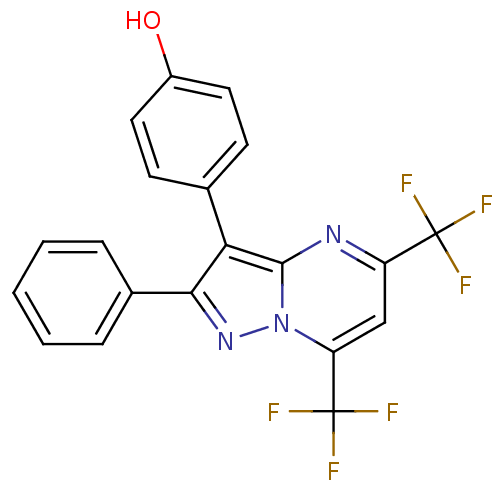 (4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 139 | -35.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19458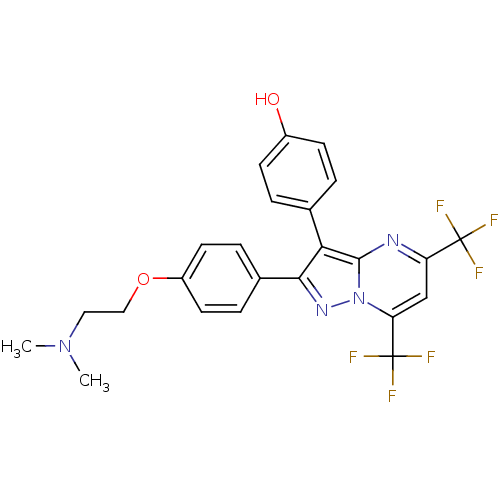 (4-(2-{4-[2-(dimethylamino)ethoxy]phenyl}-5,7-bis(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 156 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19456 (4-(3-{4-[2-(dimethylamino)ethoxy]phenyl}-5,7-bis(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 178 | -35.3 | n/a | n/a | 250 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19457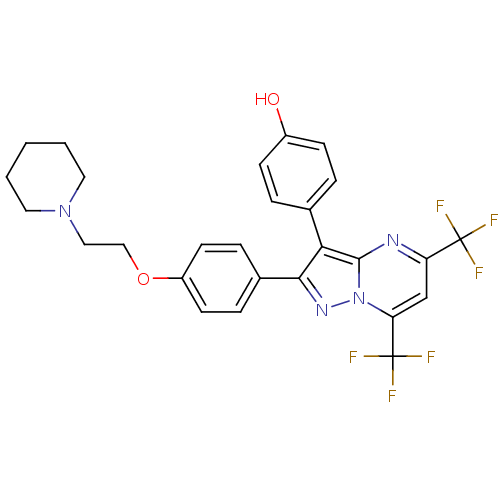 (4-(2-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-5,7-bis(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 192 | -35.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19457 (4-(2-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-5,7-bis(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 266 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19458 (4-(2-{4-[2-(dimethylamino)ethoxy]phenyl}-5,7-bis(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 408 | -33.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The enzyme kinetics were determined, wherein the arachidonic acid substrate either in the absence or presence of selected derivatives were evaluated ... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198173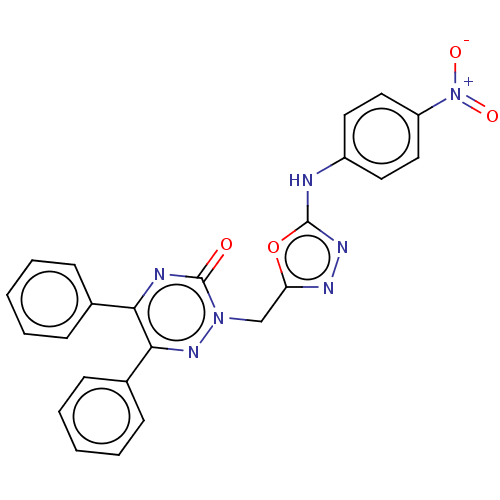 (2-((5-(4-Nitrophenylamino)-1,3,4-oxadiazol-2-yl)me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The enzyme kinetics were determined, wherein the arachidonic acid substrate either in the absence or presence of selected derivatives were evaluated ... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198172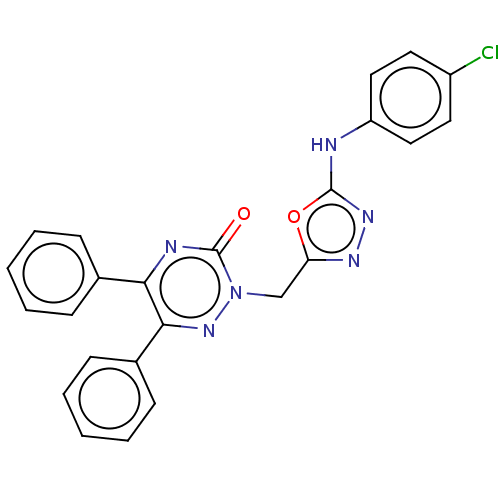 (2-((5-(4-Chlorophenylamino)-1,3,4-oxadiazol-2-yl)m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The enzyme kinetics were determined, wherein the arachidonic acid substrate either in the absence or presence of selected derivatives were evaluated ... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198171 (2-((5-(4-Methoxyphenylamino)-1,3,4-oxadiazol-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The enzyme kinetics were determined, wherein the arachidonic acid substrate either in the absence or presence of selected derivatives were evaluated ... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198174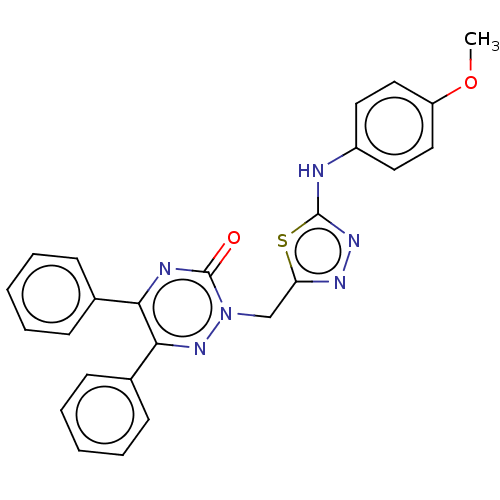 (2-((5-(4-Methoxyphenylamino)-1,3,4-thiadiazol-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The enzyme kinetics were determined, wherein the arachidonic acid substrate either in the absence or presence of selected derivatives were evaluated ... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198176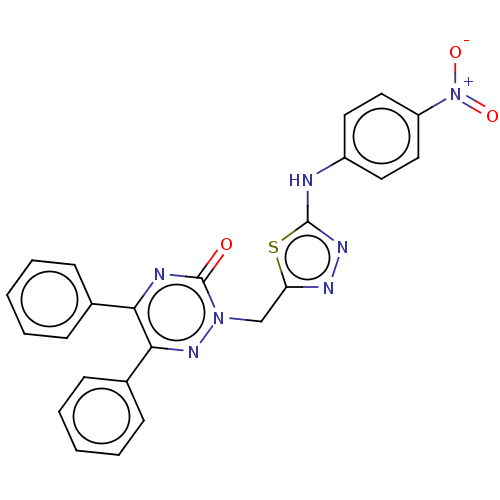 (2-((5-(4-Nitrophenylamino)-1,3,4-thiadiazol-2-yl)m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The enzyme kinetics were determined, wherein the arachidonic acid substrate either in the absence or presence of selected derivatives were evaluated ... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198175 (2-((5-(4-Chlorophenylamino)-1,3,4-thiadiazol-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The enzyme kinetics were determined, wherein the arachidonic acid substrate either in the absence or presence of selected derivatives were evaluated ... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19451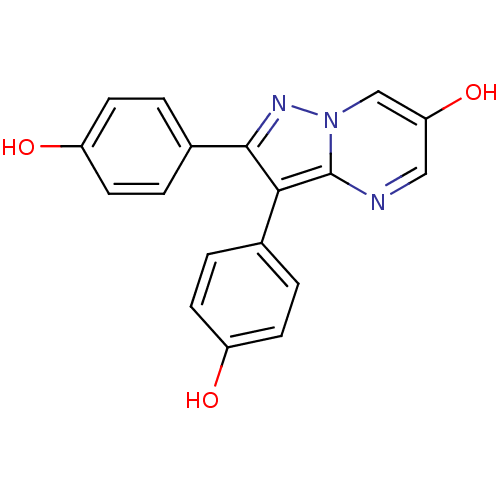 (2,3-bis(4-hydroxyphenyl)pyrazolo[1,5-a]pyrimidin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.52E+3 | -30.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19452 (4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.22E+3 | -29.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19451 (2,3-bis(4-hydroxyphenyl)pyrazolo[1,5-a]pyrimidin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.33E+3 | -28.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana | Assay Description Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... | J Med Chem 50: 399-403 (2007) Article DOI: 10.1021/jm061035y BindingDB Entry DOI: 10.7270/Q2QZ288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Competitive inhibition of ovine COX2 using arachidonic acid substrate by Ellman's spectrophotometric assay based Lineweaver-Burk double reciprocal pl... | Eur J Med Chem 101: 81-95 (2015) Article DOI: 10.1016/j.ejmech.2015.06.020 BindingDB Entry DOI: 10.7270/Q2J38VCX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50114413 (CHEMBL3608346) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Competitive inhibition of ovine COX2 using arachidonic acid substrate by Ellman's spectrophotometric assay based Lineweaver-Burk double reciprocal pl... | Eur J Med Chem 101: 81-95 (2015) Article DOI: 10.1016/j.ejmech.2015.06.020 BindingDB Entry DOI: 10.7270/Q2J38VCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50114414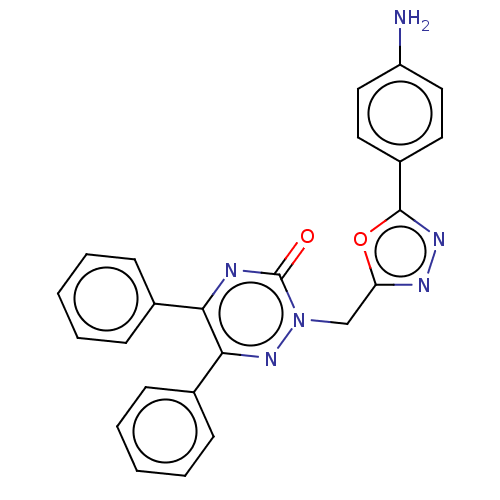 (CHEMBL3608347) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Competitive inhibition of ovine COX2 using arachidonic acid substrate by Ellman's spectrophotometric assay based Lineweaver-Burk double reciprocal pl... | Eur J Med Chem 101: 81-95 (2015) Article DOI: 10.1016/j.ejmech.2015.06.020 BindingDB Entry DOI: 10.7270/Q2J38VCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50114415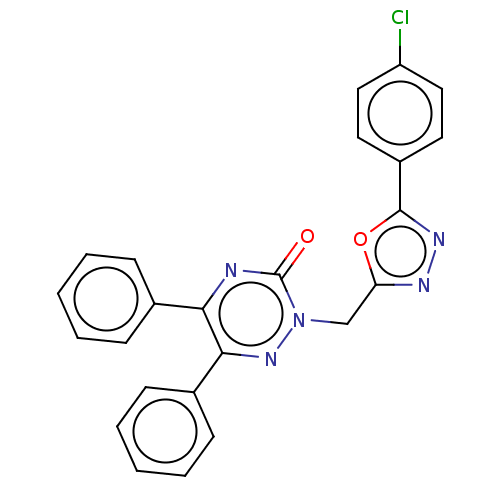 (CHEMBL3608348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Competitive inhibition of ovine COX2 using arachidonic acid substrate by Ellman's spectrophotometric assay based Lineweaver-Burk double reciprocal pl... | Eur J Med Chem 101: 81-95 (2015) Article DOI: 10.1016/j.ejmech.2015.06.020 BindingDB Entry DOI: 10.7270/Q2J38VCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50114416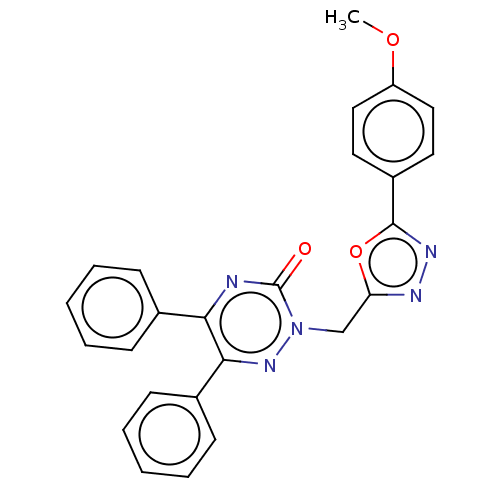 (CHEMBL3608351) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Competitive inhibition of ovine COX2 using arachidonic acid substrate by Ellman's spectrophotometric assay based Lineweaver-Burk double reciprocal pl... | Eur J Med Chem 101: 81-95 (2015) Article DOI: 10.1016/j.ejmech.2015.06.020 BindingDB Entry DOI: 10.7270/Q2J38VCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50114417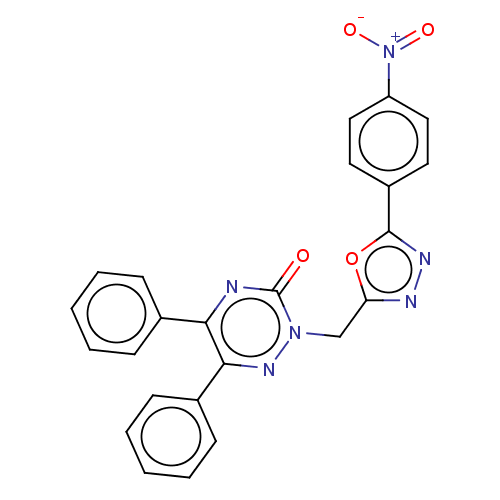 (CHEMBL3608353) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Competitive inhibition of ovine COX2 using arachidonic acid substrate by Ellman's spectrophotometric assay based Lineweaver-Burk double reciprocal pl... | Eur J Med Chem 101: 81-95 (2015) Article DOI: 10.1016/j.ejmech.2015.06.020 BindingDB Entry DOI: 10.7270/Q2J38VCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The ability of the compounds (3c-3e and 4c-4e) to inhibit ovine COX-1 and COX-2 was evaluated using a colorimetric COX (ovine) inhibitor screening as... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50215926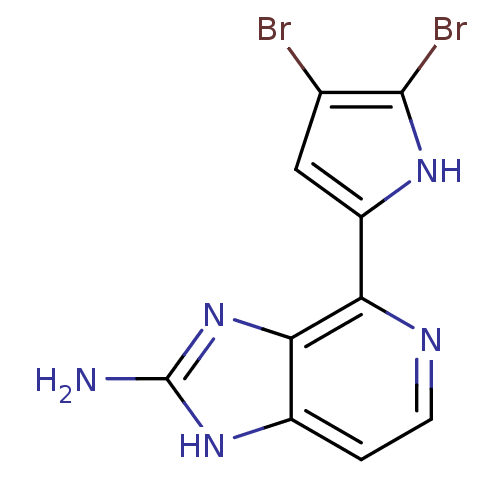 (4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University Curated by ChEMBL | Assay Description Inhibition of MMP14 using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg- NH2 as substrate preincubated for 10 mins measured after 3 hrs by fluorescence ass... | J Med Chem 54: 2492-503 (2011) Article DOI: 10.1021/jm200039m BindingDB Entry DOI: 10.7270/Q2QV3NMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198173 (2-((5-(4-Nitrophenylamino)-1,3,4-oxadiazol-2-yl)me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The ability of the compounds (3c-3e and 4c-4e) to inhibit ovine COX-1 and COX-2 was evaluated using a colorimetric COX (ovine) inhibitor screening as... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198172 (2-((5-(4-Chlorophenylamino)-1,3,4-oxadiazol-2-yl)m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The ability of the compounds (3c-3e and 4c-4e) to inhibit ovine COX-1 and COX-2 was evaluated using a colorimetric COX (ovine) inhibitor screening as... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198171 (2-((5-(4-Methoxyphenylamino)-1,3,4-oxadiazol-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The ability of the compounds (3c-3e and 4c-4e) to inhibit ovine COX-1 and COX-2 was evaluated using a colorimetric COX (ovine) inhibitor screening as... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198174 (2-((5-(4-Methoxyphenylamino)-1,3,4-thiadiazol-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The ability of the compounds (3c-3e and 4c-4e) to inhibit ovine COX-1 and COX-2 was evaluated using a colorimetric COX (ovine) inhibitor screening as... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50215926 (4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 924 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University Curated by ChEMBL | Assay Description Inhibition of MMP12 | J Med Chem 54: 2492-503 (2011) Article DOI: 10.1021/jm200039m BindingDB Entry DOI: 10.7270/Q2QV3NMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM198176 (2-((5-(4-Nitrophenylamino)-1,3,4-thiadiazol-2-yl)m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) | Assay Description The ability of the compounds (3c-3e and 4c-4e) to inhibit ovine COX-1 and COX-2 was evaluated using a colorimetric COX (ovine) inhibitor screening as... | Bioorg Chem 69: 102-120 (2016) Article DOI: 10.1016/j.bioorg.2016.10.003 BindingDB Entry DOI: 10.7270/Q2SB44K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50215926 (4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University Curated by ChEMBL | Assay Description Inhibition of MMP8 | J Med Chem 54: 2492-503 (2011) Article DOI: 10.1021/jm200039m BindingDB Entry DOI: 10.7270/Q2QV3NMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 127 total ) | Next | Last >> |